Abstract
Background
Evidence of anti-cancer properties of garlic for different cancer sites has been reported previously in in-vitro and in-vivo experimental studies but there is limited epidemiological evidence on the association between garlic and lung cancer.
Methods
We examined the association between raw garlic consumption and lung cancer in a case-control study conducted between 2005 and 2007 in Taiyuan, China. Epidemiological data was collected by face-to-face interviews from 399 incident lung cancer cases and 466 healthy controls. We used unconditional logistic regression models to estimate crude and adjusted odds ratios (aOR) and their 95% confidence intervals (CI). Adjusted models controlled for age, sex, average annual household income 10 years ago, smoking, and indoor air pollution.
Results
Compared to no intake, raw garlic intake was associated with lower risk of development of lung cancer with a dose-response pattern (aOR for <2 times per week = 0.56, 95% CI: 0.39–0.81 and aOR for ≥2 times per week = 0.50, 95% CI: 0.34 – 0.74; Ptrend = 0.0002). Exploratory analysis showed an additive interaction of raw garlic consumption with indoor air pollution and with any supplement use in association with lung cancer.
Conclusions
The results of the current study suggest that raw garlic consumption is associated with reduced risk of lung cancer in a Chinese population.
Impact
This study contributes to the limited research in human population on the association between garlic and lung cancer and advocates further investigation into the use of garlic in chemoprevention of lung cancer.
Keywords: Lung cancer, Garlic, Raw garlic, Allium sativum, Chinese population
Introduction
Garlic (Allium sativum) was used among several ancient civilizations across the world including Egypt (references as early as 3000 B.C.), Greece, Rome, India and China to treat various ailments including poisoning, respiratory and gastric ailments, abnormal growths, headache, insomnia and depression (1-3). In traditional Chinese medicine, garlic is used to improve cardiovascular health and immunity as well as to treat cancer (2, 4). Garlic was used in daily Chinese diet since around 2000 B.C. or earlier where it was consumed especially with raw meat (2). Currently, garlic is used as a popular spice in China, which is the largest producer and exporter of garlic (5).
Garlic is rich in organo-sulfur compounds (OSCs), which are responsible for most of its therapeutic properties including antibacterial, anti-protozoal, antifungal, hypolipidemic, antiatherosclerotic and anticancer properties (6, 7). The major OSCs that contribute to the anticancer properties of garlic include allicin, allixin, diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), S-allyl cysteine allylmercaptan, allylmethyldisulfide, allylmethyltrisulfide and ajoene (8-10). Several mechanisms including inhibiting cancer initiation, suppressing cancer promotion and preventing oxidative damage have been attributed to the anti-cancer properties of garlic (7-9, 11, 12). In-vitro and in-vivo experimental studies provided evidence for the anti-cancer properties of garlic against stomach, liver, colon, prostate, skin, bladder, breast and lung cancer (7, 13-17). Case-control studies conducted in different populations reported an inverse association between garlic consumption (raw/cooked) and colorectal, prostate, and head and neck cancers (18-20). However, Dutch prospective cohort studies showed no inverse association between garlic supplement intake and gastric, colorectal breast and lung cancers (21-24). Thus, consistent epidemiological evidence on the protective effects of garlic against different cancers is lacking.
In 2012, lung cancer accounted for 13% of total cancer cases and 19% of total cancer deaths worldwide (25). In China, lung cancer is the most common cancer in incidence and mortality (2011 statistics) with a higher age-standardized mortality of 28.0 per 100,000, compared to the world population (19.7 per 100,000) (25, 26). In the past 30 years, lung cancer mortality in China increased by 464.84% (27). Smoking, exposure to secondhand smoke, and air pollution (both outdoor and from indoor household fuel combustion) are the major risk factors of lung cancer in China (27). Evidence from previous epidemiological studies suggest that factors that may be protective against lung cancer are mainly related to diet, especially higher consumption of fruits and vegetables (28-30).
The existing epidemiological evidence of the association between garlic and lung cancer (Table 1) is limited and inconsistent (21, 31-35). Review of previous literature indicates that raw/cooked garlic rather than a more processed form (e.g. supplements) was inversely associated with the risk of cancer (36). Additionally, heating garlic seems to drastically diminish its anticarcinogenic properties (37-40). Therefore, raw garlic may potentially possess better anti-cancer properties compared to heated/cooked/processed garlic. Compared to the Chinese, raw garlic intake among other populations is very low, which makes it difficult to analyze disease associations (36). For example, in 1995, the annual average consumption of fresh garlic per person in Cangshan County, Shangdong Province, China was 6 kg while it was only 0.8 kg in the U.S (41). Thus, the Chinese population is probably better suited for studying the association between garlic intake and cancer.
Table 1. Review of relationship between garlic consumption and lung cancer from published epidemiological studies.
| Reference | Study design | Study population | Study sample | Garlic (form) | Garlic consumption | Results | Comments |
|---|---|---|---|---|---|---|---|
| Jin et al. (2013)a | Case-control study (population-based multi-center study) | China(Jiangsu province) | 1424 cases / 4543 controls | Raw garlic | Estimated Odds ratio |
|
|
| Never | 1.00 | ||||||
| <2 times/week | 0.92 (0.79–1.08) | ||||||
| ≥2 times/week | 0.56 (0.44–0.72) | ||||||
| Ptrend = <0.001 | |||||||
| Lin et al. (2012)b | Case-control study | China -women (Fujian province) | 226 cases / 269 controls matched on age | Raw garlic | Estimated odds ratio |
|
|
| No consumption | 1.00 | ||||||
| 1-2 times/week | 0.79 (0.49–1.28) | ||||||
| > 2 times/week | 0.37 (0.16–0.84) | ||||||
| Satia et al. (2009)c | Prospective cohort study | U.S.A. (Washington state | 665lung cancer cases / 76,460 non-lung cancer individuals | Garlic supplement pills (past ten years use) | Estimated hazard ratio |
|
|
| Non user | 1.00 | ||||||
| User | 1.09 (0.83–1.34) | ||||||
| Linseisen et al. (2007)d | Prospective cohort study | 10 European countries | 1126 lung cancer cases / 478,590 cohort members followed | Garlic vegetable | Quintiles of consumption | Estimated hazard risk |
|
| No association found | |||||||
| Le Marchand et al. (2000)e | Case-control study (population based) | Hawaii, U.S.A | 582 cases / 582 controls matched on age, sex and ethnicity | Garlic vegetable | Quartiles of consumption | Estimated odds ratio |
|
| Quartile 1 | 1.00 | ||||||
| Quartile 2 | 0.9 (0.6–1.4) | ||||||
| Quartile 3 | 0.8 (0.5–1.2) | ||||||
| Quartile 4 | 0.7 (0.4–1.1) | ||||||
| p for trend | 0.12 | ||||||
| Dorant et al. (1994)f | Case-cohort | Netherlands | 484 lung cancer cases / 3123 non-cancer controls | Garlic supplements | Estimated rate ratio |
|
|
| No supg use | 1.00 | ||||||
| Garlic supa Only | 1.78 (1.08–2.92) | ||||||
| Other supa only | 1.00 | ||||||
| Garlic and other supa | 0.93 (0.46–1.86) |
reference # 28;
reference # 30;
reference # 32;
reference # 31;
reference # 29;
reference # 21;
sup=supplements
Only two recent case-control studies in Chinese population investigated the association between raw garlic consumption and lung cancer. Both studies reported that higher consumption of raw garlic (2 or more times per week) was protective against lung cancer (31, 33). In the current study, we analyzed data collected from a case-control study conducted in Taiyuan City, Shanxi Province, China and sought to replicate the association between raw garlic consumption and lung cancer. In addition, we explored for interaction between major risk factors and raw garlic consumption in association with lung cancer.
