Abstract
In early pancreatic carcinogenesis, TGFβ acts as a tumor suppressor due to its growth-inhibitory effects in epithelial cells. However, in advanced disease, TGFβ appears to promote tumor progression. Therefore, to better understand the contributions of TGFβ signaling to pancreatic carcinogenesis, we generated mouse models of pancreatic cancer with either epithelial or systemic TGFBR deficiency. We found that epithelial suppression of TGFβ signals facilitated pancreatic tumorigenesis, whereas global loss of TGFβ signaling protected against tumor development via inhibition of tumor-associated fibrosis, stromal TGFβ1 production, and the resultant restoration of anti-tumor immune function. Similarly, TGFBR-deficient T cells resisted TGFβ-induced inactivation ex vivo, and adoptive transfer of TGFBR-deficient CD8+ T cells led to enhanced infiltration and GranzymeB-mediated destruction of developing tumors. These findings paralleled our observations in human patients, where TGFβ expression correlated with increased fibrosis and associated negatively with expression of GranzymeB. Collectively, our findings suggest that, despite opposing the proliferation of some epithelial cells, TGFβ may promote pancreatic cancer development by affecting stromal and hematopoietic cell function. Therefore, the use of TGFBR-inhibition to target components of the tumor microenvironment warrants consideration as a potential therapy for pancreatic cancer, particularly in patients who have already lost tumor suppressive TGFβ signals in the epithelium.
Introduction
While there have been several recent therapeutic advances in many GI malignancies, pancreatic ductal adenocarcinoma (PDAC) remains remarkably lethal with a 5-year survival rate of less than 7% (1). Despite the high prevalence of mutations in the Kras allele, therapeutic strategies targeting KRAS have been ineffective. Therefore, new therapeutic approaches are needed, and targeting the transforming growth factor-β (TGFβ) pathway may be one such approach.
TGFβ is a multifunctioning cytokine that has been implicated in nearly all the key steps of tumorigenesis (2). While TGFβ has potent growth inhibitory effects in benign and neoplastic epithelia, it often serves as a tumor promoter in more advanced stages. In brief, TGFβ is secreted as a latent complex and sequestered in the extracellular matrix until activated. Once biologically available to its target cells, TGFβ binds its type 2 receptor (TGFBR2), leading to the recruitment of its type 1 receptor (TGFBR1) and subsequent downstream signaling that leads to nuclear localization of the SMAD2/3/4 complex. In pancreas epithelial cells, TGFβ affects the cell cycle via upregulation of cyclin-dependent kinase inhibitors, particularly p21CIP1/WAF1 (3). In advanced tumors, this function is often lost (4) as TGFβ levels begin to correlate positively with recurrence and negatively with disease-free survival (5). Therefore, to successfully target TGFβ signals in tumors, it is imperative to identify patients with loss of the beneficial aspects of TGFβ-signals and/or those in which TGFβ actively promotes disease progression, thereby maximizing the efficacy and minimizing the risk of therapeutic TGFBR inhibition.
Recent evidence suggests that TGFβ is also a potent mediator of the tumor microenvironment, affecting several non-epithelial cell types (2). This includes stromal and hematopoietic cell types, both of which are critical to the incidence and progression of pancreatic cancer (6-8). While TGFβ is generally growth inhibitory to epithelial cells, it promotes the proliferation of mesenchymal cells such as pancreas stellate cells (9). Similarly, TGFβ has been implicated in promoting both the migration of and matrix deposition by these cells (10).
TGFβ is also a potent modifier of immune cells, particularly T lymphocytes. In CD4+ T cells, TGFβ upregulates the forkhead box transcription factor FoxP3 (11). These CD4+FoxP3+ regulatory T cells (Tregs) also generally express CD25, and serve to suppress the cytotoxic and inflammatory function of effector T cells (12). Clinically, elevated Treg populations correlate negatively with outcomes in several cancers, linking TGFβ signaling to tumor evasion of immune surveillance (2). TGFβ also directly affects CD8+ cytotoxic T lymphocytes (CTLs). In vivo experiments have also demonstrated that such lymphocytes with truncated TGFβ signals mount a robust anti-tumor immune response (13-16). Additionally, TGFβ suppresses CTL activity and differentiation, through repression of several genes involved in an anti-tumor immune response including GranzymeB (17), an anti-tumor serine protease found in CTL-associated cytotoxic granules (18, 19).
While it is well established that TGFβ is a modifier of the tumor microenvironment, the overall effects of these contributions to pancreatic carcinogenesis are poorly understood, with most studies focusing on the role of TGFβ on regulating epithelial cells. Previous in vivo studies have found that, upon conditional loss of Smad4 or Tgfbr2, Pdx1-Cre/LSL-Kras mice developed advanced pancreatic cancer with increases in the incidence and severity of developing adenocarcinoma from neoplastic lesions (20-22). However, systemic deficiency of Tgfbr1 in Elastase-KrasG12D (EL-Kras) mice (23) led to a dramatic reduction in the neoplastic phenotype (24).
To identify a mechanism that explains these diametrically opposed findings, we combined a well-established mouse model of neoplastic disease of the pancreas with those having reduced TGFβ signaling. We have extended our previous study using EL-Kras mice, in which expression of mutant human KRASG12D is restricted to the pancreas acinar compartment via a rat elastase promoter (23). At two to three months of age, EL-Kras mice present with acinar-ductal metaplasia (ADM), also commonly observed in human pancreatic cancer samples, and thought to be predecessors to neoplastic disease. Interestingly, ADM lesions lose elastase expression, yet retain promoter autonomous expression of mutant KRASG12D (23). At four to five months, EL-Kras mice present with predominantly cystic disease, closely resembling human intraductal papillary mucinous neoplasms (IPMNs), also with promoter autonous KRASG12D expression (23). El-Kras mice also develop occasional lesions resembling pancreatic intraepithelial neoplastic (PanIN) disease, and both IMPN and PanIN lesions can predate PDAC in human patients (23).
El-Kras mice were crossed to those with suppressed TGFβ signaling via epithelial expression of a dominant negative TGFBR2 (MT-TGFBR2DN) (25) and/or globally haploinsufficient in Tgfbr1 (Tgfbr1+/-) (26). The selection of mice heterozygous for MT-TGFBR2DN provides strictly epithelial suppression of TGFβ signaling, particularly in the pancreas (25). This system uses a control of a modified metallothionein 1 (MT) promoter, which allows for cation-inducible expression of the TGFBR2DN transgene in the pancreas, GI tract, and liver due to the abundance of endogenous zinc in these organs (25). In contrast, neither the spleen nor the thymus exhibit expression of the TGFBR2DN transcript, even when the mice were administered a large dose of zinc (25). This specificity was demonstrated ex vivo in our recent study (Principe et al., Cancer Research – In Review). This approach allows for TGFβ signaling inhibition in both acinar and ductal cells, thereby maintaining diminished TGFβ signals in acinar cells and during ADM and the establishment of ductal lesions. Furthermore, this bi-allelic system best recapitulates locally reduced TGFβ signaling observed in the pancreas of heterozygous Tgfbr1+/- mice. The choice of Tgfbr1+/- is seamless with our initial study (24) and extends the original findings from this model in a clean B6 background, while including mechanistic data that defines the function of TGFβ in non-parenchymal tissue compartments.
