Abstract
The GABAA α4 subunit exists in two distinct populations of GABAA receptors. Synaptic GABAA α4 receptors are localized at the synapse and mediate phasic inhibitory neurotransmission, while extrasynaptic GABAA receptors are located outside of the synapse and mediate tonic inhibitory transmission. These receptors have distinct pharmacological and biophysical properties that contribute to interest in how these different subtypes are regulated under physiological and pathological states. We utilized subcellular fractionation procedures to separate these populations of receptors in order to investigate their regulation by protein kinases in cortical cultured neurons. Protein kinase A (PKA) activation decreases synaptic α4 expression while protein kinase C (PKC) activation increases α4 subunit expression, and these effects are associated with increased β3 S408/409 or γ2 S327 phosphorylation respectively. In contrast, PKA activation increases extrasynaptic α4 and δ subunit expression, while PKC activation has no effect. Our findings suggest synaptic and extrasynaptic GABAA α4 subunit expression can be modulated by PKA to inform the development of more specific therapeutics for neurological diseases that involve deficits in GABAergic transmission.
INTRODUCTION
GABAA-Rs are ligand-gated ion channels that mediate the majority of inhibitory neurotransmission in the CNS. GABAA-Rs are normally heteropentamers that are composed of two α(1–6), two β(1–3), and either a γ (1–3) or δ subunit. The presence of either the γ or δ subunit in the assembled receptor influences receptor localization and consequentially the type of GABAAergic neurotransmission. GABAA-Rs containing the γ subunit, are located synaptically and mediate phasic inhibition1,2. Conversely, the δ-containing GABAA-Rs are located exclusively extrasynaptically and mediate tonic inhibition2,3. Both synaptic and extrasynaptic GABAA-Rs are crucial to maintaining overall neuronal excitability3.
The α4 subunit is present in both synaptic and extrasynaptic GABAA-Rs in the mammalian cerebral cortex. The α4γ and α4δ-containing GABAA-Rs have unique physiological and pharmacological properties. α4γ2-containing GABAA-Rs have a lower affinity for GABA but faster desensitization than α4δ-containing GABAA-Rs4–6. In addition, other endogenous modulators such as GABAAergic neuroactive steroids, exhibit higher potency at α4δ-containing GABAA-Rs than α4γ2-containing GABAA-Rs4. Both α4-containing GABAA-Rs assemblies are insensitive to classic benzodiazepine agonists such as diazepam4 although the structurally related benzodiazepine derivatives, imidazobenzodiazepines, such as Ro15-4513 display activity at both receptor subtypes7. α4δ GABAA-Rs are also potentiated by low millimolar concentrations of ethanol while α4γ2 GABAA-Rs require a higher concentration7–9, although this data is controversial, as not all labs have found that δ-containing GABAA-Rs are sensitive to low concentrations of ethanol10. Thus, differences in the pharmacological and physiological characteristics of these GABAA-Rs have generated considerable interest in the contributions of these receptors to both physiological and pathological disease states.
Both α4-containing GABAA-R populations have been implicated in multiple disease states, such as alcohol dependence, fragile X syndrome, epilepsy, schizophrenia, and depression11. In some disease states, such as alcohol dependence12, epilepsy13,14, and schizophrenia15, downregulation of the δ subunit is accompanied by upregulation of α4γ2-containing GABAA-Rs, suggesting that this change in overall GABAA-R population may be important to the pathogenesis of these diseases. In non-pathological states, genetic ablation of the δ subunit also resulted in an increase in γ2 subunit expression in the cerebellar granule cells16. Despite these observations, the intracellular mechanisms that regulate changes in expression of the α4δ and α4γ2 receptors are still largely unknown.
PKA and PKC have long been known to regulate GABAA-R expression either through direct phosphorylation of GABAA-R subunits or through proteins associated with GABAA-Rs17–19. PKA is known to modulate expression and function of GABAA-Rs through direct phosphorylation on the β3 subunit serine site S408/40920. PKC has been shown to phosphorylate sites on the GABAA subunits at α4 S44319, β2 S410, β3 S408/40920, and γ2 S32721. Phosphorylation on these sites contributes to different trafficking22, stabilization23, internalization24, or expression25, depending on both the phosphorylation site and the composition of the GABAA-R26. In addition to direct regulation by protein kinases, indirect regulation of signal transduction by G-coupled protein receptors has also been shown to effect GABAA-R expression and function27–29.
However, it is still unknown whether PKA and PKC regulate both synaptic and extrasynaptic populations of α4-containing GABAA-Rs. Therefore, we were interested in the role of these two kinases in GABAA-R subunit expression. Previous work in our lab has suggested that PKA and PKC have opposing effects on GABAA α4 subunit expression in the presence of ethanol in cortical neurons25,30. Thus, the present study sought to determine if PKC and PKA were involved in regulation of both the synaptic and extrasynaptic populations of α4 GABAA-Rs in the absence of ethanol.
MATERIALS AND METHODS
Primary cortical neuron cell culture and treatments
All experiments were conducted in accordance with guidelines from the National Institutes of Health and Institutional Animal Care and Use Committee at the University of North Carolina. Postnatal day 0–1 Sprague Dawley rat pups of both sexes were decapitated and cortices were isolated and cultured as previously described31. Neurons were maintained in vitro for 18 days in DMEM, B27 (1%, Invitrogen), and penicillin/streptomycin (15 days, 50 U, Invitrogen). On day 18, drugs were diluted in ddH2O or DMSO. PKA was activated with Sp-Adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt (Sp-cAMPs, 50 μM, Sigma Aldrich) and inhibited with Rp-Adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt (Rp-cAMPS, 50 μM, Sigma Aldrich). PKC was activated with Phorbol 12,13-dibutyrate (PDBu, 100 nM, Sigma Aldrich) and inhibited with Calphostin C (CalC, 300 nM, Sigma Aldrich, St. Louis) All control experiments were exposed to equal volume ddH2O. All drug exposures were for one hour since previous experiments showed PKA and PKC both alter GABAA receptors at this time point25,30.
Quantitative PCR
Following treatment, cells were homogenized in Trizol according to manufacturers instructions. RNA was purified using Direct-zol RNA miniprep kits (Zymo) and 260/280 and 260/230 ratios >1.8 were determined using a Nanodrop 1000 (ThermoScientific). RNA was reversed transcribed into cDNA using High Capacity RNA-to-cDNA kit (Applied Biosystems). qPCR was performed using 10 ng of cDNA per reaction, TaqMan Gene Expression Assays (Life Technologies), and TaqMan Gene Expression Master Mix (Life Technologies). Each reaction was run in duplicate and analyzed with the ΔΔCt method with glyceraldehyde-3-phosphate dehydrogenase (Gapdh) as a loading control.