Materials and Methods
Study population
Cases were recruited between 2005 and 2007 from Shanxi Tumor Hospital, where about 70% of cancer patients from Taiyuan city sought treatment. Eligible cases were newly diagnosed lung cancer patients who were aged 20 years or older, lived in Taiyuan city for 10 years or more, in stable medical condition and willing to participate in the study. Controls were randomly selected from resident lists of 13 communities covering most of Taiyuan city to match cases according to the distribution of age and gender. Eligibility criteria for controls were same as the cases but controls had no history of cancer or any other serious chronic disease. A total of 399 cases (89% response rate) and 466 controls (85% response rate) participated in the study. Informed consent was obtained from study participants before completing the study questionnaire. A detailed description regarding participant recruitment was previously published (42).
Data collection
Epidemiologic data was collected by trained study personnel using a structured questionnaire to conduct face-to-face interviews of all cases at the hospital and all controls at community health centers. The questionnaire included information on demographic characteristics, residence and housing history, living and cooking habits, smoking, alcohol drinking, tea drinking and personal and family medical history. “Ever smokers” were defined as those who smoked at least 100 cigarettes during their lifetime. Dietary intake 1 year ago was evaluated using 96-item food frequency questionnaire and other questions on specific items. Raw garlic intake was assessed by asking “How often do you eat raw garlic?” Participants chose one of the following responses: (1) never (2) occasionally (<2 times/week) (3) often (≥2 times/week) (4) do not know. We assessed overall exposure to indoor air pollution (IAP) 10 years prior to the date of interview by creating an IAP index, which was based on an individual's exposure to five major risk factors including solid fuel use for cooking and heating, ventilation in kitchen, opening of windows in winter and secondhand smoking. Exposure to each risk factor was scored as ‘1’ and based on the number of the above five risk factors that an individual was exposed to; an IAP index of 0-5 was assigned.
Statistical analysis
We used chi-square and t tests to test the differences in distribution of selected demographic characteristics (age, sex, education, average annual household income 10 years ago), body mass index (BMI), smoking, IAP exposure (IAP index of 0 = none, 1-2 = low and 3-5 = high) alcohol drinking, tea drinking and supplement use (intake of one or more of the following – vitamin A, beta-carotene, vitamin C, vitamin E, calcium, selenium, zinc or multivitamin) among cases and controls. We also tested differences in the distribution of the above characteristics by raw garlic consumption (never, <2 times per week and ≥2 times per week) using chi-square and one-way ANOVA tests. For the analysis in the current study, we excluded 7 participants who did not report their intake of raw garlic. Unconditional logistic regression models were used to estimate crude and adjusted odds ratios (aOR) and 95% confidence intervals (CI) for the association between raw garlic intake and lung cancer. Based on prior knowledge and review of previous relevant literature, selected covariables including education, average annual household income 10 years ago, BMI, pack years of smoking, IAP, alcohol drinking, tea drinking and supplement use were tested for association with the exposure and/or the disease in the study population. Education, BMI, pack years of smoking, IAP and supplement use were associated with lung cancer but not with raw garlic intake. Average annual household income 10 years ago was the only variable that was associated with both raw garlic intake and lung cancer. Age and sex being frequency matching variables, were included in the multivariate model. We generated two multivariate models to present the results of logistic regression. Model 1 adjusted for age, sex and average annual household income. Model 2 additionally adjusted for smoking and IAP considering that they were among the strongest risk factors for lung cancer in the Chinese population. The results from a saturated multivariable model that included all covariables were similar to the results from Model 2. We chose to use Model 2 as the final multivariate model. The association between raw garlic consumption and lung cancer was estimated in the overall population as well as in subgroups of age, sex, smoking, IAP, alcohol drinking, tea drinking, supplement use, and by histological subtypes of lung cancer. We also evaluated for multiplicative and additive interaction of raw garlic consumption with age (0 = <55 years, 1 = ≥55 years), smoking status (0 = never, 1 = ever), IAP (0 = no/low, 1 = high), alcohol drinking (0 = no, 1 = yes), tea drinking (0 = never, 1 = ever) and supplement use (0 = no, 1 = yes) in association with lung cancer. Raw garlic consumption was operationalized as 0 = “never” and 1 = “any” intake for interaction analysis. Multiplicative interaction was evaluated by including the main effect variables and their product term in logistic regression models. A ratio of odds ratios (ROR) estimate of the product term was considered statistically significant if the confidence interval did not include ‘1.00’. Additive interaction was assessed by calculating relative excess risk of interaction (RERI) and inclusion of ‘0.00’ within the CIs of RERI estimate indicated absence of more than additivity or no additive interaction. All analyses were performed using SAS 9.4 software.
Results
Approximately 56% of study participants were aged 55 years or older and approximately half of the participants were males. Differences in distribution of study characteristics between cases and controls are presented in Table 2. Cases had a higher mean age and a higher proportion of cases were exposed to high IAP. Education, average annual household income 10 years ago, BMI, and supplement use were higher among controls. Approximately 55% of cases (93% males and 17% females) were ever-smokers compared to 39% of controls (71% males and 6% females). The smoking prevalence among female cases is similar to that observed in previous studies in Chinese population (43-45).
Table 2. Distribution of study characteristics among lung cancer cases and cancer-free controls.
| Variables | Cases (N=399) |
Controls (N=466) |
Pa |
|---|---|---|---|
| N (%) | N (%) | ||
| Age (years) | |||
| ≤ 44 | 59 (14.8) | 83 (17.8) | 0.07 |
| 45 - 54 | 96 (24.1) | 139 (29.8) | |
| 55 - 64 | 111 (27.8) | 116 (24.9) | |
| ≥ 65 | 133 (33.3) | 128 (27.5) | |
| Mean (SD) | 58.1 (11.9) | 56.1 (11.3) | 0.01 |
| Sex | |||
| Male | 202 (50.6) | 234 (50.2) | 0.90 |
| Female | 197 (49.4) | 232 (49.8) | |
| Education | |||
| Illiteracy | 43 (10.8) | 23 (4.9) | < 0.0001 |
| Primary School | 106 (26.6) | 81 (17.4) | |
| Middle School | 124 (31.1) | 175 (37.5) | |
| High School | 68 (17.0) | 120 (25.8) | |
| College and above | 58 (14.5) | 67 (14.4) | |
| Average annual income/person 10 years ago (RMB)b | |||
| < 1000 | 104 (26.1) | 106 (22.7) | < 0.0001 |
| 1000 – 2499 | 236 (59.1) | 197 (42.3) | |
| 2500 – 4999 | 37 (9.3) | 116 (24.9) | |
| ≥ 5000 | 22 (5.5) | 47 (10.1) | |
| Mean (SD) | 1994.5 (2678.9) | 2539.4 (3193.7) | 0.007 |
| BMI (Kg/m2) | |||
| < 18.5 | 22 (5.8) | 9 (2.0) | < 0.0001 |
| 18.5 – 24.9 | 250 (66.3) | 259 (56.3) | |
| 25 – 29.9 | 90 (23.9) | 162 (35.2) | |
| ≥ 30 | 15 (4.0) | 30 (6.5) | |
| Mean (SD) | 23.4 (3.6) | 24.8 (3.9) | <0.0001 |
| Pack years of smoking | |||
| Never smokersc | 179 (44.9) | 285 (61.2) | <0.0001 |
| < 30 | 64 (16.0) | 107 (22.9) | |
| ≥ 30 | 156 (39.1) | 74 (15.9) | |
| Indoor air pollutiond | |||
| None | 38 (10.8) | 90 (19.7) | <0.0001 |
| Low | 145 (41.2) | 253 (55.2) | |
| High | 169 (48.0) | 115 (25.1) | |
| Alcohol drinkinge | |||
| Never | 298 (74.7) | 345 (74.0) | 0.83 |
| Ever | 101 (25.3) | 121 (26.0) | |
| Tea drinking | |||
| Never | 242 (60.7) | 263 (56.4) | 0.21 |
| Ever | 157 (39.3) | 203 (43.6) | |
| Supplement usef | |||
| No | 342 (87.0) | 355 (77.2) | 0.0002 |
| Yes | 51 (13.0) | 105 (22.8) |
from two sided χ2 test for categorical variables and from t test for continuous variables. p-values in bold represent statistical significance;
RMB = Renminbi, the Chinese currency;
smoked less than 100 cigarettes during lifetime;
Indoor air pollution exposure was calculated by summarizing participant's exposure to solid fuel for cooking and heating, ventilation in kitchen, opening of windows in winter and second hand smoking (an index of 0 = none, 1-2 = low and 3-5 = high indoor air pollution).