Using mouse, human, and in vitro models, we explored the role of TGFβ signaling in epithelial and immune cells during pancreatic carcinogenesis. In the pancreas, epithelial disruption of TGFBR leads to advanced disease corroborating earlier reports. Yet, global TGFBR deficiency protected against tumor development by impeding tumor evasion of immune surveillance and enhancing cell-mediated cytotoxicity against developing neoplastic lesions. Therefore, these findings may support therapeutic targeting of TGFBRs in a subset of pancreatic cancers with epithelial loss of TGFβ signals (i.e. DPC4/SMAD4), yet sustained pathological TGFβ signaling in the tumor microenvironment.
Materials and Methods
Cell lines and Co-Cultures
Human pancreatic stellate cells (hPSC) and human pancreatic caner cells (PANC1) were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 units/mL), and streptomycin (100 μg/mL). Primary peripheral blood mononuclear cell cultures were maintained in AIM-V media supplemented with 20% heat-inactivated fetal bovine serum (FBS), penicillin (100 units/mL), and streptomycin (100 μg/mL). All cells were cultured in a 37 °C incubator with 5% CO2.
PANC1 cells were purchased from the American Type Culture Collection (ATCC) and used less than six months from purchase, and kept under passage 10. Early passage hPSC cells were provided by the original lab that isolated these cells. Cells are routinely validated by negative western blotting for E-Cadherin and positive western blotting for αSMA. All cell lines in the laboratory are tested for mycoplasma every six momths via LookOut Mycoplasma PCR Detection Kit (Sigma Aldrich, St. Louis, MO) and, if positive, treated with w/ Plasmocin (InvivoGen, SanDiego, CA) until mycoplasma could not be detected with the aforementioned kit.
Co-cultures were established by seeding epithelial cells in the bottom of six well plates, and tissue-corresponding stromal cell lines in transwell inserts in separate plates. Cells were allowed to adhere in their own media for 24 hours, then the stroma containing transwell inserts were added to the 6 well plates containing the epithelial monolayers. Cells were given fresh media, allowed to acclimate for 24 hours, and starved of serum overnight prior to TGFβ treatment.
Mice
EL-Kras, MT-TGFBR2DN, and Tgfbr1+/− mice were generated. Cohorts of nongenic, MT-TGFBR2DN, or Tgfbr1+/- (N=5 per group), EL-Kras, EL-Kras-MT-TGFBR2DN, or EL-Kras-Tgfbr1+/− (N>10 per group) were euthanized after one year. For euthanasia, animals were anesthetized with xylazine/ketamine until unresponsive to toe tap and/or agonal breathing. Thoracotomy served as the primary method of euthanasia and exsanguination the secondary method. All animal studies were approved by the IACUC of Northwestern University.
Histology, immunohistochemistry/immunofluorescence, and slide scoring
Age-matched control, MT-TGFBR2DN, Tgfbr1+/-, EL-Kras, EL-Kras-MT-TGFBR2DN, EL-Kras-Tgfbr1+/-, mice were euthanized and subjected to pathological examination the pancreas, colon, small bowel, liver, and spleen. Tissues were fixed in 10% formalin, paraffin-embedded, and sections at 4μm interval were cut from each tissue, and stained with H&E, trichrome (Sigma Aldrich), or via immunohistochemistry (IHC)/immunofluorescence (IF).
For IHC, slides were deparaffinized by xylenes and rehydrated by ethanol gradient, then heated in a pressure cooker using DAKO retrieval buffer. Endogenous peroxidases were quenched in 3% hydrogen peroxide in methanol for 30 min. Tissues were blocked with 0.5% BSA in PBS for 30 min and incubated with primary antibodies against cleaved-Caspase 3 (Cell Signaling), p21, SMAD4 (Santa Cruz), αSMA, and Interferon γ (abcam) at 1:50-1:1000 overnight at 4°C. Slides were developed using either Streptavidin or secondary antibodies, followed by DAB substrate/buffer (DAKO, Carpinteria, CA).
For IF, slides were heated via pressure cooker in DAKO retrieval buffer and tissues blocked with .5% BSA in PBS for 1 hour at room temperature. Sections were exposed to primary antibodies against CK19 (University of Iowa Hybridoma Bank), PCNA, CD3, CD8 (Santa Cruz), TGFβ1, Foxp3, GranzymeB (abcam) at 1:50-1:200 overnight at 4°C. Slides were developed using alexaflour 488 or 594 conjugated secondary antibodies (1:200-1:1000, abcam), mounted in DAPI containing media (Santa Cruz), exposed to DAPI, FITC, and Texas Red filters, and images superimposed. All tissue counts and scores were performed by two blinded investigators. For any contradicting scores, a third investigator was consulted.
Statistical analysis
Data were analyzed by two-way ANOVA and fit to a general linear model in Minitab16, the validity of which was tested by adherence to the normality assumption and the fitted plot of the residuals. Results were arranged by the Tukey method, and were considered significant at p<0.05. In vitro results are presented as ± S.D., and in vivo/clinical results are presented as mean ± S.E.M unless otherwise noted.
Study approval
All experiments involving the use of mice were performed following protocols approved by the Institutional Animal Care and Use Committee at the Northwestern University. Patient slides and information was obtained in a de-identified fashion from the Northwestern University Pathcore and following local IRB approval (IRB#STU00007180).
Western blot analysis, flow cytometry, ELISA, and CD8 adoptive transferwere standard procedures. See supplement for more details.
Results
Global TGFBR Deficiency Protects Against Pancreatic Tumorigenesis Despites Epithelial Deficits in Downstream TGFβ Signaling
We have previously shown that mice with global Tgfbr1 haploinsufficiency are protected against mtKRASG12D-induced pancreatic tumorigenesis (24). To further understand the role of TGFβ signaling in vivo, we first utilized the EL-KrasG12D (KRAS) model (Figure 1a), continuous with our previous study (26). We compared 1-year old KRAS mice to those with either conditional suppression of TGFβ signaling in the epithelium (KTE; where TE represents MT-TGFBR2DN mice, Figure 1b), or global suppression of TGFβ signaling (KTG; where TG represents Tgfbr1+/- mice, Figure 1c). For a phenotypic description of these models see Figure S1. All mice were of the C57BL/6 strain, and neither TE nor TG mice demonstrated histological abnormalities in the pancreas (Figure S2).
Figure 1. Global TGFBR Deficiency Protects Against Pancreatic Tumorigenesis Despites Epithelial Deficits in Downstream TGFβ Signaling.
(a) El-Kras (KRAS) mice with mutant KRASG12D expression is restricted to the pancreas acinar compartment via a rat elastase promoter were employed as a model of early pancreatic tumorigenesis. (b) KRAS mice were crossed to mice conditionally expressing a dominant negative TGFBR2 in epithelial tissues (TE) to form KTE mice. (c) KRAS mice were next crossed to mice with heterozygous deletion of Tgfbr1 (TG) to form KTG. (d,e) At one year, 100% of KRAS animals present with cystic papillary neoplasia (yellow arrows), though gland architecture is predominantly intact. The KTE cohort has noticeable increases in lesion size (yellow arrows), frequency, and severity, contrasted by KTG mice where the majority of the gland is normal (N=15). (f,g) Tissues were stained with anti-CK19, and pancreatic acini in KRAS and KTE begin to take on a more ductal morphology demonstrating acinar-to-ductal metaplasia. In KTG, a majority of animals express CK19 solely in normal ducts. (h,i) Tissues were next stained with an antibody specific to mutant RASG12D, the expression of which was restricted to the E-Cadherin positive epithelial cells in all three models. (j-k) Proliferation was assessed by IHC staining for PCNA. Proliferation was at an intermediate level in KRAS mice compared to higher levels in KTE mice, and overall lower levels in KTG animals. (l-o) Tissue sections from were next stained with anti-pSMAD2 and anti-p21, two downstream targets of TGFβ. These results confirm the deficiency of these signals in the neoplastic epithelia of KRAS and KTE, as well as in the normal epithelia of KTG. (*, P < 0.05. N=5 mice per group unless otherwise specified).
