Abstract
Background
Distinguishing pediatric bipolar disorder (BD) from attention-deficit hyperactivity disorder (ADHD) can be challenging. Hyperactivity is a core feature of both disorders, but severely disturbed sleep and circadian dysregulation are more characteristic of BD, at least in adults. We tested the hypothesis that objective measures of activity, sleep and circadian rhythms would help differentiate pediatric subjects with BD from ADHD and typically developing controls.
Methods
Unmedicated youths (N=155, 97 males, age 5-18) were diagnosed using DSM-IV criteria with Kiddie-SADS PL/E. BD youths (n=48) were compared to typically developing controls (n=42) and children with ADHD (n=44) or ADHD plus comorbid depressive disorders (n=21). Three-to-five days of minute-to-minute belt-worn actigraph data (Ambulatory Monitoring Inc.), collected during the school week, were processed to yield 28 metrics per subject, and assessed for group differences with analysis of covariance. Cross-validated machine learning algorithms were used to determine the predictive accuracy of a four-parameter model, with measures reflecting sleep, hyperactivity and circadian dysregulation, plus Indic's bipolar vulnerability index (VI).
Results
There were prominent group differences in several activity measures, notably mean 5 lowest hours of activity, skewness of diurnal activity, relative circadian amplitude and VI. A predictive support vector machine model discriminated bipolar from non-bipolar with mean accuracy of 83.1±5.4%, ROC area of 0.781±0.071, kappa of 0.587±0.136, specificity of 91.7±5.3% and sensitivity of 64.4±13.6%.
Conclusions
Objective measures of sleep, circadian rhythmicity and hyperactivity were abnormal in BD. Wearable sensor technology may provide bio-behavioral markers that can help differentiate children with BD from ADHD and healthy controls.
Keywords: Actigraphy, ADHD, bipolar disorder, child, circadian rhythms, sleep
(Childhood) Mania is characterized by the following cardinal symptoms: (1) pathological jocularity (hyperthymia), (2) accelerated thinking, and (3) increased psychomotor activity. Directly related to the increased psychomotor activity and flight-of-ideas is sleep disturbance that almost always occurs in childhood mania. In severe cases nearly complete insomnia can be observed.
Theodor Ziehen, 1917 (Baethge et al., 2004).
Introduction
Bipolar disorder (BD) is a recurrent or chronic dysregulation of mood, activity and sleep that causes significant disruption of educational, vocational and interpersonal functioning. BD is associated with elevated rates of morbidity and mortality (Murray and Lopez, 1996). Type-I and -II BD together have an estimated lifetime prevalence of 2.1% in adults (Merikangas et al., 2007), and a recent meta-analysis (Van Meter et al., 2011) confirmed rates of 1.8% in children.
BD frequently emerges before adulthood (Perlis et al., 2009, Leverich et al., 2007). Its presentation in youths includes early clinical features that are often inconsistent with the adult phenotype (Leverich et al., 2007, Geller et al., 2002, Faedda et al., 1995, Faedda et al., 2004, Birmaher et al., 2009). Pediatric BD presents with symptoms of fluctuating intensity and duration, often too short to be classified as ‘episodes’, and a tumultuous course of illness referred to as ‘rapid cycling’ (Faedda et al., 1995, Faedda et al., 2004, Birmaher et al., 2009, Axelson et al., 2011).
Differences in clinical presentation make the diagnosis of BD in children a challenging task. To potentially aid in this task a new diagnostic entity called Disruptive Mood Dysregulation Disorder (DMDD) was added to DSM-5 (American Psychiatric Association, 2013) to separate children with severe non-episodic irritability from those with a more episodic or classical presentation. Further, Criterion A for manic and hypomanic episodes was modified to require both an abnormally and persistently elevated, expansive or irritable mood plus an abnormal and persistent increase in goal-directed activity or energy (American Psychiatric Association, 2013). Nevertheless, the diagnosis is still problematic as some criteria B symptoms of mania, such as grandiosity, flight of ideas, increase in goal directed activity and excessive involvement in activities with a high potential for painful consequences (e.g., sex, spending) are difficult to assess in children (Geller et al., 2002, Faedda et al., 1995, American Psychiatric Association, 2013), while other criterion B symptoms including distractibility, talkativeness and psychomotor agitation are also prominent features of ADHD (American Psychiatric Association, 2013). Hence, meticulous attention needs to be directed to those features that are especially characteristic of BD.