lifetime alcohol drinking status.
supplement use includes intake of one or more of the following: vitamin A, beta-carotene, vitamin C, vitamin E, calcium, selenium, zinc or multivitamins.
Table 3 shows the distribution of study characteristics by participants' raw garlic consumption. We did not find differences in the distribution of any of the study characteristics except for average annual household income 10 years ago. High income participants (≥2500 Renminbi) consumed higher amounts of raw garlic (≥2 times/week) compared to those with lower income. Smoking exposure did not differ by raw garlic consumption.
Table 3. Distribution of study characteristics by categories of participants' consumption of raw garlic.
| Variable | Raw garlic consumption | Pa | ||
|---|---|---|---|---|
| Never (N=357) |
<2 times/week (N=244) |
≥2 times/week (N=239) |
||
| N (%) | N (%) | N (%) | ||
| Age (years) | ||||
| ≤ 44 | 53 (14.9) | 50 (20.5) | 39 (16.3) | 0.46 |
| 45 - 54 | 100 (28.0) | 65 (26.6) | 69 (28.9) | |
| 55 - 64 | 101 (28.3) | 64 (26.3) | 55 (23.0) | |
| ≥ 65 | 103 (28.8) | 65 (26.6) | 76 (31.8) | |
| Mean (SD) | 57.2 (11.2) | 56.1 (11.7) | 56.7 (12.1) | 0.53 |
| Sex | ||||
| Male | 175 (49.0) | 115 (47.1) | 126 (52.7) | 0.46 |
| Female | 182 (51.0) | 129 (52.9) | 113 (47.3) | |
| Education | ||||
| Illiteracy | 23 (6.4) | 16 (6.6) | 24 (10.0) | 0.48 |
| Primary School | 88 (24.7) | 45 (18.4) | 48 (20.1) | |
| Middle School | 122 (34.2) | 94 (38.6) | 78 (32.6) | |
| High School | 74 (20.7) | 55 (22.5) | 53 (22.2) | |
| College and above | 50 (14.0) | 34 (13.9) | 36 (15.1) | |
| Average annual household income 10 years ago (RMB)b | ||||
| < 1000 | 106 (29.7) | 53 (21.7) | 48 (20.1) | 0.0006 |
| 1000 – 2499 | 181 (50.7) | 133 (54.5) | 106 (44.3) | |
| 2500 – 4999 | 47 (13.2) | 39 (16.0) | 58 (24.3) | |
| ≥ 5000 | 23 (6.4) | 19 (7.8) | 27 (11.3) | |
| Mean (SD) | 2033.7 (2590.5) | 2289.5 (3237.3) | 2685.0 (3330.4) | 0.04 |
| BMI (Kg/m2) | ||||
| < 18.5 | 17 (4.9) | 6 (2.6) | 8 (3.5) | 0.82 |
| 18.5 – 24.9 | 215 (61.4) | 147 (62.8) | 137 (59.6) | |
| 25 – 29.9 | 99 (28.3) | 70 (29.9) | 73 (31.7) | |
| ≥ 30 | 19 (5.4) | 11 (4.7) | 12 (5.2) | |
| Mean (SD) | 23.9 (4.1) | 24.2 (3.6) | 24.3 (3.7) | 0.53 |
| Pack years of smoking | ||||
| Never smokersc | 195 (54.6) | 135 (55.3) | 124 (51.8) | 0.89 |
| < 30 | 67 (18.8) | 46 (18.9) | 52 (21.8) | |
| ≥ 30 | 95 (26.6) | 63 (25.8) | 63 (26.4) | |
| Indoor air pollutiond | ||||
| None | 46 (13.6) | 31 (13.6) | 46 (20.9) | 0.11 |
| Low | 162 (47.9) | 119 (52.2) | 100 (45.5) | |
| High | 130 (38.5) | 78 (34.2) | 74 (33.6) | |
| Alcohol drinkinge | ||||
| Never | 274 (76.8) | 188 (77.0) | 168 (70.3) | 0.14 |
| Ever | 83 (23.2) | 56 (23.0) | 71 (29.7) | |
| Tea drinking | ||||
| Never | 212 (59.4) | 146 (59.8) | 134 (56.1) | 0.65 |
| Ever | 145 (40.6) | 98 (40.2) | 105 (43.9) | |
| Supplement usef | ||||
| No | 294 (83.8) | 191 (78.9) | 197 (83.5) | 0.27 |
| Yes | 57 (16.2) | 51 (21.1) | 39 (16.5) | |
from two sided χ2 test for categorical variables and from ANOVA for continuous variables. p-values in bold represent statistical significance;
RMB = Renminbi, the Chinese currency;
smoked less than 100 cigarettes during lifetime;
Indoor air pollution exposure was calculated by summarizing participant's exposure to solid fuel for cooking and heating, ventilation in kitchen, opening of windows in winter and second hand smoking (an index of 0 = none, 1-2 = low and 3-5 = high indoor air pollution).
lifetime alcohol drinking status.
Supplement use includes intake of one or more of the following: vitamin A, beta-carotene, vitamin C, vitamin E, calcium, selenium, zinc or multivitamins.
The overall association of raw garlic consumption with lung cancer in crude and adjusted models is presented in Table 4. Raw garlic consumption in all categories (any intake, <2 times per week, ≥2 times per week) was inversely associated with lung cancer in the crude and both the multivariable models after adjusting for potential confounders. Compared to no intake, raw garlic consumption was inversely associated with lung cancer (<2 times/week: aOR = 0.56, 95% CI: 0.39–0.81; ≥2 times/week: aOR = 0.50, 95% CI: 0.34 – 0.74) with a dose-response relationship (Ptrend = 0.0002). We did not find notable differences between the results from the two multivariate models.