At one year of age, KRAS mice present with multiple intraductal papillary neoplasms (IPNs), mild acinar degeneration, and acinar-to-ductal metaplasia (ADM). However, reduced epithelial TGFβ signaling in KTE mice was associated with highly advanced disease, consistent with TGFβ functioning as an early stage tumor suppressor. KTE mice displayed increased IPN development with extensive parenchymal loss and ADM. In contrast, a majority of KTG mice retained normal tissue architecture with no tissue abnormality, dysplasia or ADM. In fact, only 40% of these mice presented with neoplastic or metaplastic lesions (Figure 1d,e, N=15 per group).
These findings were corroborated by CK19 staining, a ductal marker that is ectopically expressed in acinar tissue having undergone ADM. Despite the targeting of mtKRASG12D to pancreatic acinar cells, 100% of KRAS and KTE mice had lesions positive for the ductal marker CK19, indicating dedifferentiation consistent with acinar-to-ductal metaplasia (ADM). However, since KTG mice have relatively few lesions, CK19 staining was confined to normal pancreatic ducts in a majority of these animals, and the same 40% presenting with ADM (Figure 1f,g). Consistent with our previous observations in the EL-KRAS model, despite losing their acinar state, mutant RASG12D was highly expressed in the ductal lesions of all three groups (Figure 1h,i).
Given these opposing phenotypes, we next assessed the status of TGFβ-induced cell cycle arrest in KTE and KTG animals (N=5 per group). Increased proliferating cell nuclear antigen (PCNA) staining was observed throughout the entire pancreas of KTE mice, but overall decreased PCNA staining was observed in KTG mice (Figure 1j,k). This indicates that epithelial, not global, TGFBR-deficiency increases proliferation in the KRAS background. However, inconsistent with these changes in proliferation, KTE and KTG mice had similar loss of pSMAD2 in the epithelial cells when compared to KRAS mice (Figure 1l,m). Similarly, both KTE and KTG mice also had reduced epithelial staining for p21 (Figure 1n,o), a transcriptional target of the SMAD2/3/4 complex and surrogate marker of TGFβ-induced growth arrest.
Epithelial and Global TGFBR Deficiency Have Opposing Effects on Tumor Associated Fibrosis
As TGFβ signaling is similarly perturbed in the epithelium of KTE and KTG mice, and mice with combined epithelial and global TGFBR-suppression (KTEG) are also protected against lesion formation (Figure S3), we hypothesized that the protective phenotype seen in the KTG mice was due to diminished TGFBR expression in non-epithelial cells. Thus, we next examined the effects of reduced TGFβ signaling in cells present in the tumor microenvironment.
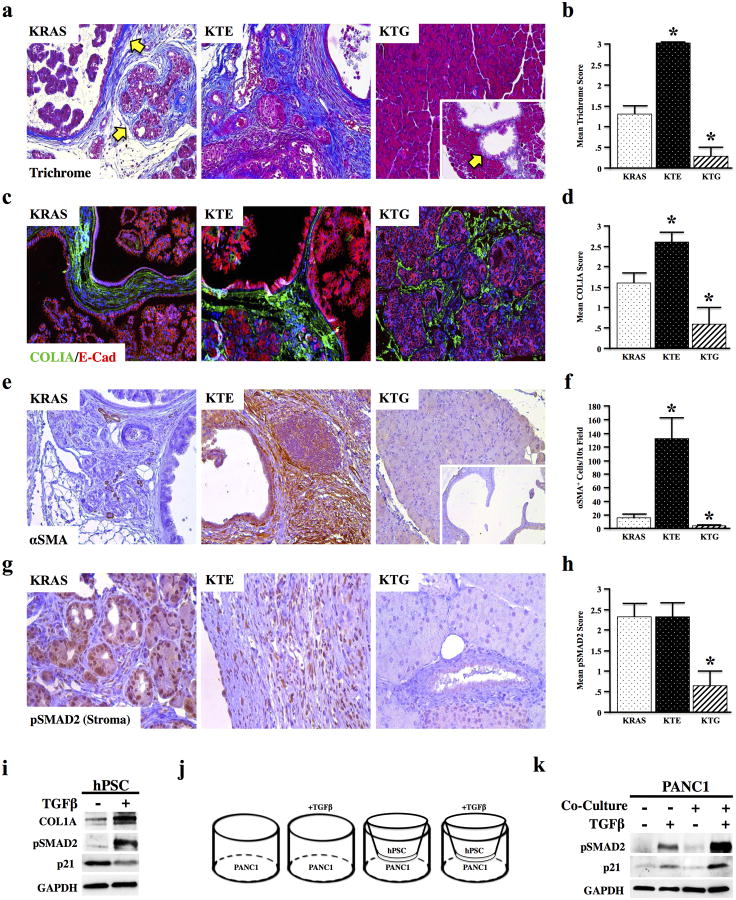
The most striking histological change in KRAS and KTE mice was the expansion of the pancreatic stroma. Lesions in KTE mice were accompanied by more severe fibrosis (as assessed by Mason's trichrome staining) and heavy matrix deposition (HABP staining), while both stains were minimal in the KTG cohort, even in the few mice that developed lesions (Figure 2a,b and Figure S4). Similarly, KTE mice also had increased Collagen IA staining compared to KRAS, which was decreased in KTG animals (Figure 2c,d). Expression of alpha smooth muscle actin (αSMA), a marker of the collagen producing pancreatic stellate cells, correlated with the extent of fibrosis, staining very strongly in KTE mice, yet was nearly absent in KTG mice (Figure 2e,f). Additionally, the pancreas stellate cell-rich stroma of KTE mice was highly positive for pSMAD2, indicative of active TGFβ signaling, while that of KTG mice was deficient in pSMAD2, consistent with globally reduced TGFβ signaling (Figure 2g,h).
Figure 2. Conditional and Global TGFBR Deficiency Have Opposing Effects on Tumor Associated Fibrosis.
(a,b) Fibrosis was assessed via trichrome staining, and KRAS mice have an intermediate phenotype with respect to KTE, where fibrosis is increased, and KTG, where fibrosis is decreased. N=4 per group. (c,d) Tissue sections were next dual stained for Collagen IA (green) and the epithelial marker E-Cadherin, indicating increased matrix deposition in KTE mice, and reduced matrix in KTG mice. (e,f) Tissue sections were next dual stained for αSMA, a marker of pancreas stellate cells. The stroma of KRAS mice had modest αSMA expression, though KTE mice had strong αSMA staining and KTG mice had very little αSMA expression, even near neoplastic lesions (*, P < 0.05. N=5 per group). (g,h) Tissue sections were next restrained for pSMAD2 and the stroma evaluated, showing high pSMAD2 staining in the stroma of KRAS and KTE mice, yet little to no staining in KTG. (i) hPSCs were incubated with recombinant TGFβ1 and lysed after 24 hours. After TGFβ1 incubation, these cells had an increase in collagen IA deposition as well as a reduction in p21, consistent with increased fibrosis and stellate cell proliferation, respectively. (j,k) PANC1 cells were co-cultured with hPSCs in transwell inserts. The co-culture was pulsed with TGFβ1. After 24 hours, these cells had a dramatic increase in downstream TGFβ-signaling, namely increased pSMAD2 and p21.