Altered locomotor activity is one of the most prominent signs of psychopathology (Teicher, 1995). It is also a primary observable behavioral measure of the Arousal and Regulatory Systems Domain as described in the Research Domain Criteria (RDOC) (NIMH, 2012) (NIMH, 2012). Technology for assessing activity has evolved from hospital-based telemetry systems (Kupfer et al., 1974), to reliable ambulatory instruments (Teicher, 1995), and now to inexpensive consumer products such as the Nike+ Fuelband and Apple Watch. Actigraphy provides quantitative, repeatable, non-invasive measures of bodily movements (accelerations) and provides an accepted paradigm for assessing the three Arousal and Regulatory Systems Domain RDOC Constructs of: arousal; circadian rhythms; and sleep-wakefulness.
Prior studies have provided objective evidence of psychomotor agitation and retardation during manic and depressed phases, respectively in adults (Teicher, 1995, Kupfer et al., 1974, Wehr et al., 1980). Circadian dysregulation in the rest-activity rhythm has also been proposed as a prominent feature of both unipolar and bipolar disorder in adults and children (Teicher, 1995, Wehr et al., 1980, Teicher et al., 1993, Teicher et al., 1997). BD may also be characterized by episodic fluctuations in activity levels. The most impressive effort in this regard was by Indic et al (Indic et al., 2011) who developed a wavelet-based vulnerability index (VI) for BD characterized by amplitude fluctuations across different time scales.
Additionally, actigraphy provides measures of sleep versus wakefulness (but not sleep states) that accord well with polysomnography (PSG) in children and non-elderly adults (Jean-Louis et al., 2001, Sadeh et al., 1991, Acebo et al., 1999). Use of this technique has been validated for assessing sleep/wake in infants (Sadeh et al., 1991), children and adolescents (Sadeh et al., 1991, Acebo et al., 1999), as well as in adults with bipolar disorder (Kaplan et al., 2012). Therefore, actigraphy provides a convenient assessment of total sleep time and can help determine whether loss of sleep is accompanied by daytime fatigue, as might be expected in typically developing children, or by heightened levels of activity, as in mania.
Sleep disturbance is a diagnostic feature of bipolar disorder not ADHD, and circadian dysregulation appears to be strongly linked to affective disorders (Teicher, 1995). However, there is a subset of individuals with ADHD who have sleep and circadian abnormalities (Van der Heijden et al., 2005, Van der Heijden et al., 2007, Arns and Kenemans, 2014, Singh and Zimmerman, 2015). Nevertheless, individuals with ADHD and bipolar disorder appear to differ in the frequency with which sleep and circadian abnormalities occur and in the nature of their expression. In a direct comparison Geller et al. (Geller et al., 2002) reported that significant sleep disturbances were 6.5-fold more prevalent in children with BPD than ADHD. Further the primary sleep and circadian disturbance in children with ADHD appears to be sleep onset insomnia characterized by a ~ 45 minute phase delay without attenuation of circadian amplitude or rhythmicity (Van der Heijden et al., 2005) and with inconclusive effects on sleep maintenance and duration as assessed using actigraphy or polysomnography (Van der Heijden et al., 2005, Snitselaar and Smits, 2014, Arns and Kenemans, 2014, Singh and Zimmerman, 2015). In contrast, bipolar disorder in children is typically associated with blunted circadian amplitude (Teicher, 1995) and diminished sleep efficiency and duration (Mehl et al., 2006). Hence, actigraphic measures related to circadian strength and sleep duration may have value in distinguishing between children with ADHD and bipolar disorder.
The correct identification of children with BD is essential as mood stabilizing agents and atypical antipsychotic may be beneficial for children with early onset BD but are unlikely to enhance attention in children with ADHD, and are associated with serious side-effects. Conversely, stimulant medications have been shown to provide marked benefits in children with ADHD, but cause disruptions in circadian rhythms and sleep that may negatively affect mood regulation.