Membrane, synaptic and extrasynaptic fractionation
Membrane, synaptic and extrasynaptic fractions were produced as described previously5,32–35. Following drug exposures, cells were lysed by brief sonication in PBS containing 0.32M sucrose. The nuclear fraction and cell debris were removed by centrifugation at 1,000×g for 10 minutes at 4 °C. The membrane fraction was produced by centrifugation of the supernatant at 12,000×g for 30 minutes at 4 °C. The resulting pellet was resuspended in 0.32M sucrose PBS buffer containing 0.5% (v/v) Triton-X100 and incubated at 4°C under rotation for 20 minutes. The synaptic fraction was produced by centrifugation at 32,000×g for 20 minutes at 4 °C. The resulting pellet containing the synaptic fraction was resuspended in PBS containing protease and phosphatase inhibitors. The supernatant containing the extrasynaptic fraction was incubated in acetone (1:8 v/v) overnight at −20°C to insolubilize and concentrate the protein. This solution was pelleted by centrifugation at 3000×g for 15 min at 4 °C. The resulting pellet containing the extrasynaptic fraction was resuspended in PBS containing protease and phosphatase inhibitors (Halt™, ThermoScientific).
Biotinylation for isolation of surface proteins
Isolation of surface proteins with biotinylation was performed using the Cell Surface Protein Isolation Kit (Pierce) according to manufacturers instructions. An aliquot was taken before avidin pulldown in order to analyze expression in the total fraction. The eluted biotinylated fraction was then subjected to western blot analysis. Surface expression was analyzed as the ratio of α4 in the biotinylated fraction over α4 in the total fraction. β-actin was probed in the biotinylated fraction as a control to insure that there were no intracellular proteins in the biotinylated fraction. Results were then normalized to the control for each fraction.
Western blot analysis
Protein concentrations were determined using BCA assay (Pierce). 30–50μg of protein was electrophoresed on 4–16% Tris-Glycine polyacrylamide gels (Biorad) and transferred to iBlot PVDF membranes (Invitrogen), blocked for 1 h in 1–5% BSA and incubated overnight at 4 °C with either anti-GABAA α4 (Abcam, #ab117080, 1:500), anti-GABAA β2 (Novus, #NB300-198, 1:1000), anti-GABAA β3 (Novus, #NBP1-47613, 1:1000), anti-GABAA δ (Novus, #3002-200, 1:750), anti-GABAA γ2 (Novus, #NB300-190, 1:1000), anti-GABAA phospho-γ2 (Ser327)(PhosphoSolutions, p1130-327, 1:1000), anti-GABAA phosphor-β3 (Novus, NBP2-29508, 1:1000) anti-PSD95 (Novus, #NB300-198, 1:2000), anti-Gephyrin (BD Transduction, #610584, 1:1000), anti-neuroligin2 (Alomone Labs, ANR-036, 1:1000) or β-actin (Novus, #NB600-501, 1:3000) Membranes were then incubated with peroxidase-labeled secondary antibodies (Mouse, rabbit, goat, Jackson Laboratories, 1:10000) and signals were developed using ECLPrime (GE) on a LAS 4000 Imager (GE). Bands were quantified using GE ImageQuant software and normalized to β-actin to control for loading.
Statistical analysis
Student’s t test was used to determine significance for all comparisons between two groups. One-way ANOVA was used to determine significance for more than two groups. Tukey’s posthoc test was used to determine significance between groups after one-way ANOVA. All analysis was performed using GraphPad Prism (version 6).
RESULTS
PKA and PKC activation have opposite effects on α4 GABAA-R subunit expression
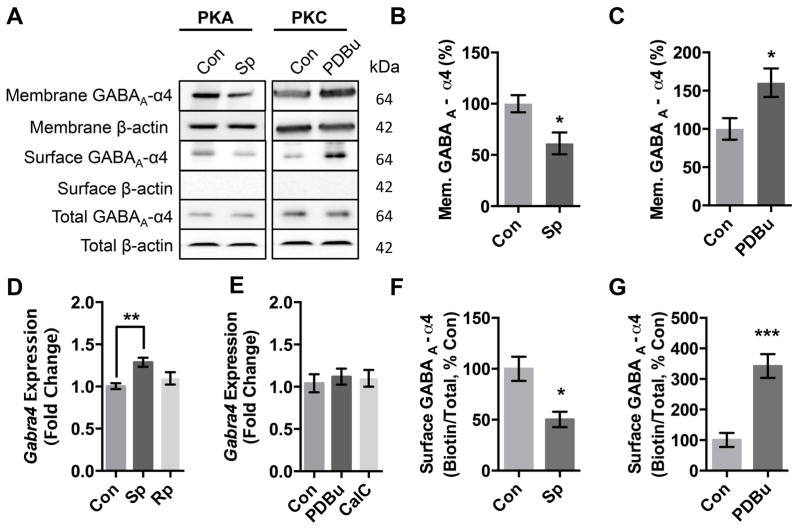
PKA and PKC activation are known to have opposite effects on GABAA-R function and expression cerebral cortical neurons in the presence of ethanol25,30. To determine if PKA and PKC activation cause changes in α4 expression independent of ethanol, we activated PKA with Sp-cAMPs and activated PKC with PBDu then prepared membrane fractions and analyzed α4 expression using western blots (Fig 1A). Our results indicate that PKA activation decreases α4 expression (Fig 1B) while PKC activation increases α4 expression (Fig 1C) in the membrane fraction. We next used quantitative PCR to determine if there were also changes in gene transcription. PKA activation increases Gabra4 expression (Fig 1D) while PKC activation caused no change in Gabra4 expression (Fig 1E). We also used qPCR to analyze Gabrd and Gabrg2 expression after all four drug treatments and found no significant changes in either transcript (Gabrd: Sp 1.21±0.19; Rp 0.83±24; PDBu 1.22±0.45; CalC 1.14±0.36. Gabrg2: Sp 1.18±0.22; Rp 1.00±0.08; PDBu 1.20±0.41; CalC 1.17±0.11). To determine if PKA and PKC activation caused changes in surface expression we used biotinylation to isolate surface receptors. Our results indicate that PKA activation decreases α4 surface expression (Fig 1F) while PKC activation increases α4 surface expression (Fig 1G). There was no change in total α4 expression levels after either PKA or PKC activation (data not shown).
Figure 1. PKA and PKC activation have opposite effects on GABAA α4 subunit expression.