Therefore, to examine the role of TGFβ on regulating stellate cell function, immortalized human pancreatic stellate cells (hPSC) were incubated with control media or recombinant TGFβ1. After 24 hours, TGFβ1 treatment led to increased Collagen IA production and down-regulation of p21 (Figure 2i), consistent with increased fibrosis. The next step was to establish the effects of stellate cells on epithelial TGFβ signaling. Transwell co-cultures with PANC1 and hPSC cells (Figure 2j) demonstrated an increase in epithelial TGFβ signaling, as evidenced by markedly enhanced levels of pSMAD2 and p21 (Figure 2k) suggesting a synergistic effect of the stroma to epithelial TGFβ signaling.
Pancreatic Stromal Cells Mediate TGFβ Overexpression in the TME
Consistent with the increased epithelial pSMAD2 response, media from PANC1/hPSC co-cultures contained a significantly higher concentration of the TGFβ1 ligand than the sum of TGFβ1 isolated from PANC1 and hPSCs (Figure 3a, P=0.0001). To assess the mechanism underlying the change in TGFβ1 expression, hPSCs were incubated for 2 hours with the TGFBR inhibitor Galunisertib, which neutralized TGFβ-induced SMAD2 phosphorylation (Figure 3b). After 24 hours, Galunisertib also reduced endogenous TGFβ1 production (Figure 3c), suggesting TGFβ1 induces/maintains its own expression in pancreatic stellate cells through a positive feedback loop.
Figure 3. Pancreatic Stromal Cells Mediate TGFβ Overexpression in the TME.
(a) Media from isolated PANC1 and hPSCs was compared to media from PANC1/hPSC co- cultures. In the co-culture media, the amount of TGFβ1 was significantly (P=0.0001. N=4) higher than the sum of that in isolated cell media. (b) hPSCs were treated with the TGFBR-inhibitor Galunisertib, and inhibitor efficacy was assessed by western blotting for pSMAD2. (c) hPSCs were treated with Galunisertib for 24 hours, indicating that TGFBR-inhibition reduces endogenous TGFβ1 production. (d-e) hPSCs were pretreated with Galunisertib prior to co-culture with untreated PANC1 cells. hPSC and PANC1 cells were lysed separately. In the co-culture, Galunisertib pretreated hPSCs had dramatically lower levels of endogenous TGFβ1. PANC1 cells cultured in the presence of TGFβ-insensitive stroma (Stromal-Gal) had a normal response to exogenous TGFβ1, with no change in the amount of endogenous TGFβ1 ligand. These results suggest that the stroma is responsible for the increase in TGFβ1 in the co-culture setting. (f,g) TGFβ1 expression was analyzed via IHC staining in KRAS, KTE, and KTG cohorts. KRAS and particularly KTE mice had localization of TGFβ1 to the stroma, where KTG had no detectable staining in the stroma, even near neoplastic lesions (yellow arrow). (h) T cells were localized to the TGFβ-rich stroma in KRAS and KTE, and the TGFβ-low stroma of KTG mice by dual staining for Vimentin and CD3.
PANC1/hPSC co-cultures cells were then repeated with Galunisertib pretreated hPSCs, using co-cultures with untreated hPSCs as controls. Galunisertib-pretreated hPSCs produced less TGFβ1 in the co-culture than untreated controls (Figure 3d). Co-culture of PANC1 cells with the TGFβ-insensitive stroma elicited a more normal response to exogenous TGFβ1, which was measured by reduced SMAD2 phosphorylation and a reduction in p21 expression. However, there was no change in endogenous TGFβ1 production by PANC1 cells (Figure 3e). This observation suggests that increased epithelial TGFβ signaling observed in the co-culture setting is a function of the stroma, and not the epithelial cells.
Given these results, we hypothesized that the TGFβ-responsive stromal populations in KRAS and KTE animals may have elevated TGFβ biosynthesis, though this should not be observed in KTG animals where the stroma are TGFBR-deficient, and therefore, the described positive feedback mechanism is disrupted. Therefore, sections were stained for expression of TGFβ1. Expectedly, KRAS and KTE animals had strong stromal staining for TGFβ1, consistent with increased production by stellate cells. Contrastingly, there was weak to no staining in KTG mice, suggesting reduced stromal TGFβ1 biosynthesis (Figure 3f,g). This is of particular importance, as though the epithelium of KTE and KTG are both unresponsive to stromal derived TGFβ1, additional cells residing in the stroma, such as CD3+ T lymphocytes (Figure 3h), are likely affected and may further contribute to the divergent phenotypes in KTE and KTG mice.
Global TGFBR Deficiency Promotes a Cytotoxic Response Against Pancreatic Lesions
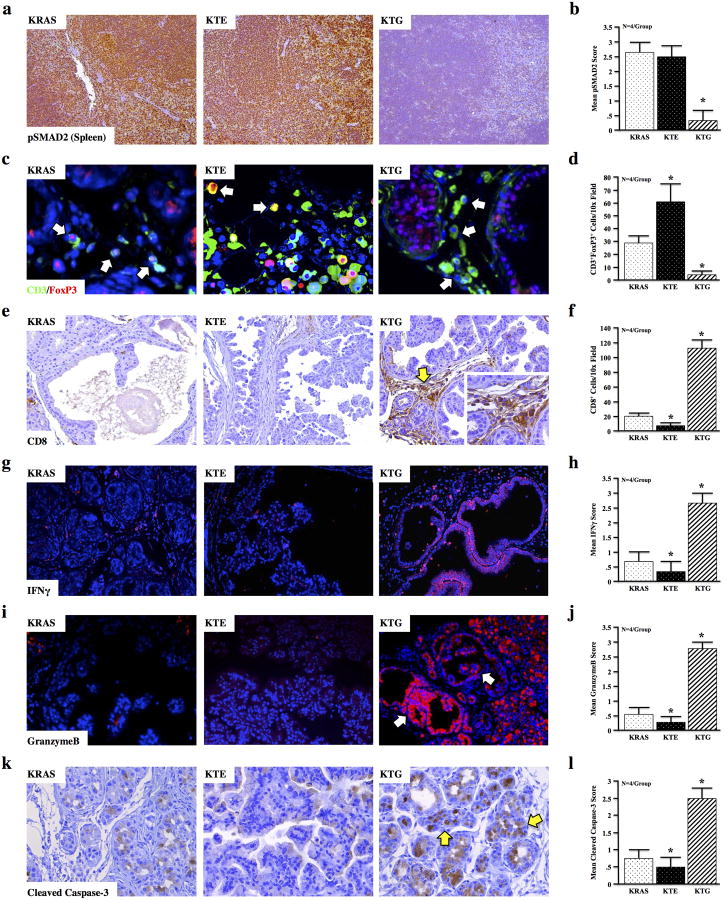
T lymphocytes are highly TGFβ-responsive and have been implicated in pancreatic cancer development (27). We found that in KRAS and KTE mice there was robust pSMAD2 expression in primary lymphoid tissues (where lymphoid TGFBR expression was not altered), yet pSMAD2 was significantly reduced in that of the globally TGFBR-deficient KTG mice (Figure 4a,b). Therefore, to assess their contribution to the tumor-protective phenotype seen in KTG mice, we next assessed the CD3+ T cell infiltrate near pancreatic lesions for downstream targets of TGFβ signaling. While total T cell infiltration was increased in both KTE and KTG animals, a large population of these CD3+ cells in KTE and KRAS were also positive for both pSMAD2 and FoxP3, a target of TGFβ and marker of suppressive/anergic regulatory T lymphocytes (Tregs). CD3/pSMAD2+ and CD3/FoxP3+ cells were almost completely absent in the pancreas of KTG mice (Figure S5 and Figure 4c,d). Interestingly, there were no significant changes between Tregs in the lymph nodes or peripheral blood of KRAS and KTG animals (Figure S6), suggesting that these Tregs are being induced by TGFβ at the site of the tumor.