A key aim of the present study was to ascertain whether objective measures of locomotor activity can potentially aid in the differential diagnosis of pediatric BD. To test this hypothesis, measures of activity, sleep and circadian rhythmicity were compared in children diagnosed with BD, ADHD, ADHD with a comorbid depressive disorder, and in typical developing controls. In addition to evaluating group differences, predictive analytics were used to assess how well a small subset of activity measures could discriminate children with versus without BD in a way that would likely generalize to independent samples.
Methods
Subjects
Children (age 5-18) were recruited either from consecutive referrals to a private outpatient specialty clinic or via advertisements and assessment at a teaching Hospital. Parents provided written informed consent and children provided informed verbal assent for this IRB approved study
Diagnostic assessment
Trained mental health professionals (psychiatrists, psychologists or clinical nurse specialists) interviewed all subjects and their parents. Diagnostic assessments were made using the Kiddie-SADS by trained raters at the master's, M.D. or Ph.D. level, at the private clinic with K-SADS-PL (Kaufman et al., 1996), and at teaching hospital initially with K-SADS-E (Orvaschel and Puig-Antich, 1994) (79%) and later K-SADS-PL. Scores were reviewed to obtain DSM IV-TR Axis I past, current and lifetime diagnoses, and to assess whether subjects met Course and Outcome of Bipolar Youth (COBY) criteria for BD-NOS (Birmaher et al., 2009). We included subjects with current or lifetime diagnoses of BD, ADHD (with or without comorbid depressive disorders) and typically developing controls. Children with BD more often than not meet criteria for ADHD (Kowatch et al., 2005) and children were included in the BD group regardless of comorbid ADHD symptoms.
Actigraphy
Children were fitted with a belt-worn actigraphs (mini-motionlogger or motionlogger watch, Ambulatory Monitoring, Inc.) for 3-5 days during the school week. Parents completed daily logs with bedtimes, rise times, times of device removal (e.g., showers), and times of unusual activity (e.g., horseback riding, driving in a car on a bumpy road), helpful for identifying and rejecting artifacts, which was done by hand prior to data analysis by a skilled technician blind to diagnosis.
Actigraph analysis
Activity was recorded in zero-crossing mode in one-minute epochs. Data were downloaded and analyzed using routines written in R (The R Foundation for Statistical Computing, version 3.0.0) or MATLAB (Mathworks, version R2013a) to provide conventional metrics used in prior reports of activity disturbances in children (Glod et al., 1997, Teicher et al., 1993, Glod and Teicher, 1996) plus Indic et al. bipolar vulnerability index (Indic et al., 2011). Measures either focused on extent of diurnal activity (Teicher, 1995), levels of nocturnal activity or sleep efficiency (Acebo et al., 1999), circadian regulation (Teicher and Barber, 1990, Halberg and Panofsky, 1961) or scaling properties (Indic et al., 2011). The final set consisted of 28 separate parameters (five additional measures were eliminated that correlated r > 0.9 with the included parameters). Definitions of measures used, and degree of cross-correlation, are provided in the online Appendix.
Statistical analysis
Between group differences in activity parameters were assessed using analysis of covariance (ANCOVA) with age and gender as covariates. Paired differences between groups were assessed using Tukey's HSD test with modifications to handle unequal n's. Benjamini and Hochberg's (Hochberg and Benjamini, 1990) false discovery rate was used to correct for multiple comparisons, adjusting for the total number of ANCOVA and Tukey HSD comparisons made (m=252).
Predictive modeling techniques (R library caret (Kuhn, 2008)) were used to evaluate the capacity of activity parameters to distinguish bipolar from non-bipolar children. Typically, biomarker studies analyze collected data to determine the degree to which subjects of interest can be discriminated. This approach is prone to overfit the data and to overestimate discriminability. What is important is not how well a predictive model discriminates subjects in the data set on which it was derived, but how well it discriminates subjects in an independent sample. Hence, we used cross validation to estimate predictive accuracy. The sample was divided into a training set (75% of subjects) and test set (25%). A best-fitting model was then developed on the training set using machine-learning strategies including: random forest classification (RFC); artificial neural networks (ANN); support vector machines (SVM), multinomial regression (MNR) and partial least squares (PLS) (see online Appendix for description of these techniques). The test set was then used to evaluate the goodness of fit of the model, as indicated by accuracy, kappa and area under the receiver operating characteristic curve (ROC-AUC). This process was repeated 500 times on different random splits of the data to obtain average levels of fit across the different splits.