(A) Cortical neurons (DIV 18) were treated 1 hr with either ddH2O(Con), PKA activator Sp-cAMPs (Sp, 50μM) or PKC activator PDBu (PDBu, 100 nM), followed by membrane fractionation or surface biotinylation and western blot.
(B) Quantification of membrane expression of GABAA α4 subunit following PKA activation.
(C) Quantification of membrane expression of GABAA α4 subunit following PKC activation.
(D) qPCR for Gabra4 in cortical neurons (DIV 18) treated by PKA activators and inhibitors.
(E) qPCR for Gabra4 in cortical neurons (DIV 18) treated by PKA activators and inhibitors.
(F) Quantification of surface expression of GABAA α4 subunit following PKA activation.
(G) Quantification of surface expression of GABAA α4 subunit following PKC activation.
Values are relative to control. *p<0.05, **p<0.01. Error bars indicate ± SEM. n = 3–8 independent experiments.
Synaptic and extrasynaptic receptors can be separated by detergent Triton X-100
Since the GABAA α4 subunit is present in both synaptic and extrasynaptic populations of GABAA-Rs, we utilized a biochemical approach previously used for glutamate32 and GABAA receptors5,33 to investigate both synaptic and extrasynaptic receptors. We validated this approach for separation of GABAA receptors in cortical cultured neurons by probing for synaptic markers neuroligin 2, gephyrin, postsynaptic density protein-95 and for the GABAA δ subunit that is exclusively localized extrasynaptically (Fig 2)3. The GABA specific synaptic markers, neuroligin 2 and gephyrin were highly enriched in the synaptic fraction, along with postsynaptic density protein 95, while the GABAA-R δ subunit was enriched exclusively in the extrasynaptic fraction. Of note, the GABAA-R α4, α1, and γ2 subunits were found in both fractions as expected from previous studies1–3,5.
Figure 2. Synaptic and extrasynaptic populations of α4 containing GABAA receptors can be separated with Triton X-100.
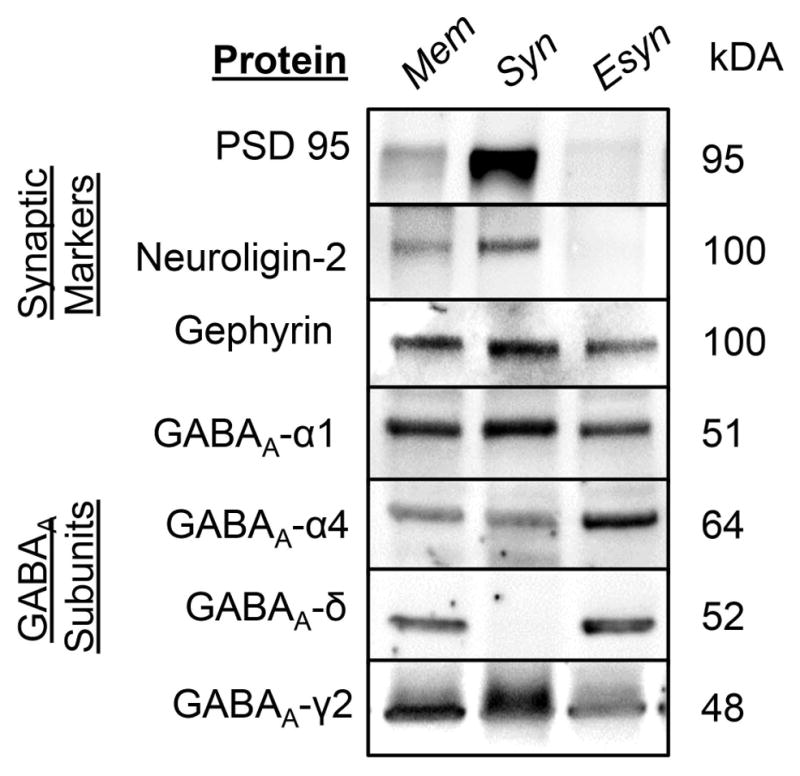
Representative blots showing TritonX-100 (0.5%) enriches the synaptic fraction for synaptic markers, neuroligin 2, PSD-95, and gephyrin, while the extrasynaptic fraction is enriched for GABAA δ subunit.
PKA and PKC regulation of synaptic α4 GABAA-Rs
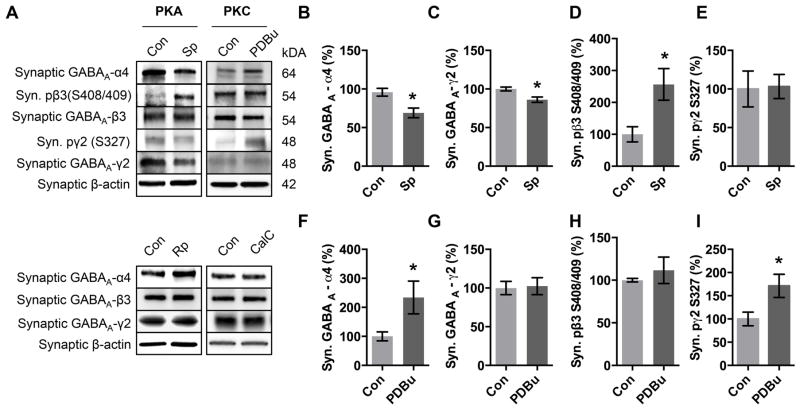
Following validation of our strategy to separate synaptic and extrasynaptic populations of GABAA-Rs, we determined if PKA and PKC regulate the two different populations of α4-containing GABAA-Rs. Activation of PKA decreased synaptic α4 expression (Fig 3B) while activation of PKC increased synaptic α4 expression (Fig 3F). We next analyzed γ2 expression, and found that PKA activation caused a decrease in γ 2 expression (Fig 3C) while PKC activation did not alter γ2 expression (Fig 3G). We found that neither PKA activation nor PKC activation caused a change in β3 expression (Fig 3A, Sp-cAMPs 110.4±14.9 % control; PdBU 99.9±23.4 % control).
Figure 3. PKA and PKC have opposing effects on synaptic α4 subunit expression.
(A) Cortical neurons (DIV 18) were treated 1 hr with either ddH2O(Con), PKA activator Sp-cAMPs (Sp, 50μM), PKC activator PDBu (PDBu, 100 nM), PKA inhibitor Rp-cAMPs (Rp, 50 μM), or PKC inhibitor Calphostin C (CalC, 300nM) followed by isolation of the synaptic fraction and western blot.
(B) Quantification of synaptic expression of GABAA α4 subunit following PKA activation.