Figure 4. Global TGFBR Deficiency Promotes a Cytotoxic Response Against Pancreatic Lesions.
(a,b) Spleens from KRAS, KTE, and KTG mice were stained for pSMAD2, suggesting TGFβ signaling is intact in the hematopoietic cells of KRAS and KTE mice, but not in KTG (c,d) When compared to KRAS, T cell infiltration was increased near the lesions of both KTE and KTG mice. However, in KTE, these cells are largely FoxP3+, suggesting increased peripheral tolerance, consistent with a strong TGFβ presence. This was not observed in KTG mice, where nearly all CD3+ cells did not express FoxP3. (e-l) Functional cell-mediated cytotoxicity was evaluated by lesion-specific staining of CD8, Interferon gamma (IFNγ), GranzymeB, and Cleaved Caspase-3. Each of these signals was absent in neoplastic lesions of KRAS and KTE mice, yet stained very strongly in the few neoplastic and metaplastic lesions observed in KTG mice. N=4 mice per group.
In accordance with these observations, there was little detectable staining for CD8, a marker for cytotoxic T-lymphocytes (CTLs), near the lesions in KRAS and KTE mice, yet the few lesions that developed in KTG mice had strong CD8+ infiltration (Figure 4e,f). When stained for GranzymeB, which is secreted by active CD8+ cells to induce apoptosis in their targets, pancreatic lesions in KRAS and KTE mice stained very poorly. Yet, KTG mice displayed increased Interferon gamma (IFNγ) and GranzymeB expression throughout the pancreas, particularly in the few metaplastic and neoplastic lesions that were observed (Figure 4g-j). Interestingly, while the TG transgene led to a robust GranzymeB-response in the colon, there were no noticeable immunological changes in other organs (Figure S7). In the pancreas, Cleaved Caspase-3, a direct downstream effector of GranzymeB, localized strongly with both CD8 and GranzymeB/IFNγ, again being most pronounced in KTG mice and augmented in the metaplastic and neoplastic regions (Figure 4k,l).
TGFBR Deficient T Cells Resist Inactivation ex Vivo
These results appear to suggest that, even at the metaplastic stage, these mutant KRAS-induced lesions have evaded the cytotoxic program or the adaptive immune system in a TGFβ-dependent manner. To assess this hypothesis, KRAS mice were regenerated and crossed to animals with genetic ablation of CD4 (deficient in all T helper cells), CD8 (deficient in cytotoxic T cells), or Rag1 (loss of all adaptive immunity). Interestingly, there was no change in lesion development or GranzymeB deposition in either model (Figure S8), suggesting that the lymphoid compartment is heavily compromised, and restored upon global TGFβ signaling deficiency.
Therefore, to further evaluate the restoration of cell-mediated immunity observed in KTG mice, mesenteric lymph nodes from KTG and KRAS control mice were evaluated by flow cytometry. Examination of CD4+ and CD8+ populations revealed a marked expansion of CD8+ populations in KTG when compared to KRAS (Figure 5a). This change was consistent across several animals (N=4 per group), and was highly significant (P=0.0001), with a mean 30-fold increase in the number of live CD8+ cells in the mesenteric lymph nodes of KTG as compared to KRAS control mice (Figure 5b). Live CD8+ cells in KTG mice were also highly positive for the activation marker CD69 (Figure S9a), though the majority of CD8+CD69+ cells in KRAS animals were not viable (Figure S9b). Similar changes were also detected in the peripheral blood of KTG animals (Figure S9c,d). Additionally, media from cultured nongenic and TG peripheral blood mononuclear cells revealed a near four-fold increase in the amount of GranzymeB secreted by TG cells, further demonstrating that the TGFBR-deficient mononuclear cells have enhanced cytotoxic activity (Figure 5c).
Figure 5. TGFBR Deficient Cytotoxic T Cells Resist Inactivation ex Vivo.
(a-b) The presence of live CD4 and CD8 cells in the mesenteric lymph nodes of KRAS and KTG mice was detected via flow cytometry, indicating an approximate 30-fold increase in the number of live CD8+ cells in KTG compared to KRAS mice. (*, P < 0.05. N=4 per group) (c) 1 million peripheral blood mononuclear cells (PBMCs) were cultured ex vivo from control and TG animals. Media was subject to GranzymeB ELISA, revealing a 3-fold increase in GranzymeB secretion by TG PBMCs consistent with increased cytotoxic activity (*, P < 0.05. N=3). (d) PBMC cultures from control and TG mice and maintained ex vivo and pulsed with recombinant TGFβ1. CD8 activity was assessed by CD8+CD69+ dual staining. TG PBMCs resisted TGFβ1-induced inactivation. Control PBMCs pre-treated with Galunisertib displayed similar resistance to TGFβ1-induced inactivation. (e) Ex vivo PBMC cultures of control mice were pulsed with recombinant TGFβ1. After 24 hours, CD4+ cells displayed an increase in CD25 and FoxP3 expression, consistent with an induced Treg phenotype. This experiment was repeated with cells from TG animals, which resisted TGFβ1-induced CD25 and FoxP3 expression. Additionally, nongenic cells pre-incubated with the TGFBR-inhibitor Galunisertib behaved like TG lymphocytes, and resulted an induced Treg phenotype. (*, P < 0.05. N=3).
To determine if absence of TGFBR in T cells confers resistance to TGFβ-induced inactivation, T cells were cultured ex vivo and treated with recombinant TGFβ1. In contrast to T cells isolated from TG mice, nongenic CD8+ cells treated with TGFβ displayed a decrease in CD8+CD69+ population, consistent with a reduced numbers of active CD8 cells. Blocking the TGFBRs in nongenic cells with Galunisertib displayed no change in CD8+CD69+ expression following TGFβ1 incubation (Figure 5d).
To further assess whether Tgfbr1 haploinsufficiency impedes tumor-associated evasion of immune surveillance through direct modification of the lymphoid compartment, ex vivo T cell cultures from nongenic and TG mice were reestablished and treated with recombinant TGFβ1. When treated with TGFβ1, nongenic CD4 cells displayed an increase in CD25+FoxP3+ population. This response was not observed in CD4 cells isolated from TG animals, suggesting that Tgfbr1 haploinsufficiency confers resistance to an induced CD25+FoxP3+ Treg phenotype. This was also recapitulated in nongenic CD4 cells pretreated with the TGFBR inhibitor Galunisertib (Figure 5e).
Adoptive Transfer of TGFBR-Deficient CD8+ T Cells Promotes a Cytotoxic Response Against Pancreatic Neoplasms
While global TGFBR-deficiency promotes a CD8-mediated cytotoxic response against neoplastic lesions in KRAS mice (predominantly IPN with few mPanINs), similar lesions (intraductal papillary mucinous neoplasms or IPMN) are only present in 15-20% of patients, with the majority having disease originating from pancreatic intraepithelial neoplasms (PanINs) (28). Therefore, to examine the ability of TGFBR-deficient T cells (TG) to infiltrate and destroy mPanIN lesions, the Ptf1a-Cre/LSL-KRAS (KC) model was utilized (Figure 6a). These mice have an almost exclusively mPanIN histotype, and present with lesions as early as two weeks (29).
Figure 6. Adoptive Transfer of TGFBR-Deficient CD8+ T Cells Promotes a Cytotoxic Response Against PanIN Lesions.