Table 4. Association of raw garlic consumption with lung cancer.
| Raw garlic consumption | Cases (N=392) |
Controls (N=448) |
cOR (95% CI) | Model 1a aOR (95% CI) | Model 2b aOR (95% CI) |
|---|---|---|---|---|---|
| N (%) | N (%) | ||||
| Never | 197 (50.3) | 160 (35.7) | 1.00 | 1.00 | 1.00 |
| Any | 195 (49.7) | 288 (64.3) | 0.55 (0.42–0.73) | 0.59 (0.44–0.78) | 0.52 (0.38–0.72) |
| < 2 times/week | 104 (26.5) | 140 (31.3) | 0.60 (0.43–0.84) | 0.61 (0.44–0.86) | 0.56 (0.39–0.81) |
| ≥ 2 times/week | 91 (23.2) | 148 (33.0) | 0.50 (0.36–0.70) | 0.56 (0.40–0.80) | 0.50 (0.34–0.74) |
| Ptrend | <0.0001 | 0.0006 | 0.0002 |
cOR = crude odds ratio aOR = adjusted odds ratio;
adjusted for age (years), sex, and average household income 10 years ago (<1000 = 1, 1000-2499 = 2, 2500-4999 = 3, ≥5000 = 4).
adjusted for pack years of smoking (continuous) and indoor air pollution (an index of 0 = none, 1-2 = low and 3-5 = high indoor air pollution) in addition to the covariates in Model 1.
Table 5 shows the association between raw garlic consumption and lung cancer among any garlic consumers as well as those who consumed <2 times/week and ≥2 times/week, stratified by age, sex, smoking status, IAP, alcohol drinking, tea drinking, supplement use and association with specific histo-pathological subtypes of lung cancer. The association between raw garlic consumption and lung cancer was stronger in the younger age group, among females and those exposed to IAP, whereas the association did not differ by smoking status, alcohol drinking and tea drinking. The association with adenocarcinoma and squamous cell carcinoma subtypes was statistically significant. When these analyses were further stratified by sex, the associations seemed to be more prominent among women (Supplementary Table S1). However, these observations may not be sufficient to draw explicit conclusions because of the small sample sizes within the strata.
Table 5. Association between raw garlic consumption and lung cancer stratified by major risk factors.
| Variable | Never | Any | <2 times/week | ≥2 times/week | Ptrenda | |||
|---|---|---|---|---|---|---|---|---|
| Ca/Co | Ca/Co | aORa (95% CI) | Ca/Co | aORa (95% CI) | Ca/Co | aORa (95% CI) | ||
| Age (years) | ||||||||
| <55 | 88/65 | 67/156 | 0.36 (0.23–0.58) | 34/81 | 0.33 (0.19–0.58) | 33/75 | 0.40 (0.23–0.72) | <0.001 |
| ≥55 | 109/95 | 128/132 | 0.69 (0.45–1.08) | 70/59 | 0.82 (0.49–1.39) | 58/73 | 0.57 (0.33–0.99) | 0.049 |
| Sex | ||||||||
| Males | 92/83 | 103/138 | 0.64 (0.40–1.03) | 54/61 | 0.68 (0.39–1.20) | 49/77 | 0.60 (0.34–1.08) | 0.071 |
| Females | 105/77 | 92/150 | 0.46 (0.30–0.72) | 50/79 | 0.49 (0.29–0.81) | 42/71 | 0.43 (0.25–0.75) | 0.001 |
| Smoking status | ||||||||
| Never | 96/99 | 82/177 | 0.49 (0.32–0.76) | 49/86 | 0.59 (0.35–0.97) | 33/91 | 0.41 (0.24–0.71) | <0.001 |
| Ever | 101/61 | 113/11 | 0.56 (0.34–0.91) | 55/54 | 0.44 (0.20–0.96) | 58/57 | 0.63 (0.28–1.41) | 0.063 |
| Indoor air pollution | ||||||||
| None | 18/28 | 20/57 | 0.53 (0.21–1.36) | 11/20 | 0.73 (0.24–2.23) | 9/37 | 0.35 (0.11–1.10) | 0.075 |
| Low | 74/88 | 66/153 | 0.52 (0.33–0.83) | 39/80 | 0.58 (0.34–0.99) | 27/73 | 0.45 (0.25–0.82) | 0.006 |
| High | 88/42 | 80/72 | 0.49 (0.29–0.84) | 40/38 | 0.44 (0.24–0.83) | 40/34 | 0.62 (0.32–1.19) | 0.077 |
| Alcohol Drinking | ||||||||
| No | 148/126 | 146/210 | 0.57 (0.40–0.82) | 83/105 | 0.65 (0.42–0.99) | 63/105 | 0.51 (0.32–0.81) | 0.003 |
| Yes | 49/34 | 49/78 | 0.41 (0.21–0.82) | 21/35 | 0.34 (0.15–0.81) | 28/43 | 0.53 (0.23–1.21) | 0.078 |
| Tea Drinking | ||||||||
| Never | 121/91 | 117/163 | 0.55 (0.36–82) | 65/81 | 0.63 (0.39–1.00) | 52/82 | 0.46 (0.29–0.77) | 0.002 |
| Ever | 76/69 | 39/59 | 0.46 (0.27–0.78) | 39/59 | 0.42 (0.22–0.80) | 39/66 | 0.50 (0.26–0.95) | 0.019 |
| Supplement useb | ||||||||
| No | 171/123 | 167/221 | 0.49 (0.34–0.69) | 86/105 | 0.51 (0.33–0.77) | 81/116 | 0.46 (0.30–0.72) | <0.001 |
| Yes | 23/34 | 26/64 | 0.78 (0.33–1.87) | 17/34 | 0.87 (0.32–2.31) | 9/30 | 0.76 (0.24–2.35) | 0.622 |
| Histo-pathology | ||||||||
| ACC | 53/160 | 54/288 | 0.55 (0.34–0.89) | 33/140 | 0.67 (0.38–1.16) | 21/148 | 0.43 (0.22–0.81) | <0.001 |
| SqCC | 47/160 | 42/288 | 0.44 (0.25–0.77) | 17/140 | 0.32 (0.15–0.67) | 25/148 | 0.59 (0.30–1.17) | 0.050 |
| SmCC | 26/160 | 31/288 | 0.61 (0.33–1.13) | 17/140 | 0.60 (0.29–1.24) | 14/148 | 0.58 (0.26–1.28) | 0.136 |
Ca/Co = cases/controls; aOR = adjusted odds ratio; ACC = adenocarcinoma, SqCC = squamous cell carcinoma, SmCC = small cell carcinoma.
adjusted for age (in years except for subgroups of age), sex (except for subgroups of sex), average annual household income 10 years ago (<1000 = 1, 1000-2499 = 2, 2500-4999 = 3, ≥5000 = 4), pack years of smoking (continuous, except for subgroups of smoking), and indoor air pollution (an index of 0 = none, 1-2 = low and 3-5 = high indoor air pollution except for subgroups of indoor air pollution).
supplement use includes intake of one or more of the following: vitamin A, beta-carotene, vitamin C, vitamin E, calcium, selenium, zinc or multivitamins. Statistically significant OR estimates and p-values are presented in bold font.
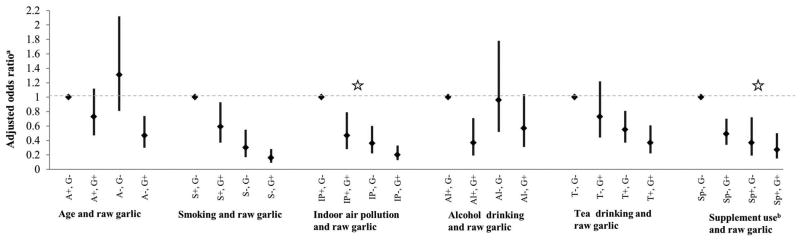
Figure 1 depicts the results of the exploratory interaction analyses between raw garlic consumption and selected risk factors in association with lung cancer. We observed additive interaction between IAP and raw garlic consumption (aRERI = 0.37, 95% CI: 0.05–0.68) as well as supplement use and raw garlic consumption (aRERI = 0.41, 95% CI: 0.08–0.74) in association with lung cancer.