Results
Data were obtained on 155 unmedicated children (97M/58F, 9.2 ± 3.1 years old). The sample included 48 children with bipolar disorder (23M/25F, 10.1 ± 3.4 years), 42 typically developing controls (23M/19F, 9.0 ± 3.2 years), 44 subjects with ADHD (33M/11F, 8.4 ± 2.2 years), and 21 subjects with ADHD and a comorbid depressive disorder (MDD or dysthymia, 18M/3F, 9.5 ± 3.1 years). Differences between groups in age fell slightly short of significant F3,151 = 2.53, p < 0.06. Bipolar subjects were neither classically depressed nor manic at the time of assessment but were best described as mixed or ultra rapidly cycling with frequent fluctuations in mood, including periods of irritability, hyperactivity and dysphoria.
Actigraphy was easily obtained, as most subjects and parents/guardians were cooperative. There were no adverse events. Complaints were limited to discomfort during sleep or fear of the device being noticed at school. At the teaching hospital there were 12 children who refused to wear the device, 8 children who agreed but did not fully follow through (e.g., did not wear the monitor to bed), 2 instances when the device failed to record any data, and two participating families did not return the actigraphs. These subjects were not included in the study, which is limited to the 155 subjects with actigraph measures.
Table 1 shows the least square mean and SD values for the different activity parameters across groups, and main effects of group, age and gender. (See online Appendix for representative plots). Overall, groups differed significantly on 22 of 28 measures. The most significant differences were in time dependent coefficient of variance, diurnal skew, mean nocturnal activity, percent time during the day at low activity levels, relative circadian amplitude and vulnerability index. There were also strong main effects of age on 21 of 28 measures (especially absolute and relative circadian amplitude, acrophase, total sleep time, fit to 3 oscillator cosine model and diurnal skew). No statistical effects of gender survived correction for multiple comparisons.
Table 1.
Group differences in activity parameters showing least square means and standard deviations after removing variance attributable to age and sex plus analysis of covariance results
| Measures | Bipolar | ADHD | ADHD+ Mood | Controls | F-Group | p-Groups | F-Age | p-Age | F-Sex | p-Sex |
|---|---|---|---|---|---|---|---|---|---|---|
| Activity Levels | ||||||||||
| 10 Most Active Hrs | 14640±1780 | 14503±1767 | 14276±1767 | 13342±1746 | 4.87 | p<0.02 | 13.56 | p<0.002 | 1.44 | p>0.4 |
| Mesor 3 Osc. Model | 840±118 | 792±117 | 785±117 | 685±115 | 13.88 | p<10−6 | 5.92 | p<0.05 | 1.07 | p>0.4 |
| Time Dependent COV | 0.239±0.063 | 0.271±0.062 | 0.308±0.062 | 0.342±0.062 | 22.73 | p<10−9 | 19.67 | p<0.0002 | 0.00 | p>0.9 |