(a) Ptf1a-Cre/LSL-KRAS (KC) mice were generated to provide a model of PanIN-disease. (b,c) CD8+ T cells were isolated by dyanabeads, and purity measured by flow cytometry. 2 million CD8+ cells were then transferred to two-month-old KC animals. (d) One month after adoptive transfer, KC animals with control CD8+ cells (KCAT) or TG CD8+ cells (KCAT-TG) were euthanized and subject to pathological analysis by H&E. Lesion frequency and leukocyte infiltration (yellow arrows) was quantified per high power field (*, P < 0.05). (f) T cell infiltration was measured by CD3/E-Cadherin dual staining, or staining with anti-CD8, revealing an increased cytotoxic presence in KCAT-TG. (g) Functional cell-mediated cytotoxicity was evaluated by lesion-specific staining of CD3/E-Cadherin, CD8, CK19/GranzymeB, and Cleaved Caspase-3. The increase in GranzymeB and Cleaved Caspase-3 in abnormal tissues and the surrounding stroma suggest an increase in anti-tumor cytotoxicity in KCAT-TG animals.
Rather than employ the global Tgfbr1+/- transgene in this model, CD8+ T cells were instead isolated from the mesenteric lymph from control and TG animals by dynabead assay and evaluated for CD4 contamination by flow cytometry. We next transferred 2 million CD8+CD4- cells from either control or TGFBR-deficient animals into to three-month-old KC mice via the retroorbital vein (Figure 6b,c). This approach restricts TGFβ signaling deficiency to the CD8+ cells, allowing more a more functional analysis of the effects of lymphoid TGFBR-deficiency on already established mPanIN lesions.
While adoptive transfer of control CD8+ cells (KCAT) did not significantly effect PanIN development in KC mice, those that received TGFBR-deficient CD8+ T cells (KCAT-TG) had impaired lesion development and enhanced leukocyte infiltration (Figure 6c-e). KCAT-TG mice also displayed higher CD3+ T cell involvement, particularly in the stroma near developing PanINs (Figure 6f). Additionally, these T cells were also mainly CD8 positive, consistent with an enhanced cytotoxic presence (Figure 6f). KCAT-TG mice also presented with increased GranzymeB deposition in CK19+ neoplastic tissues, as well as increased Cleaved Caspase-3, suggested increased apoptosis in abnormal tissues (Figure 6g).
The Tumor Promoting Effects of TGFβ in the Tumor Microenvironment are Independent of Epithelial TGFβ Signaling
Our data to this point suggests that stromal-derived TGFβ promotes pancreatic cancer development by inhibiting a tumor-specific T cell response. To test the clinical relevance of these findings, we next assessed 73 human primary pancreatic cancer specimens for expression of the TGFβ1 ligand, epithelial expression of SMAD4 and p21 (surrogate markers of TGFβ-induced growth arrest in epithelial cells (30)), as well as GranzymeB (a marker of cell mediated cytotoxicity (31)) and fibrosis via Mason's trichrome staining (Figure 7a). Each set of stains was then independently scored from 0-3+ by two blinded investigators.
Figure 7. The Tumor Promoting Effects of TGFβ in the Tumor Microenvironment are Independent of Epithelial TGFβ Signaling.
(a-f) Human pancreatic cancer sections were stained for TGFβ1, SMAD4, p21, GranzymeB, and Mason's Trichrome. Stains were then and independently scored by two blinded investigators from 0-3+ based on intensity. The number of patients in each scoring category is displayed via histogram. (g) Two-way ANOVA revealed a highly significant (P=0.0001) interaction between epithelial staining for SMAD4 and p21, yet none (p>0.05) between SMAD4 and GranzymeB. (h) Two-way ANOVA also revealed a highly significant (P=0.0006) inverse correlation between TGFβ1 and GranzymeB expression, and a significant (P=0.041), positive association between TGFβ1 and Trichrome staining.
While nearly all patients in the examined cohort had at least some expression of the TGFβ1 ligand (Figure 7b), the majority (approximately 70%) presented with weak or no SMAD4 staining (Figure 7c). Most retained moderate cytoplasmic and some nuclear expression of p21 (Figure 7d). The majority of patients were at least somewhat deficient (0-1+ staining) in tumor specific GranzymeB staining (Figure 7e), though the overwhelming majority presented with strong (2-3+) Trichrome staining (Figure 7f).
We found a highly significant association (P=0.0001) between epithelial expression of SMAD4 and p21, further substantiating the hypothesis that SMAD4 is necessary for TGFβ-induced cell cycle arrest in epithelial cells. However, there was no observed relationship (P=0.198) between SMAD4 and GranzymeB expression (Figure 7g). However, while GranzymeB expression was unrelated to the status of epithelial TGFβ signaling, we found that TGFβ1 expression correlates inversely with GranzymeB expression in the tumor microenvironment (Figure 7h, P=0.0006). Additionally, high TGFβ1 expression was associated with increased trichrome staining (Figure 7i, P=0.041), further suggesting that the anti-cytotoxic and pro-fibrotic effects of TGFβ in the tumor microenvironment are independent of its growth inhibitory effects on epithelial cells.
Discussion
While there is clear evidence suggesting that TGFβ contributes to the progression of several solid tumors, clinical trials targeting the TGFβ pathway have had mixed success (32). This outcome is likely due to the dual function of TGFβ as a contextual tumor suppressor and promoter. While the majority of in vivo experiments suggest that TGFβ is a classical tumor suppressor in early tumorigenesis, the transgenic models used in these reports relied almost entirely on epithelial targeting systems (21, 22, 33-36).
These conditional models of TGFβ signaling deficiency are physiologically relevant, and mimic somatic mutations akin to those in patients with sporadic pancreatic tumors. TGFβ-pathway inactivating mutations are highly prevalent in epithelial cells, and up to 55% of pancreatic cancer patients present with loss of SMAD4 (37). Indeed, in advanced pancreatic cancer, the contribution of TGFβ to a metastatic phenotype is being corroborated (38, 39). TGFβ signaling may be abrogated in a significant subset of patients or already be serving a tumor-promoting role, which would need to be taken into account when stratifying patients for future targeted therapy with TGFBR inhibitors.
In addition, models of epithelial TGFβ signaling deficiency do not provide a platform for the global contributions of TGFβ to a dynamic tumor microenvironment, nor do they accurately predict the effects of a globally administered TGFBR inhibitor. This is evident in animals with global deficiency of TGFBR1 that are protected against mutant KRAS-induced pancreatic tumorigenesis, suggesting that, while TGFβ may serve as a tumor suppressor in isolated epithelial systems, its global role in the tumor microenvironment may actually facilitate early pancreatic tumorigenesis.
Despite these divergent phenotypes, the equivalent loss of pSMAD2 and p21 in both KTE and KTG mice suggest that TGFβ-induced growth arrest is similarly disrupted in animals with respective conditional and global TGFBR-deficiency, regardless of the genetic alteration in either receptor type. Yet this is not reflected in proliferation, as KTG mice had very low mitotic indices, suggesting that the protective effect observed in this animal may be due to TGFBR-deficiency in non-epithelial cells. Perhaps the most compelling evidence in support of this hypothesis is that KRAS mice with combined epithelial and global TGFBR-deficiency were not phenotypically distinct from those with solely global loss of TGFBR.
As the protective mechanism seen in globally TGFBR deficient models of pancreatic cancer seems to involve both the stromal and T cell compartments, we propose that in early pancreatic carcinogenesis, TGFβ facilitates tumor development via its effects in the TME. Our in vitro experiments suggest that the pancreatic stroma, when in the presence of the epithelia, increases TGFβ biosynthesis. Autocrine TGFβ signaling further increases TGFβ expression by the stroma, which in turn promotes matrix deposition, increases the presence/activity of Tregs, and inactivates tumor reactive lymphocytes in the microenvironment. These oncogenic effects appear to overwhelm the anti-proliferative function(s) of TGFβ in the neoplastic epithelium, furthering tumor progression (Figure S10).