Figure 1.
Charts depicting the joint effects of raw garlic consumption and selected risk factors in association with lung cancer. G+: any raw garlic intake; G-: no raw garlic intake; A+: age ≥55 years; A-: age <55 years; S+: ever smokers; S-: never smokers; IP+: high indoor air pollution; IP-: low/no indoor air pollution; Al+: alcohol ever drinkers; Al-: alcohol never drinkers; T+: Tea ever drinkers; T-: Tea never drinkers; Sp+: supplement users; Sp-: Supplement non-users. a adjusted for age (in years, except for interaction with age), sex, average household income 10 years ago (<1000 = 1, 1000-2499 = 2, 2500-4999 = 3, ≥5000 = 4), pack years of smoking (continuous, except for interaction with smoking), and indoor air pollution (an index of 0 = none, 1-2 = low and 3-5 = high indoor air pollution, except for interaction with indoor air pollution); b supplemental use includes intake of one or more of the following: vitamin A, beta-carotene, vitamin C, vitamin E, calcium, selenium, zinc or multivitamins. ✩ additive interaction between indoor air pollution and raw garlic consumption (RERI = 0.37, 95% CI: 0.05–0.68) as well as supplement use and raw garlic consumption (RERI = 0.41, 95% CI: 0.08–0.74)
Discussion
In the current study, we observed that raw garlic consumption was inversely associated with lung cancer with a dose-response relationship. The association between raw garlic consumption and lung cancer did not differ by smoking status, alcohol drinking and tea drinking. We observed additive interaction between raw garlic consumption and IAP as well as between raw garlic consumption and supplement use in association with lung cancer.
Only two recent publications examined the association between raw garlic consumption and lung cancer and observed statistically significant inverse associations (31, 33). A case-control study conducted in Fujian Province, China reported that compared to no intake, consuming raw garlic more than 2 times per week was inversely associated with lung cancer (aOR = 0.37, 95% CI: 0.16–0.84). This association was slightly stronger than what we observed in the current study. The smaller sample size (226 cases and 269 controls), consisting of primarily non-smoking women and the assessment of raw garlic consumption (no consumption, 1-2 times/week, >2 times/week) that is somewhat different from the current study could have contributed to the differences. The Fujian study did not report an association in the lower raw garlic consumption category (1-2 times/week).
Another case-control study with larger sample size (1424 cases and 4543 controls) conducted in Jiangsu Province, China reported an inverse association between raw garlic intake and lung cancer among those who consumed raw garlic ≥2 times/week (aOR = 0.56, 95% CI: 0.44–0.72) (31). This association was similar to what we found in the current study. Similar to the Fujian study, the Jiangsu study did not find an association in the low garlic consumption category (<2 times/week). This may reflect the differences in the consumption of raw garlic in different geographic regions in China. The percentage of ever-garlic consumption was lower in the Jiangsu study (47.6%) compared to the current study (55.83%) and the use of garlic as an ingredient in the spices was not common among the residents of that province (31). Although there are some differences, the current study reiterated the findings of the previous two studies that raw garlic consumption of 2 or more times per week may be protective against lung cancer.
In the current study, we observed a slightly stronger association between raw garlic consumption and lung cancer among women and the younger participants (<55 years). Differences in smoking characteristics may have played a role in this observation. A dose-response relationship between raw garlic consumption and lung cancer was observed in never-smokers, but not in ever smokers. Approximately 90% of women were never-smokers and the majority of participants in the younger age group were women and never-smokers. Although it may seem that the protective association between raw garlic consumption may be restricted to certain subgroups like women and never-smokers, the observations from the stratified analysis may not be sufficient to derive definite conclusions because of the limited sample size in the individual strata.
We observed that the association between raw garlic consumption and lung cancer was modified by IAP and supplement use on an additive scale. IAP is a major risk factor for lung cancer in China, with the major sources being fuel used for cooking and indoor heating (27, 42). Our finding indicates that the reduced risk of lung cancer associated with low/no exposure to IAP and consumption of raw garlic might be greater than the risk reduction associated with any one of the factors alone. We also observed an additive interaction between supplement use and raw garlic consumption in association with lung cancer. A recent review of clinical trials that investigated drugs for preventing lung cancer suggested that intake of vitamin and mineral supplements either alone or in combination do not reduce either incidence or mortality due to lung cancer (46). Other studies did not report consistent evidence for associations between lung cancer and the supplements taken by the majority of the participants in the current study (vitamin C, vitamin E and calcium) who reported any supplement use (47-50). We do not have additional information to attribute the observed additive interaction between supplement use and raw garlic intake to any single supplement. Further targeted investigation with larger study samples is necessary to confirm potential interactions of garlic with other risk or protective factors in association with lung cancer.
Although the anti-cancer properties are mainly attributed to the bioactive OSCs, garlic contains other nutrients including flavonoids, oligosaccharides, arginine and potassium, vitamin C and selenium that may contribute to its overall beneficial effects (51, 52). The major mechanisms contributing to the anti-cancer potential of garlic are: (i) inhibition of cancer initiation by suppressing the formation, intercepting metabolic activation and enhancing enzyme-detoxification of carcinogens (e.g. nitrosamines) as well as by inhibiting the formation of DNA-adducts; (ii) blocking cancer promotion through anti-proliferating activities including regulation of cell cycle arrest in G2/M phase and promoting apoptosis in cancer cells; (iii) antioxidant activity formation and/or scavenging of free radicals. Other mechanisms include the inhibition of cancer cell growth by modification of histone acetylation, inhibition of angiogenesis, immunomodulation and anti-inflammatory activity (8-12, 51, 53-56). The results of the current study align well with the anticancer mechanisms of garlic as described. When garlic is ingested, the active volatile compounds that are released in the stomach diffuse into the lung tissues (4) where the OSCs may act through the mechanisms described above to protect against lung cancer. Epidemiological evidence based on results of meta-analyses show that higher consumption of fruits and vegetables may also protect against lung cancer (16, 30, 57). The association between raw garlic consumption and lung cancer seems to be stronger than the association with consumption of fruits [pooled OR = 0.80; 95% CI: 0.74–0.88] and consumption of vegetables [pooled OR = 0.74; 95% CI: 0.67–0.82] (57). The numerous anti-cancer properties of garlic including high antiproliferative and antioxidant properties (58), which are mediated through multiple mechanisms, may explain the potential stronger protection effect of garlic alone against lung cancer. Further studies are needed to substantiate the evidence observed in the current study.