| Diurnal Skew | −.837±0.413 | −.508±0.41 | −.281±0.41 | −.263±0.405 | 17.44 | p<10−7 | 25.08 | p<10−4 | 0.02 | p>0.9 |
| % Very Low Activity | 0.43±1.08 | 0.61±1.07 | 0.76±1.07 | 1.79±1.06 | 14.25 | p<10−6 | 7.87 | p<0.02 | 2.53 | p>0.2 |
| % Low Activity | 4.27±5.77 | 5.82±5.73 | 8.77±5.73 | 13.5±5.66 | 22.31 | p<10−9 | 14.78 | p<0.0009 | 0.48 | p>0.7 |
| % Moderate Activity | 15.6±7.82 | 17.5±7.76 | 21.2±7.76 | 23.3±7.67 | 8.77 | p<0.0002 | 18.91 | p<0.0002 | 0.61 | p>0.6 |
| % High Activity | 32.8±9.95 | 33.9±9.89 | 28.2±9.88 | 31.5±9.76 | 1.74 | p>0.3 | 0.63 | p>0.6 | 0.64 | p>0.6 |
| % Very High Activity | 47.0±16.7 | 42.7±16.6 | 41.5±16.6 | 31.6±16.4 | 6.88 | p<0.002 | 16.04 | p<0.0006 | 0.18 | p>0.8 |
| Sleep-Related Measures | ||||||||||
| 5 Least Active Hrs | 626±253 | 385±251 | 336±251 | 289±248 | 15.36 | p<10−6 | 5.70 | p<0.05 | 3.27 | p>0.1 |
| Nocturnal Activity | 106±32.6 | 74.9±32.4 | 71.1±32.4 | 54.8±32 | 19.69 | p<10−8 | 0.77 | p>0.6 | 0.62 | p>0.6 |
| % Nocturnal Activity | 4.95±1.53 | 3.72±1.52 | 3.71±1.52 | 3.1±1.5 | 11.86 | p<10−5 | 0.13 | p>0.9 | 0.53 | p>0.6 |
| Total Sleep (min.) | 475±59 | 523±58.6 | 524±58.6 | 543±57.9 | 10.82 | p<10−4 | 38.91 | p<10−7 | 1.94 | p>0.3 |
| Sleep Latency (min.) | 16.5±7.64 | 14.4±7.58 | 12.5±7.58 | 12.8±7.49 | 2.32 | p>0.1 | 7.34 | p<0.03 | 1.18 | p>0.4 |
| Sleep Efficiency | 0.846±0.066 | 0.888±0.066 | 0.893±0.066 | 0.921±0.065 | 9.99 | p<10−4 | 2.68 | p>0.2 | 0.70 | p>0.6 |
| Rhythms and Fluctuations | ||||||||||
| Circadian Variance | 37.3±5.8 | 36.4±5.75 | 33.5±5.76 | 36±5.69 | 2.14 | p>0.2 | 1.43 | p>0.4 | 1.73 | p>0.3 |
| 12:24 Hr Variance | 10.9±6.68 | 6.71±6.64 | 8.33±6.64 | 6.98±6.56 | 3.72 | p<0.04 | 17.13 | p<0.0004 | 0.21 | p>0.8 |
| Circadian Amplitude | 747±116 | 780±115 | 748±115 | 691±114 | 4.37 | p<0.02 | 37.86 | p<10−6 | 0.14 | p>0.9 |
| Rel. Circadian Ampl. | 88.9±8.56 | 98.5±8.5 | 96±8.5 | 101±8.39 | 17.52 | p<10−7 | 46.56 | p<10−6 | 0.60 | p>0.6 |
| Hemicircadian Ampl. | 207±69.7 | 181±69.2 | 208±69.2 | 149±68.4 | 6.34 | p<0.002 | 4.00 | p>0.1 | 0.11 | p>0.9 |
| Ultradian Amplitude | 195±39.4 | 200±39.1 | 198±39.1 | 184±38.6 | 1.36 | p>0.4 | 22.69 | p<10−4 | 0.05 | p>0.9 |
| 12:24 Hr Amplitude | 29.4±10.9 | 23.9±10.8 | 27.3±10.8 | 21.7±10.6 | 4.35 | p<0.02 | 19.68 | p<0.0002 | 0.00 | p>0.9 |
| 3 Oscillator Fit (r) | 0.848±0.045 | 0.84±0.0449 | 0.808±0.045 | 0.807±0.044 | 8.94 | p<0.0002 | 36.18 | p<10−6 | 0.00 | p>0.9 |
| Cosinor Acrophase | 0.613±0.043 | 0.598±0.042 | 0.59±0.0423 | 0.601±0.042 | 1.71 | p>0.3 | 36.97 | p<10−6 | 1.91 | p>0.3 |
| Entrainment Error | 13.9±15 | 13.8±14.9 | 14.3±14.9 | 17±14.7 | 0.44 | p>0.9 | 15.42 | p<0.0007 | 2.13 | p>0.2 |