This is observed in globally TGFBR-deficient KTG mice that, despite the loss of TGFβ–induced cell cycle arrest, present with a marked decrease in pancreatic cancer-associated fibrosis, reduced stromal TGFβ1 expression, and the restoration of CD8-mediated anti-tumor immunity, culminating in a protective phenotype. Furthermore, ablation of TGFβ signals in T cells, either genetically or pharmacologically, prevented functional inactivation, and TGFBR-deficient CD8+ T cells were sufficient to generate a GranzymeB-mediated cytotoxic response against KRAS-induced neoplastic lesions. While it remains to be seen if these findings are applicable to metastatic or late stage models of pancreatic carcinogenesis, in human patients we found that expression of the TGFβ1 ligand correlated positively with tumor-associated fibrosis and inversely with GranzymeB expression, further substantiating TGFβ1 as a critical pro-fibrotic and anti-cytotoxic cytokine. The impact of these findings is further substantiated by studies showing the strong prognostic value of these biomarkers by showing that TGFβ1 expression (5), enhanced fibrosis (40), and reduced CD8/GranzymeB-mediated immunity (41) are each independently associated with worse outcomes.
To this end, there are several emerging therapies targeting the pancreatic stroma and associated fibrosis. In addition to providing soluble factors that facilitate tumor growth, the desmoplastic pancreatic cancer stroma has also been implicated in the resistance to cytotoxic therapies (42). Blockade of the pro-fibrotic Hedgehog pathway has shown promise in vivo, transiently increasing tumor vascularity and gemcitabine delivery, temporarily stabilizing disease (43). Given the pro-fibrotic effects of TGFβ, there may be merit in targeting the TGFβ pathway to mitigate the fibrotic response. In vivo studies to this effect have shown early promise, as mice with defective TGFβ signals are protected against cerulean-induced fibrosis (44, 45). Additionally, we have shown that in patients TGFβ1 expression is associated with increased fibrosis.
One limitation of this study is that the animal models used present with predominantly neoplastic disease. While systemic TGFBR1 deficiency protected against lesion development in vivo, and the adoptive transfer of TGFBR1-deficient CD8 cells rescued against established lesions, in the cancer condition there are many factors that must be considered. The most notable is the Programmed Death (PD-1) pathway, and its ligand such as PDL1 (46). PD-1 is a coinhibitory T cell receptor, and when bound to its ligands induces inhibitory responses and host tolerization (47). PD-L1 is largely upregulated in the tumor microenvironment (48, 49). These, and many other factors unique to the tumor microenvironment must be considered, and may be synergistic with TGFBR-inhibition therapy.
Another therapeutic approach currently being investigated in pancreatic cancer is the restoration of cytotoxic immunity. Such therapies show similar promise, as blockade of cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), particularly when combined with GM-CSF cell-based vaccines (GVAX) lead to improved survival (50). Additionally, the combination of an antibody agonizing the co-stimulatory molecule CD40 combined with PD-1 and/or CTLA-4 inhibition lead to regression of subcutaneous tumors in mice, and protected against multiple tumor re-challenges (51).
As we have demonstrated that systemic inhibition of TGFβ signaling attenuates pancreatic fibrosis as well as evasion of the cytotoxic response, there may be merit to exploring TGFBR-inhibition therapy to simultaneously target both stromal and immune compartments in pancreatic cancer. Yet this approach requires caution, as patients with intact tumor suppressive TGFβ signals may exhibit increased tumor proliferation upon TGFBR inhibition, as we observed in KTE mice. However, pancreatic cancer patients that already harbor loss of the growth-inhibitory effects of TGFβ, such as those with loss of SMAD4, are unlikely to have such side effects from TGFBR inhibitors, particularly in light of our observation that SMAD4 loss was associated with reduced p21 expression in human patients. Should these patients also present with poor tumor-specific GranzymeB staining and/or enhanced tumor-associated fibrosis, TGFBR-inhibition therapies may be efficacious and warrant further study.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Olga Volpert for use of her fluorescent microscope. We thank Dr. Mark Rovedo and colleagues at Eli Lilly for providing TGFBR inhibitor used in this manuscript, as well as Paul Mehl, Jeff Nelson, and Dr. Suchitra Swaminathan at the Northwestern University RHLCC Flow Core for their assistance. We thank Dr. David Gius for his insight into our figures, Dr. Nancy Krett for her critical review of this manuscript, and Ronald McKinney for his technical assistance. We also thank undergraduate student Hanah L. Miller for her hard work and dedication.
Grant Support: This work was supported by the Barnum and Zell Family Foundations at Northwestern University to P.J. Grippo, by the NIH grant R01CA141057 to BJ, and by the NIH grant R01CA186885 to HM.
Abbreviations
- TGFβ
Transforming Growth Factor-β
- TGFBR
Transforming Growth Factor β Receptor
- TME
Tumor Microenvironment
- GI
Gastrointestinal
- EL
Elastase
- KRAS
EL-KrasG12D
- TE
Epithelial TGFBR-deficient
- TG
Globally TGFBR-deficient
- KTE
KRAS-TE
- KTG
KRAS-TG
- Gal
Galunisertib
- KC
Ptf1a-Cre/LSL-KRAS
- KCAT
KC-Adoptive Transfer Control
- KCAT-TG
KCAT-TG, KC-Adoptive Transfer TG
- IPNs
intraductal papillary neoplasms
- PanINs
pancreatic intraepithelial neoplasms
Footnotes
Disclosures: The authors have no conflicts to disclose.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L, Pasche B, Lee C, Grippo PJ. TGF-beta: Duality of Function Between Tumor Prevention and Carcinogenesis. Journal of the National Cancer Institute. 2014;106(2):djt369. doi: 10.1093/jnci/djt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer J, Sporn JC, Cabral J, Gomez J, Jung B. Effects of activin and TGFbeta on p21 in colon cancer. PLoS One. 2012;7(6):e39381. doi: 10.1371/journal.pone.0039381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friess H, Yamanaka Y, Buchler M, Ebert M, Beger HG, Gold LI, Korc M. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993;105(6):1846–56. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- 6.Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29(3):179–87. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. The Journal of clinical investigation. 2007;117(1):50–9. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer research. 2007;67(19):9518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 9.Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44(4):534–41. doi: 10.1136/gut.44.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shek FW, Benyon RC, Walker FM, McCrudden PR, Pender SL, Williams EJ, Johnson PA, Johnson CD, Bateman AC, Fine DR, et al. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. The American journal of pathology. 2002;160(5):1787–98. doi: 10.1016/s0002-9440(10)61125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Chen D, Zhang H, Ding Y, Bromberg JS. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 - precursors. Am J Transplant. 2004;4(10):1614–27. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 12.Corthay A. How do regulatory T cells work? Scandinavian journal of immunology. 2009;70(4):326–36. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7(10):1118–22. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Yang X, Pins M, Javonovic B, Kuzel T, Kim SJ, Parijs LV, Greenberg NM, Liu V, Guo Y, et al. Adoptive transfer of tumor-reactive transforming growth factor-beta-insensitive CD8+ T cells: eradication of autologous mouse prostate cancer. Cancer research. 2005;65(5):1761–9. doi: 10.1158/0008-5472.CAN-04-3169. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Yang XJ, Kundu SD, Pins M, Javonovic B, Meyer R, Kim SJ, Greenberg NM, Kuzel T, Meagher R, et al. Blockade of transforming growth factor-{beta} signaling in tumor-reactive CD8(+) T cells activates the antitumor immune response cycle. Mol Cancer Ther. 2006;5(7):1733–43. doi: 10.1158/1535-7163.MCT-06-0109. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Wen W, Yuan J, Helfand B, Li Y, Shi C, Tian F, Zheng J, Wang F, Chen L, et al. Immunotherapy for human renal cell carcinoma by adoptive transfer of autologous transforming growth factor beta-insensitive CD8+ T cells. Clin Cancer Res. 2010;16(1):164–73. doi: 10.1158/1078-0432.CCR-09-1758. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer cell. 2005;8(5):369–80. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Rousalova I, Krepela E. Granzyme B-induced apoptosis in cancer cells and its regulation (review) International journal of oncology. 2010;37(6):1361–78. doi: 10.3892/ijo_00000788. [DOI] [PubMed] [Google Scholar]