(C) Quantification of synaptic expression of GABAAγ2 subunit following PKA activation.
(D) Quantification of extrasynaptic expression of GABAA β3 S408/409 phosphorylation following PKA activation.
(E) Quantification of synaptic expression of GABAAγ2 S327 phosphorylation following PKA activation.
(F) Quantification of synaptic expression of GABAA α4 subunit following PKC activation.
(G) Quantification of synaptic expression of GABAAγ2 subunit following PKC activation.
(H) Quantification of extrasynaptic expression of GABAA β3 S408/409 phosphorylation following PKC activation.
(I) Quantification of synaptic expression of GABAAγ2 S327 phosphorylation following PKC activation.
Values are relative to control. *p<0.05, Error bars indicate ± SEM. n = 4–9 independent experiments.
We next determined if inhibition of PKA or PKC caused changes in synaptic α4 expression. We inhibited PKA with the cAMP derivative Rp-cAMPs and found that there was no change in GABAA α4 expression (Fig 3A). Inhibition of PKC with CalC also caused no change in expression (Fig 3A).
The β3 subunit has two known PKA and PKC phosphorylation sites on S408 and S409, therefore we were interested if activation of PKA or PKC caused direct phosphorylation of β3 S408/409. Interestingly, we found that PKA activation increased phosphorylation on β3 S408/409 (Fig 3D), but PKC activation did not (Fig 3H). Intrigued by the lack of PKC-induced phosphorylation of β3 S408/409 on the β3 subunit, we next tested to see if there was increased phosphorylation on γ2 S327, another known GABAA-R site phosphorylated by PKC but not PKA. PKC activation increased phosphorylation of γ2 S327 (Fig 3I). As expected, PKA activation did not increase γ2 S327 phosphorylation (Fig 3E).
PKA and PKC regulation of extrasynaptic α4 GABAA-Rs
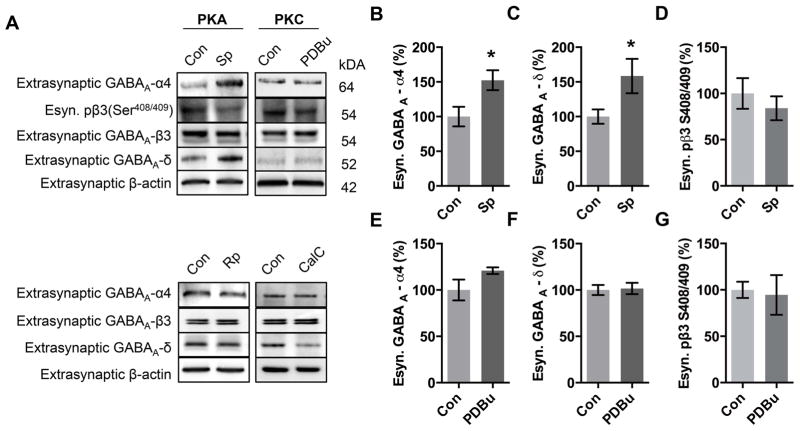
Regulation of extrasynaptic GABAA-Rs by protein kinases is still poorly understood, and to date, no definitive phosphorylation site has been identified on the δ subunit despite the intracellular loop (316–410) of the GABAA δ subunit containing putative serine phosphorylation sites for PKA and PKC at δ S305/404 and δ S364/390 respectively (http://www.cbs.dtu.dk/services/NetPhos/, accessed 3/4/15)36. In the present study, we found that activation of PKA caused an increase in the expression of α4 (Fig 4B) and δ subunits (Fig 4C) in the extrasynaptic fraction, while activation of PKC did not change in either extrasynaptic α4 (Fig 4C) or δ expression (Fig 4F). We then inhibited PKC with CalC and analyzed the extrasynaptic fraction by western blot. Interestingly, we found that inhibition of PKC resulted in a decrease in δ subunit expression (51.9±10.4 % control, Fig 4A) but no change in α4 expression (109.4±12.34 % control, Fig 4A), suggesting that PKC inhibition may alter other extrasynaptic δ-containing GABAA-Rs. We found that neither PKA nor PKC activation altered β3 S408/409 phosphorylation (Fig 4A, D), or β3 expression (Fig 4A; Sp-cAMPs 111.4±7.2 % control; PDBu 103.4±15.0 % control) in the extrasynaptic fraction.
Figure 4. PKA, but not PKC activation, increases extrasynaptic α4 subunit expression.
(A) Cortical neurons (DIV 18) were treated 1 hr with either ddH2O(Con), PKA activator Sp-cAMPs (Sp, 50μM), PKC activator PDBu (PDBu, 100 nM), PKA inhibitor Rp-cAMPs (Rp, 50 μM), or PKC inhibitor Calphostin C (CalC, 300nM) followed by isolation of the extrasynaptic fraction and western blot.
(B) Quantification of extrasynaptic expression of GABAA α4 subunit following PKA activation.
(C) Quantification of extrasynaptic expression of GABAA δ subunit following PKA activation.
(D) Quantification of extrasynaptic expression of GABAA β3 S408/409 phosphorylation following PKA activation.
(E) Quantification of extrasynaptic expression of GABAA α4 subunit following PKC activation.
(F) Quantification of extrasynaptic expression of GABAA δ subunit following PKC activation.
(G) Quantification of extrasynaptic expression of GABAA β3 S408/409 phosphorylation following PKC activation.
Values are relative to control. *p<0.05, Error bars indicate ± SEM. n = 4–9 independent experiments.