The current study has the following limitations: 1. Sample size: Our study results are based on a relatively small sample size but they do reiterate the findings from previous published studies regarding the relationship between raw garlic consumption and lung cancer. Our exploration for interaction with known risk factors for lung cancer may have been affected by the small sample sizes in subgroup analyses. Hence, we suggest interpreting the interaction results from the current study with caution. Raw garlic consumption may differ considerably by geographic region. Jin et al. (2013) reported that garlic consumption patterns differed even between the two counties in the Jiangsu province where the study was conducted, which may have influenced the magnitude of adjusted association compared to the crude model, more than smoking and other factors (31). Thus, more studies involving different populations and larger study samples are justified to verify the association between raw garlic consumption and lung cancer. 2. Bias: As in any case-control study, the results of the current study may have been affected by bias. Incident lung cancer cases were recruited from Shanxi Tumor Hospital, visited by 70% of the cancer patients from Taiyuan city for treatment, while controls were selected from 13 communities of the same source population. The response rates for both cases and controls were high (89% for cases and 86% for controls). These design measures may have contributed to minimize selection bias. There are two possibilities for information bias. Firstly, study personnel conducted face-to-face interviews with lung cancer patients in the hospital and controls were interviewed in community centers. The use of structured questionnaires and trained interviewers would have minimized any potential bias. Secondly, differential recall of raw garlic intake by cases and controls could have resulted in biased estimates. However, in both instances, as raw garlic intake was not thought to be associated with lung cancer at the time of data collection (31), any potential information bias would be non-differential. This may have attenuated the estimate towards the null, which would make the current observed estimates conservative. 3. Cooked garlic intake: We did not assess consumption of cooked garlic. Heating garlic seems to drastically diminish its anticarcinogenic properties (8, 38, 40). If we assume that a certain amount of anti-cancer potential is preserved in cooked garlic and participants in the “never raw garlic” group actually ate cooked garlic, it may lead to exposure misclassification. If the misclassification was non-differential, the association between raw and cooked garlic and lung cancer would probably bias towards the null. Alternatively, higher proportion of cases than controls being misclassified would probably weaken the association whereas a higher proportion of controls than cases being misclassified would probably make the association stronger than the current observations. Considering the limited anti-cancer potential, we do not expect the association of raw and cooked garlic with lung cancer to significantly deviate from the current observed results. 4. Raw garlic intake quantity: Quantity of raw garlic intake was not assessed in the current study. A previous study in a Chinese population (Jiangsu Province) reported a similar pattern of association between raw garlic intake quantity and lung cancer when compared to the association using raw garlic consumption frequency (results were not presented) (31). Although, we cannot make distinct conclusions based on the current study without the required data, we do not expect this to significantly impact our study results.
Despite the above-mentioned limitations, the current study provides epidemiological evidence to substantiate the protective effect of raw garlic against lung cancer, which have been indicated in previous in-vitro studies that investigated the effects of garlic OSCs on human lung cancer cell lines (e.g. Calu-1, A-549 cells) (14-17, 59, 60) as well as in animal models (61-63). Garlic OSCs were successfully tested for chemotherapy of cancers of various organs including breast (13), prostate (64) and colon (65). Currently, many OSCs are being tested for their potential role in lung cancer treatment (14). A randomized phase IIb trial conducted in former and current smokers with a history of at least 30 pack years showed that participants who received the OSC anethole dithiolethione (ADT) as treatment had a higher reduction in progression of bronchial dysplasia and/or appearance of new lesions compared to those who received a placebo (66). Hence, garlic and garlic derived OSCs show promise for lung cancer chemoprevention and chemotherapy. Further intervention trials and prospective studies are needed to verify the feasibility of using garlic or garlic OSCs in lung cancer prevention. In this regard, it is important to develop stable OSCs that can be successfully used in chemotherapy. Consuming raw garlic itself might provide a cost-effective method to prevent lung cancer. It would certainly seem more beneficial to promote intake of garlic as part of a healthy daily diet among never smokers compared to ever smokers. However, the magnitudes of association in the current and previous study (31) indicate that raw garlic consumption may be protective against lung cancer among never-smokers as well as ever smokers.
Among Chinese men, the prevalence of cigarette smoking is extremely high (52.9%) (67) and the percentage of ever smokers who have quit (12.6%) (67) is among the lowest in the world (67, 68). Encouraging smoking cessation through programs and policies reduces disease risk and helps to protect non-smokers from the deleterious effects of secondhand smoke (69-71) and should be the first priority. It would also be prudent to promote consumption of raw garlic as a preventive approach against lung cancer, among both ever smokers and never-smokers.
In conclusion, our results indicate that raw garlic consumption may protect against lung cancer in a Chinese population. As evidence from epidemiological studies can be utilized to promote dietary modification in lung cancer prevention, further studies are warranted to confirm the observed association among different populations and with larger study sample sizes to establish the role and utility of garlic in lung cancer chemoprevention.
Supplementary Material
Acknowledgments
The authors would like to thank Xiaoyou Han, Baoxing Zhao and Jianping Shi, who were part of the original data collection but were unable to be reached to contribute to this manuscript.
Financial support: This work was supported in part by the National Nature Science Foundation of China grant award to L. Mu (NSFC-30500417). The work is also partially supported by NIH grants (ES06718, CA09142, DA11386) awarded to Z-F.Zhang and the Alper Research Center for Environmental Genomics of the UCLA Jonsson Comprehensive Cancer Center.
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest
References
- 1.Milner JA. A historical perspective on garlic and cancer. The Journal of nutrition. 2001;131:1027S–31S. doi: 10.1093/jn/131.3.1027S. [DOI] [PubMed] [Google Scholar]
- 2.Rivlin RS. Historical perspective on the use of garlic. The Journal of nutrition. 2001;131:951S–4S. doi: 10.1093/jn/131.3.951S. [DOI] [PubMed] [Google Scholar]
- 3.Hahn G. In: Garlic: The Science and Therapeutic Application of Allium Sativum L and Related Species. 2nd. Koch HP, Lawson LD, editors. Baltimore, M.D: Williams & Wilkins; 1996. [Google Scholar]
- 4.O'Brien P. Garlic in Traditional Chinese Medicine. Spezzatino. 2007-2010:26–9. [Google Scholar]
- 5.Nations FFaAOotU. Average production of top 5 produces of Garlic from 1993-2013. 2015 [Google Scholar]