- 19.Cullen SP, Brunet M, Martin SJ. Granzymes in cancer and immunity. Cell death and differentiation. 2010;17(4):616–23. doi: 10.1038/cdd.2009.206. [DOI] [PubMed] [Google Scholar]
- 20.Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, Wright CV, Moses HL. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20(22):3147–60. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM, Deng CX, Hruban RH, Adsay NV, Tuveson DA, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer cell. 2007;11(3):229–43. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Kojima K, Vickers SM, Adsay NV, Jhala NC, Kim HG, Schoeb TR, Grizzle WE, Klug CA. Inactivation of Smad4 accelerates Kras(G12D)-mediated pancreatic neoplasia. Cancer research. 2007;67(17):8121–30. doi: 10.1158/0008-5472.CAN-06-4167. [DOI] [PubMed] [Google Scholar]
- 23.Grippo PJ, Nowlin PS, Demeure MJ, Longnecker DS, Sandgren EP. Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer research. 2003;63(9):2016–9. [PubMed] [Google Scholar]
- 24.Adrian K, Strouch MJ, Zeng Q, Barron MR, Cheon EC, Honasoge A, Xu Y, Phukan S, Sadim M, Bentrem DJ, et al. Tgfbr1 haploinsufficiency inhibits the development of murine mutant Kras-induced pancreatic precancer. Cancer research. 2009;69(24):9169–74. doi: 10.1158/0008-5472.CAN-09-1705. [DOI] [PubMed] [Google Scholar]
- 25.Bottinger EP, Jakubczak JL, Roberts IS, Mumy M, Hemmati P, Bagnall K, Merlino G, Wakefield LM. Expression of a dominant-negative mutant TGF-beta type II receptor in transgenic mice reveals essential roles for TGF-beta in regulation of growth and differentiation in the exocrine pancreas. The EMBO journal. 1997;16(10):2621–33. doi: 10.1093/emboj/16.10.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng Q, Phukan S, Xu Y, Sadim M, Rosman DS, Pennison M, Liao J, Yang GY, Huang CC, Valle L, et al. Tgfbr1 haploinsufficiency is a potent modifier of colorectal cancer development. Cancer research. 2009;69(2):678–86. doi: 10.1158/0008-5472.CAN-08-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–34. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 28.Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Annals of surgery. 2004;239(6):788–97. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 97-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer cell. 2003;4(6):437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 30.Nicolas FJ, Hill CS. Attenuation of the TGF-beta-Smad signaling pathway in pancreatic tumor cells confers resistance to TGF-beta-induced growth arrest. Oncogene. 2003;22(24):3698–711. doi: 10.1038/sj.onc.1206420. [DOI] [PubMed] [Google Scholar]
- 31.Pinkoski MJ, Waterhouse NJ, Heibein JA, Wolf BB, Kuwana T, Goldstein JC, Newmeyer DD, Bleackley RC, Green DR. Granzyme B-mediated apoptosis proceeds predominantly through a Bcl-2-inhibitable mitochondrial pathway. The Journal of biological chemistry. 2001;276(15):12060–7. doi: 10.1074/jbc.M009038200. [DOI] [PubMed] [Google Scholar]
- 32.Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGF-beta targeted cancer therapy. International journal of biological sciences. 2012;8(7):964–78. doi: 10.7150/ijbs.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amendt C, Schirmacher P, Weber H, Blessing M. Expression of a dominant negative type II TGF-beta receptor in mouse skin results in an increase in carcinoma incidence and an acceleration of carcinoma development. Oncogene. 1998;17(1):25–34. doi: 10.1038/sj.onc.1202161. [DOI] [PubMed] [Google Scholar]
- 34.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20(22):3130–46. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forrester E, Chytil A, Bierie B, Aakre M, Gorska AE, Sharif-Afshar AR, Muller WJ, Moses HL. Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer research. 2005;65(6):2296–302. doi: 10.1158/0008-5472.CAN-04-3272. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura T, Kometani K, Hashida H, Matsunaga A, Miyoshi H, Hosogi H, Aoki M, Oshima M, Hattori M, Takabayashi A, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007;39(4):467–75. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- 37.Liu F. SMAD4/DPC4 and pancreatic cancer survival. Commentary re: M. Tascilar et al., The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin. Cancer Res., 7: 4115-4121, 2001. Clin Cancer Res. 2001;7(12):3853–6. [PubMed] [Google Scholar]
- 38.Gore AJ, Deitz SL, Palam LR, Craven KE, Korc M. Pancreatic cancer-associated retinoblastoma 1 dysfunction enables TGF-beta to promote proliferation. The Journal of clinical investigation. 2014;124(1):338–52. doi: 10.1172/JCI71526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostapoff KT, Kutluk Cenik B, Wang M, Ye R, Xu X, Nugent D, Hagopian MM, Topalovski M, Rivera LB, Carroll KD, et al. Neutralizing murine TGFbetaR2 promotes a differentiated tumor cell phenotype and inhibits pancreatic cancer metastasis. Cancer research. 2014 doi: 10.1158/0008-5472.CAN-13-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haqq J, Howells LM, Garcea G, Metcalfe MS, Steward WP, Dennison AR. Pancreatic stellate cells and pancreas cancer: current perspectives and future strategies. European journal of cancer. 2014;50(15):2570–82. doi: 10.1016/j.ejca.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Karakhanova S, Ryschich E, Mosl B, Harig S, Jager D, Schmidt J, Hartwig W, Werner J, Bazhin AV. Prognostic and predictive value of immunological parameters for chemoradioimmunotherapy in patients with pancreatic adenocarcinoma. British journal of cancer. 2015;112(6):1027–36. doi: 10.1038/bjc.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liss AS, Thayer SP. In: Pancreatic Cancer and Tumor Microenvironment. Grippo PJ, Munshi HG, editors. Trivandrum (India): 2012. [Google Scholar]
- 43.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He J, Sun X, Qian KQ, Liu X, Wang Z, Chen Y. Protection of cerulein-induced pancreatic fibrosis by pancreas-specific expression of Smad7. Biochimica et biophysica acta. 2009;1792(1):56–60. doi: 10.1016/j.bbadis.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Yoo BM, Yeo M, Oh TY, Choi JH, Kim WW, Kim JH, Cho SW, Kim SJ, Hahm KB. Amelioration of pancreatic fibrosis in mice with defective TGF-beta signaling. Pancreas. 2005;30(3):e71–9. doi: 10.1097/01.mpa.0000157388.54016.0a. [DOI] [PubMed] [Google Scholar]
- 46.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual review of immunology. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(19):12293–7. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 50.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Jr, Donehower RC, Jaffee EM, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. Journal of immunotherapy. 2013;36(7):382–9. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, et al. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer immunology research. 2015;3(4):399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.