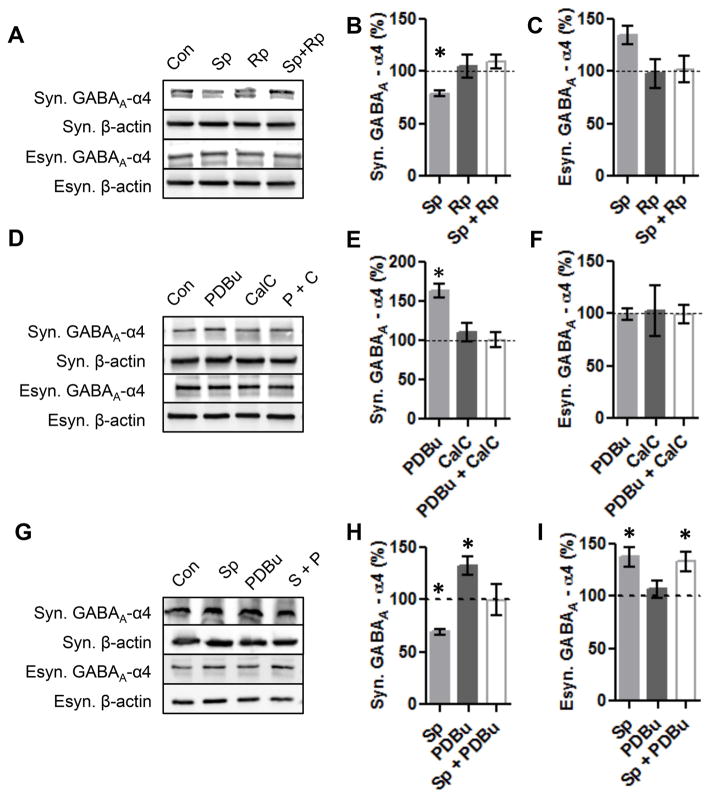
Effects of PKA and PKC activation can be prevented
Since pharmacological activation of PKA and PKC may have off target or downstream effects, we next wanted to test that the activators were selective for PKA and PKC. Therefore, we simultaneously activated and inhibited PKA and analyzed α4 expression in the synaptic and extrasynaptic fractions. Our results indicate that simultaneous activation and inhibition of PKA prevented changes in α4 expression in the synaptic and extrasynaptic fractions (Fig 5A–C). Simultaneous activation and inhibition of PKC prevented the increase of α4 expression in the synaptic fraction, and had no effect on the extrasynaptic α4 expression (Fig 5B–E).
Figure 5. Effects of PKA and PKC activation on synaptic and extrasynaptic α4 expression can be blocked by PKA or PKC inhibitors.
(A) Cortical neurons (DIV 18) were treated 1 hr with either ddH2O(Con), PKA activator Sp-cAMPs (Sp, 50μM), PKA inhibitor Rp-cAMPs (Rp, 50 μM), or both followed by fractionation and western blot.
(B) Quantification of synaptic GABAA α4 expression following simultaneous PKA activation and inhibition.
(C) Quantification of extrasynaptic GABAA α4 expression following simultaneous PKA activation and inhibition.
(D) Cortical neurons (DIV 18) were treated 1 hr with either ddH2O(Con), PKC activator PDBu (PDBu, 100 nM), and PKC inhibitor Calphostin C (CalC, 300nM), or both followed by fractionation and western blot.
(E) Quantification of synaptic GABAA α4 expression following simultaneous PKC activation and inhibition.
(F) Quantification of extrasynaptic GABAA α4 expression following simultaneous PKC activation and inhibition.
(G) Cortical neurons (DIV 18) were treated 1 hr with either ddH2O(Con), PKA activator Sp-cAMPs (Sp, 50μM), PKC activator PDBu (PDBu, 100 nM), or both followed by fractionation and western blot.
(H) Quantification of synaptic GABAA α4 expression following simultaneous PKA activation and PKC activation.
(I) Quantification of extrasynaptic GABAA α4 expression following simultaneous PKA activation and PKC activation.
Values are relative to control. p<0.05, Error bars indicate ±SEM. n = 4–6 independent experiments.
Since PKC and PKA appear to regulate synaptic GABAA-Rs in opposing directions (Fig 3B, F) we sought to determine if simultaneous activation of both PKA and PKC would negate the effects on synaptic GABAA-Rs. Our results indicate that simultaneous activation of PKA and PKC restores α4 expression to control levels (Fig 5G, H). In contrast, simultaneous activation of PKA and PKC did not prevent PKA-induced up-regulation of α4 expression in the extrasynaptic fraction (Fig 5G, I), consistent with the conclusion that PKC does not regulate extrasynaptic α4 receptors.
DISCUSSION
Dysfunction of GABAergic systems that contribute to the development of neurological diseases likely stems from changes in GABAA-R expression, however little is known about the underlying mechanisms that facilitates these changes. We used a pharmacological and biochemical strategy to investigate the role of PKA and PKC on GABAA-Rs containing the α4 subunit. We focused on the α4 subunit because of its unique physiological properties37 and due to its dysregulation in many disease states11,25,38–42. We focused on PKA and PKC because both kinases have long been known to modulate GABAA-R function17,20,21,30,43–45 and expression18,46–48. We found that the activation of PKA or PKC had opposite effects on α4 expression, with PKA activation decreasing α4 expression and PKC noticeably increasing α4 expression (Fig 1). Our results further indicate that changes in GABAA-R trafficking are likely responsible for changes in α4 expression, as surface expression changed, but total α4 expression did not (Fig 1). Since GABAA-R expression is thought to occur through either de novo insertion or reinsertion following internalization49 and since we didn’t observe increased Gabra4 mRNA levels following PKC activation we propose that increases in α4 surface expression are due to changes in receptor trafficking as opposed to de novo synthesis. Similarly, PKA activation increased Gabra4 expression but decreased α4 surface expression, again indicating that changes in α4 expression are likely due to changes in GABAA-R trafficking. These results indicate that PKA and PKC activation have opposite effects on α4 membrane and surface expression in cultured cortical neurons, possibly through a trafficking mechanism. Future studies will need to address if changes in surface expression of α4 occur due to stabilization, changes in insertion, or recycling of these receptors.
The α4 subunit readily assembles into two distinct receptor populations in the cortex, the primarily synaptic α42βx2γ2 and exclusively extrasynaptic α42βx2δ GABAA-Rs. We utilized and validated a biochemical strategy that had previously been utilized for separation of synaptic and extrasynaptic NMDA receptors32 and for GABAA-Rs in vivo5 and in vitro33 to determine if there was differential regulation of these two populations by protein kinases in cultured cortical neurons (Fig 2). The enrichment of synaptic marker PSD-95 and GABAergic synaptic markers neuroligin 250 and gephyrin51,52 in our synaptic preparation and the presence of the δ exclusively in our extrasynaptic fraction provide ample evidence that our protocol can be used to interrogate the expression of synaptic vs. extrasynaptic populations of GABAA-Rs in cultured neurons. The presence of gephyrin outside the synaptic fraction, was surprising, but is consistent with gephyrin’s role as a synaptic organizer52, but not located exclusively at the synapse53. We discovered that there was significant expression of γ2 in our extrasynaptic preparation suggesting that some γ2-containing receptors are localized outside of the synapse consistent with the previous studies52,54,55. Differentiation of α4-containing subtypes is important, as in epileptic and alcohol dependence models, there is a downregulation of δ-containing α4 GABAA-Rs and an upregulation of γ2-containing α4 GABAA-Rs11,12,56. These receptors mediate different forms of GABAergic inhibition3 and therefore changes in expression will have important consequences in mediating overall neuronal excitability and neurotransmission. Our methodology provides a relatively simple procedure that could be used to further investigation of endogenous populations of synaptic and extrasynaptic GABAA-Rs.