- 6.Harris JC, Cottrell SL, Plummer S, Lloyd D. Antimicrobial properties of Allium sativum (garlic) Applied microbiology and biotechnology. 2001;57:282–6. doi: 10.1007/s002530100722. [DOI] [PubMed] [Google Scholar]
- 7.Khanum F, Anilakumar KR, Viswanathan KR. Anticarcinogenic properties of garlic: a review. Critical reviews in food science and nutrition. 2004;44:479–88. doi: 10.1080/10408690490886700. [DOI] [PubMed] [Google Scholar]
- 8.Milner JA. Mechanisms by which garlic and allyl sulfur compounds suppress carcinogen bioactivation. Garlic and carcinogenesis. Advances in experimental medicine and biology. 2001;492:69–81. doi: 10.1007/978-1-4615-1283-7_7. [DOI] [PubMed] [Google Scholar]
- 9.Shukla Y, Kalra N. Cancer chemoprevention with garlic and its constituents. Cancer letters. 2007;247:167–81. doi: 10.1016/j.canlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Dorant E, van den Brandt PA, Goldbohm RA, Hermus RJ, Sturmans F. Garlic and its significance for the prevention of cancer in humans: a critical view. British journal of cancer. 1993;67:424–9. doi: 10.1038/bjc.1993.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerella C, Dicato M, Jacob C, Diederich M. Chemical properties and mechanisms determining the anti-cancer action of garlic-derived organic sulfur compounds. Anti-cancer agents in medicinal chemistry. 2011;11:267–71. doi: 10.2174/187152011795347522. [DOI] [PubMed] [Google Scholar]
- 12.Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer letters. 2008;269:305–14. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsubura A, Lai YC, Kuwata M, Uehara N, Yoshizawa K. Anticancer effects of garlic and garlic-derived compounds for breast cancer control. Anti-cancer agents in medicinal chemistry. 2011;11:249–53. doi: 10.2174/187152011795347441. [DOI] [PubMed] [Google Scholar]
- 14.Nagaraj NS, Anilakumar KR, Singh OV. Diallyl disulfide causes caspase-dependent apoptosis in human cancer cells through a Bax-triggered mitochondrial pathway. The Journal of nutritional biochemistry. 2010;21:405–12. doi: 10.1016/j.jnutbio.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto K, Lawson LD, Milner JA. Allyl sulfides from garlic suppress the in vitro proliferation of human A549 lung tumor cells. Nutrition and cancer. 1997;29:152–6. doi: 10.1080/01635589709514617. [DOI] [PubMed] [Google Scholar]
- 16.Wu XJ, Kassie F, Mersch-Sundermann V. The role of reactive oxygen species (ROS) production on diallyl disulfide (DADS) induced apoptosis and cell cycle arrest in human A549 lung carcinoma cells. Mutation research. 2005;579:115–24. doi: 10.1016/j.mrfmmm.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Xiao D, Zeng Y, Hahm ER, Kim YA, Ramalingam S, Singh SV. Diallyl trisulfide selectively causes Bax- and Bak-mediated apoptosis in human lung cancer cells. Environmental and molecular mutagenesis. 2009;50:201–12. doi: 10.1002/em.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galeone C, Pelucchi C, Levi F, Negri E, Franceschi S, Talamini R, et al. Onion and garlic use and human cancer. The American journal of clinical nutrition. 2006;84:1027–32. doi: 10.1093/ajcn/84.5.1027. [DOI] [PubMed] [Google Scholar]
- 19.Hsing AW, Chokkalingam AP, Gao YT, Madigan MP, Deng J, Gridley G, et al. Allium vegetables and risk of prostate cancer: a population-based study. Journal of the National Cancer Institute. 2002;94:1648–51. doi: 10.1093/jnci/94.21.1648. [DOI] [PubMed] [Google Scholar]
- 20.Kim JY, Kwon O. Garlic intake and cancer risk: an analysis using the Food and Drug Administration's evidence-based review system for the scientific evaluation of health claims. The American journal of clinical nutrition. 2009;89:257–64. doi: 10.3945/ajcn.2008.26142. [DOI] [PubMed] [Google Scholar]
- 21.Dorant E, van den Brandt PA, Goldbohm RA. A prospective cohort study on Allium vegetable consumption, garlic supplement use, and the risk of lung carcinoma in The Netherlands. Cancer research. 1994;54:6148–53. [PubMed] [Google Scholar]
- 22.Dorant E, van den Brandt PA, Goldbohm RA. Allium vegetable consumption, garlic supplement intake, and female breast carcinoma incidence. Breast Cancer Res Treat. 1995;33:163–70. doi: 10.1007/BF00682723. [DOI] [PubMed] [Google Scholar]
- 23.Dorant E, van den Brandt PA, Goldbohm RA. A prospective cohort study on the relationship between onion and leek consumption, garlic supplement use and the risk of colorectal carcinoma in The Netherlands. Carcinogenesis. 1996;17:477–84. doi: 10.1093/carcin/17.3.477. [DOI] [PubMed] [Google Scholar]
- 24.Dorant E, van den Brandt PA, Goldbohm RA, Sturmans F. Consumption of onions and a reduced risk of stomach carcinoma. Gastroenterology. 1996;110:12–20. doi: 10.1053/gast.1996.v110.pm8536847. [DOI] [PubMed] [Google Scholar]
- 25.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu. 2015;27:2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.She J, Yang P, Hong Q, Bai C. Lung cancer in China: challenges and interventions. Chest. 2013;143:1117–26. doi: 10.1378/chest.11-2948. [DOI] [PubMed] [Google Scholar]
- 28.Key TJ. Fruit and vegetables and cancer risk. British journal of cancer. 2011;104:6–11. doi: 10.1038/sj.bjc.6606032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norat T, Aune D, Chan D, Romaguera D. Fruits and vegetables: updating the epidemiologic evidence for the WCRF/AICR lifestyle recommendations for cancer prevention. Cancer treatment and research. 2014;159:35–50. doi: 10.1007/978-3-642-38007-5_3. [DOI] [PubMed] [Google Scholar]
- 30.Research WCRFAIfC. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 31.Jin ZY, Wu M, Han RQ, Zhang XF, Wang XS, Liu AM, et al. Raw garlic consumption as a protective factor for lung cancer, a population-based case-control study in a Chinese population. Cancer prevention research. 2013;6:711–8. doi: 10.1158/1940-6207.CAPR-13-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Marchand L, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN. Intake of flavonoids and lung cancer. Journal of the National Cancer Institute. 2000;92:154–60. doi: 10.1093/jnci/92.2.154. [DOI] [PubMed] [Google Scholar]
- 33.Lin Y, Cai L. Environmental and dietary factors and lung cancer risk among Chinese women: a case-control study in southeast China. Nutrition and cancer. 2012;64:508–14. doi: 10.1080/01635581.2012.668743. [DOI] [PubMed] [Google Scholar]
- 34.Linseisen J, Rohrmann S, Miller AB, Bueno-de-Mesquita HB, Buchner FL, Vineis P, et al. Fruit and vegetable consumption and lung cancer risk: updated information from the European Prospective Investigation into Cancer and Nutrition (EPIC) International journal of cancer Journal international du cancer. 2007;121:1103–14. doi: 10.1002/ijc.22807. [DOI] [PubMed] [Google Scholar]
- 35.Satia JA, Littman A, Slatore CG, Galanko JA, White E. Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:1419–28. doi: 10.1158/1055-9965.EPI-09-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleischauer AT, Arab L. Garlic and cancer: a critical review of the epidemiologic literature. The Journal of nutrition. 2001;131:1032S–40S. doi: 10.1093/jn/131.3.1032S. [DOI] [PubMed] [Google Scholar]
- 37.Capasso A. Antioxidant action and therapeutic efficacy of Allium sativum L. Molecules. 2013;18:690–700. doi: 10.3390/molecules18010690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song K, Milner JA. The influence of heating on the anticancer properties of garlic. The Journal of nutrition. 2001;131:1054S–7S. doi: 10.1093/jn/131.3.1054S. [DOI] [PubMed] [Google Scholar]
- 39.Park JH, Park YK, Park E. Antioxidative and antigenotoxic effects of garlic (Allium sativum L.) prepared by different processing methods. Plant Foods Hum Nutr. 2009;64:244–9. doi: 10.1007/s11130-009-0132-1. [DOI] [PubMed] [Google Scholar]