PKA has been shown to modulate GABAA-R responses and expression in recombinant systems and in cortical neurons17,20,30. Consistent with previous findings in the hippocampus57, PKA activation caused a decrease in synaptic α4 expression and provides further evidence that PKA is a modulator of α4-containing GABAA-Rs (Fig 3). Interestingly, decreased synaptic α4 expression is accompanied by an increase in phosphorylation of the known PKA phosphorylation sites and positive modulator of GABAA-R function β3 S408/40917. Phosphorylation of this site has been shown to inhibit binding of GABAA receptors to the AP2 complex, preventing internalization58, which appears to be at odds with our current results. However, previous studies in our lab demonstrate that PKA activation increases α1 expression and zolpidem evoked-currents30, suggesting that PKA simultaneously down-regulates α4-containing GABAA-Rs and up-regulates α1-containing GABAA-Rs. Future studies are needed to determine whether changes in β3 S408/409 phosphorylation are associated with receptors containing both the α1 or α4 subunits following PKA activation.
Like PKA, PKC modulates GABAA-R function and expression in recombinant and neuronal systems18,21,22,59. We found that PKC activation increases synaptic α4 expression in direct opposition to our finding with PKA activation (Fig 3) but consistent with previous results that PKC increases overall α4 surface expression in COS7 cells19 and cortical neurons25. These effects are likely specific to PKC as this effect was blocked by the co-exposure with the PKC inhibitor, CalC (Fig 5). We also observed an increase in γ2 S327 phosphorylation that may account for the increase of synaptic α4 expression following PKC activation. Phosphorylation of γ2 S327 has been shown to stabilize GABAA-Rs at synaptic sites60. Further studies are needed to demonstrate that phosphorylation of γ2 S327 is required for the effects of PKC on synaptic α4 receptors.
PKA and PKC appear to be working in opposition on synaptic α4 expression as simultaneous activation of both kinases blocked changes in synaptic α4 expression (Fig 5). PKA and PKC have previously been shown to work in opposition61 and have opposite effects on GABAA-R function in a cell-type specific manner57,29. This is also consistent with previous work showing that ethanol exposure for one hour increases both PKA and PKC membrane activity and consequently there is no change in α4 expression30 suggesting that these kinases have opposite effects on synaptic α4 expression. PKA and PKC may compete for regulation of these receptors, as previous reports have found that PKA phosphorylation of β3 S408/409 is only increased when PKC is inhibited18.
In contrast to findings in the synaptic fraction, we found that PKC did not regulate extrasynaptic α4 expression (Fig 4). Previous studies examining the effects of PKC on extrasynaptic GABAA-Rs have demonstrated that PKC regulation of extrasynaptic GABAA-Rs is complex and varies depending on receptor composition, cell type, drug exposure, and experimental temperature19,23,24. In recombinant systems, pharmacological activation of PKC for twenty minutes decreased α4β2δ surface expression in HEK293 cells while other studies have shown that 10 minute pharmacological activation of PKC increases α4β3δ GABAA-Rs in COS7 cells19. PKC activation in dentate gyrus granule cells and thalamic relay neurons caused a decrease in GABAA-R tonic current24 possibly due to down-regulation of δ-containing GABAA-Rs. In the hippocampus, treatment with PDBu increased α4 surface expression19. Other studies have found that neither activating nor inhibiting PKC had any effect on GABAA-R tonic current in cerebellar granule cells62. Conflicting reports regarding PKC regulation of extrasynaptic GABAA-Rs may be due to the presence of different PKC isoforms, GABAA-R assemblies, or experimental conditions.
In contrast, we found that PKA activation increases the expression of extrasynaptic α4 GABAA-Rs (Fig 4), and this effect can be blocked by simultaneous exposure with a PKA inhibitor (Fig 5) but not a PKC activator. This suggests that in contrast to synaptic α4 GABAA-Rs, PKA and PKC do not work in opposition on extrasynaptic α4 GABAA-Rs in cultured cortical neurons. This result agrees with functional studies conducted in our laboratory showing increases in tonic current in cultured cortical neurons after exposure to a PKA activator but no change in tonic current after PKC activation33 as well as other studies conducted in the visual cortex finding that PKA activation increases tonic current63. However, other studies have shown PKA activation decreases tonic conductance in thalamocortical neurons, while activation of metabotropic Gi/o GABAB receptors or use of PKA inhibitors increases tonic conductance27 suggesting that PKA modulation of GABAA-Rs may be brain region or cell type specific. The present results suggest that PKA has differential effects on α42βx2δ and α2βx2γ2 GABAA-Rs which backs work in recombinant systems showing that PKA activation increases α42β32δ spontaneous currents, but not α42β32γ2 spontaneous currents59. Future studies will need to determine the precise mechanism of how PKA activation increases α42βx2δ expression.
The current study demonstrates that PKA and PKC are regulators of α4 expression and provides insight into how activation of these kinases may facilitate changes in α4 expression. Additional studies will be required in order to determine if changes in synaptic and extrasynaptic α4 expression are due to receptor trafficking, surface stabilization, or degradation. Unfortunately, biotinylation interferes with TritonX-100 fractionation and prevents adequate separation of the synaptic and extrasynaptic fractions. Future studies using alternative methods will be needed to determine the mechanism of changes in expression of these two populations of α4 containing GABAA-Rs. Future studies will also need to determine if changes in phosphorylation observed in the current study occur only on the α4-containing GABAA-Rs or other GABAA-R complexes assembled with a different α subunits. These studies could use the methodology that we present in the present study order to further interrogate whether these changes occur on synaptic or extrasynaptic GABAA-Rs.
Overall, our present work suggests that PKA and PKC have opposing effects on synaptic α4 expression while only PKA has effects on extrasynaptic α4 expression in cortical neurons (Fig 6). The results of this study demonstrate one regulatory mechanism for the expression of extrasynaptic GABAA-Rs in the cortex. This could inform diagnostic and therapeutic interventions for alcoholism, depression, epilepsy, stroke, and schizophrenia as well as broaden the knowledge and understanding of the regulation of extrasynaptic GABAA-Rs and inhibitory tonic current in the cortex.
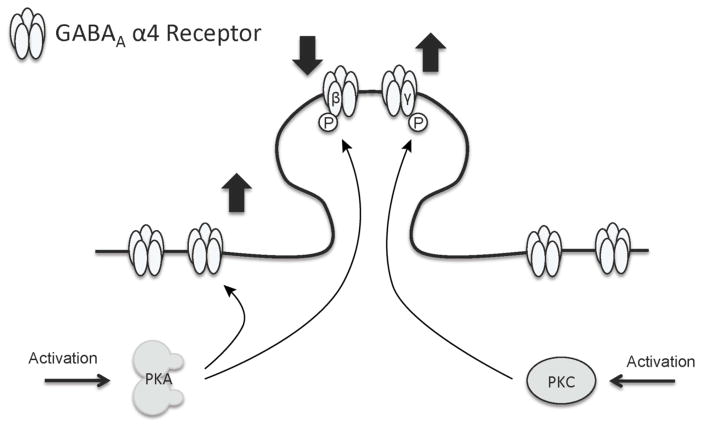
Fig 6. Model of PKA and PKC regulation of subpopulations of α4 GABAA-Rs in cortical neurons.
PKA activation decreases synaptic α4 GABAA-R subunit expression in conjunction with increased S408/409 phosphorylation of the β3 subunit, while PKA activation increases extrasynaptic α4/δ GABAA-R expression independent of β3 subunit phosphorylation, suggesting these distinct receptor subtypes are independently regulated in opposite directions by PKA. PKC activation increases synaptic α4 GABAA-R subunit expression in conjunction with increased S327 phosphorylation of the γ2 subunit, while this activation has no effect on extrasynaptic α4/δ GABAA-R expression. PKA and PKC have opposing effects on synaptic α4 GABAA-Rs.
HIGHLIGHTS.
PKA regulates the expression of synaptic and extrasynaptic α4 GABAA-Rs.
PKC regulates the expression of synaptic but not extrasynaptic α4 GABAA-Rs.
PKA and PKC have opposing effects on expression of synaptic α4-containing GABAA-Rs.
Biochemical separation of synaptic and extrasynaptic α4-containing GABAA-Rs shown.
Acknowledgments
This work was funded by NIH grant AA11605 to ALM. The authors wish to thank Todd O’Buckley and Vraj Patel for expert technical assistance and members of the Morrow lab for feedback and insight.
Abbreviations
- PKA
protein kinase A
- PKC
protein kinase C
- Sp-cAMPS
Sp-Adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt
- Rp-cAMPS
Rp-Adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt
- PDBu
Phorbol 12,13-dibutyrate
- CalC
Calphostin C
- PSD-95
postsynaptic density protein-95
Footnotes
The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nusser Z, Roberts JD, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J Neurosci. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 4.Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson SL, O’Buckley TK, Thomas R, Thiele TE, Morrow AL. Altered GABAA receptor expression and seizure threshold following acute ethanol challenge in mice lacking the RIIbeta subunit of PKA. Neurochem Res. 2014;39:1079–1087. doi: 10.1007/s11064-013-1167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchi MT, Haas KF, Macdonald RL. Structural determinants of fast desensitization and desensitization-deactivation coupling in GABAA receptors. J Neurosci. 2001;21:1127–1136. doi: 10.1523/JNEUROSCI.21-04-01127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallner M, Hanchar HJ, Olsen RW. Low-dose alcohol actions on alpha4beta3delta GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci USA. 2006;103:8540–8545. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundstrom-Poromaa I, et al. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghese CM, et al. The delta subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- 11.Whissell PD, Lecker I, Wang DS, Yu J, Orser BA. Altered expression of deltaGABAA receptors in health and disease. Neuropharmacology. 2015;88:24–35. doi: 10.1016/j.neuropharm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Liang J, et al. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABA(A) receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lund IV, et al. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal. 2008;1:ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maldonado-Avilés JG, et al. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. The American Journal of Psychiatry. 2009;166:450–459. doi: 10.1176/appi.ajp.2008.08101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tretter V, et al. Targeted disruption of the GABA(A) receptor delta subunit gene leads to an up-regulation of gamma 2 subunit-containing receptors in cerebellar granule cells. J Biol Chem. 2001;276:10532–10538. doi: 10.1074/jbc.M011054200. [DOI] [PubMed] [Google Scholar]
- 17.McDonald BJ, et al. Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci. 1998;1:23–28. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- 18.Brandon NJ, et al. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem. 2000;275:38856–38862. doi: 10.1074/jbc.M004910200. [DOI] [PubMed] [Google Scholar]
- 19.Abramian AM, et al. Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J Biol Chem. 2010;285:41795–41805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald BJ, Moss SJ. Conserved phosphorylation of the intracellular domains of GABA(A) receptor beta2 and beta3 subunits by cAMP-dependent protein kinase, cGMP-dependent protein kinase protein kinase C and Ca2+/calmodulin type II-dependent protein kinase. Neuropharmacology. 1997;36:1377–1385. doi: 10.1016/s0028-3908(97)00111-1. [DOI] [PubMed] [Google Scholar]
- 21.Krishek BJ, et al. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 22.Connolly CN, et al. Cell surface stability of gamma-aminobutyric acid type A receptors. Dependence on protein kinase C activity and subunit composition. J Biol Chem. 1999;274:36565–36572. doi: 10.1074/jbc.274.51.36565. [DOI] [PubMed] [Google Scholar]
- 23.Abramian AM, et al. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proc Natl Acad Sci U S A. 2014;111:7132–7137. doi: 10.1073/pnas.1403285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bright DP, Smart TG. Protein kinase C regulates tonic GABA(A) receptor-mediated inhibition in the hippocampus and thalamus. Eur J Neurosci. 2013;38:3408–3423. doi: 10.1111/ejn.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werner DF, et al. PKCgamma is required for ethanol-induced increases in GABA(A) receptor alpha4 subunit expression in cultured cerebral cortical neurons. J Neurochem. 2011;116:554–563. doi: 10.1111/j.1471-4159.2010.07140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura Y, Darnieder LM, Deeb TZ, Moss SJ. Regulation of GABAARs by Phosphorylation. Adv Pharmacol. 2015;72:97–146. doi: 10.1016/bs.apha.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connelly WM, et al. GABAB Receptors Regulate Extrasynaptic GABAA Receptors. J Neurosci. 2013;33:3780–3785. doi: 10.1523/JNEUROSCI.4989-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen MJ, Ade KK, Fu Z, Vicini S. Dopamine modulation of GABA tonic conductance in striatal output neurons. J Neurosci. 2009;29:5116–5126. doi: 10.1523/JNEUROSCI.4737-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunig I, Sommer M, Hatt H, Bormann J. Dopamine receptor subtypes modulate olfactory bulb gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 1999;96:2456–2460. doi: 10.1073/pnas.96.5.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson SL, Kumar S, Werner DF, Comerford CE, Morrow AL. Ethanol activation of protein kinase A regulates GABAA alpha1 receptor function and trafficking in cultured cerebral cortical neurons. J Pharmacol Exp Ther. 2013;345:317–325. doi: 10.1124/jpet.112.201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar S, et al. Ethanol reduces GABAA alpha1 subunit receptor surface expression by a protein kinase Cgamma-dependent mechanism in cultured cerebral cortical neurons. Mol Pharmacol. 