- 40.Shin JH, Ryu JH, Kang MJ, Hwang CR, Han J, Kang D. Short-term heating reduces the anti-inflammatory effects of fresh raw garlic extracts on the LPS-induced production of NO and pro-inflammatory cytokines by downregulating allicin activity in RAW 264.7 macrophages. Food Chem Toxicol. 2013;58:545–51. doi: 10.1016/j.fct.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Koch HP, Lawson LD. Garlic: the science and therapeutic application of Allium sativum L and related species. xv. baltimore, Maryland: Williams & Wilkins; 1996. p. 329. [Google Scholar]
- 42.Mu L, Liu L, Niu R, Zhao B, Shi J, Li Y, et al. Indoor air pollution and risk of lung cancer among Chinese female non-smokers. Cancer causes & control : CCC. 2013;24:439–50. doi: 10.1007/s10552-012-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang XR, Chiu YL, Qiu H, Au JS, Yu IT. The roles of smoking and cooking emissions in lung cancer risk among Chinese women in Hong Kong. Ann Oncol. 2009;20:746–51. doi: 10.1093/annonc/mdn699. [DOI] [PubMed] [Google Scholar]
- 44.Zhi XY, Zou XN, Hu M, Jiang Y, Jia MM, Yang GH. Increased lung cancer mortality rates in the Chinese population from 1973-1975 to 2004-2005: An adverse health effect from exposure to smoking. Cancer. 2015;121(Suppl 17):3107–12. doi: 10.1002/cncr.29603. [DOI] [PubMed] [Google Scholar]
- 45.Zhong L, Goldberg MS, Gao YT, Hanley JA, Parent ME, Jin F. A population-based case-control study of lung cancer and green tea consumption among women living in Shanghai, China. Epidemiology. 2001;12:695–700. doi: 10.1097/00001648-200111000-00019. [DOI] [PubMed] [Google Scholar]
- 46.Cortes-Jofre M, Rueda JR, Corsini-Munoz G, Fonseca-Cortes C, Caraballoso M, Bonfill Cosp. Drugs for preventing lung cancer in healthy people. The Cochrane database of systematic reviews. 2012;10:CD002141. doi: 10.1002/14651858.CD002141.pub2. X. [DOI] [PubMed] [Google Scholar]
- 47.Hinds MW, Kolonel LN, Hankin JH, Lee J. Dietary vitamin A, carotene, vitamin C and risk of lung cancer in Hawaii. American journal of epidemiology. 1984;119:227–37. doi: 10.1093/oxfordjournals.aje.a113741. [DOI] [PubMed] [Google Scholar]
- 48.Luo J, Shen L, Zheng D. Association between vitamin C intake and lung cancer: a dose-response meta-analysis. Scientific reports. 2014;4:6161. doi: 10.1038/srep06161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slatore CG, Littman AJ, Au DH, Satia JA, White E. Long-term use of supplemental multivitamins, vitamin C, vitamin E, and folate does not reduce the risk of lung cancer. American journal of respiratory and critical care medicine. 2008;177:524–30. doi: 10.1164/rccm.200709-1398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takata Y, Shu XO, Yang G, Li H, Dai Q, Gao J, et al. Calcium intake and lung cancer risk among female nonsmokers: a report from the Shanghai Women's Health Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22:50–7. doi: 10.1158/1055-9965.EPI-12-0915-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicastro HL, Ross SA, Milner JA. Garlic and onions: their cancer prevention properties. Cancer prevention research. 2015;8:181–9. doi: 10.1158/1940-6207.CAPR-14-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.U.S. Departmentof Agriculture ARS. USDA National Nutrient Database for Standard Reference, Release 27. Nutrient Data Laboratory Home Page. 2014 [Google Scholar]
- 53.Schafer G, Kaschula CH. The immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anti-cancer agents in medicinal chemistry. 2014;14:233–40. doi: 10.2174/18715206113136660370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aboyade-Cole A, Darling-Reed S, Oriaku E, McCaskill M, Thomas R. Diallyl sulfide inhibits PhIP-induced cell death via the inhibition of DNA strand breaks in normal human breast epithelial cells. Oncology reports. 2008;20:319–23. [PMC free article] [PubMed] [Google Scholar]
- 55.Lu X, Ross CF, Powers JR, Aston DE, Rasco BA. Determination of total phenolic content and antioxidant activity of garlic (Allium sativum) and elephant garlic (Allium ampeloprasum) by attenuated total reflectance-Fourier transformed infrared spectroscopy. Journal of agricultural and food chemistry. 2011;59:5215–21. doi: 10.1021/jf201254f. [DOI] [PubMed] [Google Scholar]
- 56.Wilson C, Aboyade-Cole A, Newell O, Darling-Reed S, Oriaku E, Thomas R. Diallyl sulfide inhibits PhIP-induced DNA strand breaks in normal human breast epithelial cells. Oncology reports. 2007;17:807–11. [PubMed] [Google Scholar]
- 57.Wang Y, Li F, Wang Z, Qiu T, Shen Y, Wang M. Fruit and vegetable consumption and risk of lung cancer: A dose-response meta-analysis of prospective cohort studies. Lung cancer. 2015;88:124–30. doi: 10.1016/j.lungcan.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 58.Boivin D, Lamy S, Lord-Dufour S, Jackson J, Beaulieu E, Côté M, et al. Antiproliferative and antioxidant activities of common vegetables: A comparative study. Food Chemistry. 2009;112:374–80. [Google Scholar]
- 59.Hui C, Jun W, Ya LN, Ming X. Effect of Allium sativum (garlic) diallyl disulfide (DADS) on human non-small cell lung carcinoma H1299 cells. Tropical biomedicine. 2008;25:37–45. [PubMed] [Google Scholar]
- 60.Li W, Tian H, Li L, Li S, Yue W, Chen Z, et al. Diallyl trisulfide induces apoptosis and inhibits proliferation of A549 cells in vitro and in vivo. Acta biochimica et biophysica Sinica. 2012;44:577–83. doi: 10.1093/abbs/gms033. [DOI] [PubMed] [Google Scholar]
- 61.Herman-Antosiewicz A, Singh SV. Signal transduction pathways leading to cell cycle arrest and apoptosis induction in cancer cells by Allium vegetable-derived organosulfur compounds: a review. Mutation research. 2004;555:121–31. doi: 10.1016/j.mrfmmm.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 62.Hong JY, Wang ZY, Smith TJ, Zhou S, Shi S, Pan J, et al. Inhibitory effects of diallyl sulfide on the metabolism and tumorigenicity of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A/J mouse lung. Carcinogenesis. 1992;13:901–4. doi: 10.1093/carcin/13.5.901. [DOI] [PubMed] [Google Scholar]
- 63.Singh SV, Pan SS, Srivastava SK, Xia H, Hu X, Zaren HA, et al. Differential induction of NAD(P)H:quinone oxidoreductase by anti-carcinogenic organosulfides from garlic. Biochemical and biophysical research communications. 1998;244:917–20. doi: 10.1006/bbrc.1998.8352. [DOI] [PubMed] [Google Scholar]
- 64.Howard EW, Ling MT, Chua CW, Cheung HW, Wang X, Wong YC. Garlic-derived S-allylmercaptocysteine is a novel in vivo antimetastatic agent for androgen-independent prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:1847–56. doi: 10.1158/1078-0432.CCR-06-2074. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka S, Haruma K, Yoshihara M, Kajiyama G, Kira K, Amagase H, et al. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. The Journal of nutrition. 2006;136:821S–6S. doi: 10.1093/jn/136.3.821S. [DOI] [PubMed] [Google Scholar]
- 66.Lam S, MacAulay C, Le Riche JC, Dyachkova Y, Coldman A, Guillaud M, et al. A randomized phase IIb trial of anethole dithiolethione in smokers with bronchial dysplasia. Journal of the National Cancer Institute. 2002;94:1001–9. doi: 10.1093/jnci/94.13.1001. [DOI] [PubMed] [Google Scholar]
- 67.Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. The Lancet. 2012;380:668–79. doi: 10.1016/S0140-6736(12)61085-X. [DOI] [PubMed] [Google Scholar]
- 68.World Health, Organization Representative, Office C. Tobbaco in China. 2015 [cited 2015 06/22/215]; Available from: http://www.wpro.who.int/china/mediacentre/factsheets/tobacco/en/
- 69.Services USDoHaH. The Health Benefits of Smoking Cessation. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1990. pp. 90–8416. Report No.: DHHS Publication No. (CDC) [Google Scholar]
- 70.Services USDoHaH. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [PubMed] [Google Scholar]
- 71.Services USDoHaH. The Health Consequences of Smoking: 50 Years of Progress A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.