2010;77:793–803. doi: 10.1124/mol.109.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Carlson SL, Bohnsack JP, Patel V, Morrow AL. Regulation of extrasynaptic GABAA alpha4 receptors by ethanol-induced PKA, but not PKC activation in cultured rat cerebral cortical neurons. J Pharmacol Exp Ther. 2015 doi: 10.1124/jpet.115.228056. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- 36.Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- 37.Wisden W, et al. Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor alpha 4 subunit. FEBS Lett. 1991;289:227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- 38.Grabenstatter HL, et al. Effect of spontaneous seizures on GABAA receptor alpha4 subunit expression in an animal model of temporal lobe epilepsy. Epilepsia. 2014;55:1826–1833. doi: 10.1111/epi.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lovick TA, Griffiths JL, Dunn SM, Martin IL. Changes in GABA(A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131:397–405. doi: 10.1016/j.neuroscience.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Fatemi SH, et al. mRNA and protein levels for GABAA alpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. J Autism Dev Disord. 2010;40:743–750. doi: 10.1007/s10803-009-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulinello M, Orman R, Smith SS. Sex differences in anxiety, sensorimotor gating and expression of the alpha4 subunit of the GABAA receptor in the amygdala after progesterone withdrawal. Eur J Neurosci. 2003;17:641–648. doi: 10.1046/j.1460-9568.2003.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rewal M, et al. Alpha4-containing GABAA receptors in the nucleus accumbens mediate moderate intake of alcohol. J Neurosci. 2009;29:543–549. doi: 10.1523/JNEUROSCI.3199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leidenheimer NJ, McQuilkin SJ, Hahner LD, Whiting P, Harris RA. Activation of protein kinase C selectively inhibits the gamma-aminobutyric acid A receptor: role of desensitization. Mol Pharmacol. 1992;41:1116–1123. [PubMed] [Google Scholar]
- 44.Song M, Messing RO. Protein kinase C regulation of GABAA receptors. Cell Mol Life Sci. 2005;62:119–127. doi: 10.1007/s00018-004-4339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herring D, Huang R, Singh M, Dillon GH, Leidenheimer NJ. PKC modulation of GABAA receptor endocytosis and function is inhibited by mutation of a dileucine motif within the receptor beta 2 subunit. Neuropharmacology. 2005;48:181–194. doi: 10.1016/j.neuropharm.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Brandon N, Jovanovic J, Moss S. Multiple roles of protein kinases in the modulation of gamma-aminobutyric acid(A) receptor function and cell surface expression. Pharmacol Ther. 2002;94:113–122. doi: 10.1016/s0163-7258(02)00175-4. [DOI] [PubMed] [Google Scholar]
- 47.Brandon NJ, et al. A-kinase anchoring protein 79/150 facilitates the phosphorylation of GABAA receptors by cAMP-dependent protein kinase via selective interaction with receptor β subunits. Molecular and Cellular Neuroscience. 2003;22:87–97. doi: 10.1016/s1044-7431(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 48.Brandon NJ, et al. Subunit-specific association of protein kinase C and the receptor for activated C kinase with GABA type A receptors. J Neurosci. 1999;19:9228–9234. doi: 10.1523/JNEUROSCI.19-21-09228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kittler JT, McAinsh K, Moss SJ. Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol Neurobiol. 2002;26:251–268. doi: 10.1385/MN:26:2-3:251. [DOI] [PubMed] [Google Scholar]
- 50.Levinson JN, et al. Postsynaptic scaffolding molecules modulate the localization of neuroligins. Neuroscience. 2010;165:782–793. doi: 10.1016/j.neuroscience.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 51.Sassoe-Pognetto M, Panzanelli P, Sieghart W, Fritschy JM. Colocalization of multiple GABA(A) receptor subtypes with gephyrin at postsynaptic sites. J Comp Neurol. 2000;420:481–498. doi: 10.1002/(sici)1096-9861(20000515)420:4<481::aid-cne6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 52.Jacob TC, et al. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Danglot L, Triller A, Bessis A. Association of gephyrin with synaptic and extrasynaptic GABAA receptors varies during development in cultured hippocampal neurons. Molecular and Cellular Neuroscience. 2003;23:264–278. doi: 10.1016/s1044-7431(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 54.Bogdanov Y, et al. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bannai H, et al. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 56.Peng Z, et al. GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- 57.Poisbeau P, Cheney MC, Browning MD, Mody I. Modulation of synaptic GABAA receptor function by PKA and PKC in adult hippocampal neurons. J Neurosci. 1999;19:674–683. doi: 10.1523/JNEUROSCI.19-02-00674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kittler JT, et al. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci USA. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang X, Hernandez CC, Macdonald RL. Modulation of spontaneous and GABA-evoked tonic alpha4beta3delta and alpha4beta3gamma2L GABAA receptor currents by protein kinase A. J Neurophysiol. 2010;103:1007–1019. doi: 10.1152/jn.00801.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muir J, et al. NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the gamma2 subunit. Proc Natl Acad Sci USA. 2010;107:16679–16684. doi: 10.1073/pnas.1000589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Francesconi A, Duvoisin RM. Opposing effects of protein kinase C and protein kinase A on metabotropic glutamate receptor signaling: selective desensitization of the inositol trisphosphate/Ca2+ pathway by phosphorylation of the receptor-G protein-coupling domain. Proc Natl Acad Sci USA. 2000;97:6185–6190. doi: 10.1073/pnas.97.11.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaplan JS, Mohr C, Rossi DJ. Opposite actions of alcohol on tonic GABA(A) receptor currents mediated by nNOS and PKC activity. Nat Neurosci. 2013;16:1783–1793. doi: 10.1038/nn.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joo K, Yoon SH, Rhie DJ, Jang HJ. Phasic and Tonic Inhibition are Maintained Respectively by CaMKII and PKA in the Rat Visual Cortex. Korean J Physiol Pharmacol. 2014;18:517–524. doi: 10.4196/kjpp.2014.18.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]