| Interdaily Stability | 0.913±0.051 | 0.957±0.051 | 0.931±0.051 | 0.918±0.050 | 6.22 | p<0.003 | 5.67 | p<0.05 | 4.27 | p<0.1 |
| Intradaily Variability | 0.327±0.093 | 0.338±0.092 | 0.421±0.093 | 0.387±0.091 | 7.41 | p<0.0007 | 21.44 | p<10−4 | 0.00 | p>0.9 |
| Vulnerability Index | 1.880±0.226 | 1.690±0.224 | 1.630±0.224 | 1.570±0.221 | 16.16 | p<10−7 | 0.66 | p>0.6 | 1.23 | p>0.4 |
Table 2 provides corrected p-values for all possible between group contrasts. Subjects with BD differed significantly from controls, subjects with ADHD and ADHD plus depressive disorders (ADHD+Dep) on 19, 8 and 11 activity parameters, respectively. The most significant differences were in measures of nocturnal activity (mean, percent and 5 least active hours of activity, total sleep time), diurnal skew, relative circadian amplitude and vulnerability index. ADHD subjects differed from controls on 12 parameters mostly reflecting differences in activity levels. Subject with ADHD+Dep only differed from controls on 4 measures and with more modest levels of significance.
Table 2.
Significance of between group contrasts calculated using Tukey's honestly significant differences test and corrected for multiple comparisons using method of Benjamini and Hochberg
| Measures | Bipolar v ADHD | Bipolar v ADHD+Mood | Bipolar v Controls | ADHD v ADHD+Mood | ADHD v Controls | ADHD+Mood v Controls |
|---|---|---|---|---|---|---|
| Activity Levels | ||||||
| 10 Most Active Hrs | p>0.9 | p>0.9 | p<0.02 | p>0.9 | p<0.04 | p>0.3 |
| Mesor 3 Oscillator Model | p>0.4 | p>0.4 | p<10−6 | p>0.9 | p<0.0008 | p<0.03 |
| Time Dependent COV | p>0.2 | p<0.002 | p<10−9 | p>0.2 | p<10−4 | p>0.3 |
| Diurnal Skew | p<0.02 | p<10−4 | p<10−7 | p>0.2 | p<0.05 | p>0.9 |
| % Very Low Activity | p>0.9 | p>0.8 | p<10−6 | p>0.9 | p<10−4 | p<0.02 |
| % Low Activity | p>0.8 | p<0.05 | p<10−9 | p>0.3 | p<10−6 | p<0.04 |
| % Moderate Activity | p>0.9 | p<0.09 | p<0.0002 | p>0.4 | p<0.009 | p>0.9 |
| % High Activity | p>0.9 | p>0.4 | p>0.9 | p>0.2 | p>0.8 | p>0.8 |
| % Very High Activity | p>0.8 | p>0.8 | p<0.0007 | p>0.9 | p<0.03 | p>0.2 |
| Sleep-Related Measures | ||||||
| 5 Least Active Hrs | p<0.002 | p<0.002 | p<10−6 | p>0.9 | p>0.3 | p>0.9 |
| Mean Nocturnal Activity | p<0.0009 | p<0.003 | p<10−8 | p>0.9 | p<0.04 | p>0.3 |
| % Nocturnal Activity | p<0.008 | p<0.05 | p<10−5 | p>0.9 | p>0.3 | p>0.6 |
| Total Sleep (minutes) | p<0.009 | p<0.05 | p<10−4 | p>0.9 | p>0.4 | p>0.8 |
| Sleep Latency (minutes) | p>0.8 | p>0.3 | p>0.2 | p>0.9 | p>0.9 | p>0.9 |
| Sleep Efficiency | p<0.06 | p>0.1 | p<10−4 | p>0.9 | p>0.1 | p>0.5 |
| Rhythms and Fluctuations | ||||||
| Circadian Variance | p>0.9 | p>0.1 | p>0.9 | p>0.4 | p>0.9 | p>0.6 |
| 12:24 Hr. Rhythm Variance | p<0.07 | p>0.7 | p<0.09 | p>0.9 | p>0.9 | p>0.9 |
| Circadian Amplitude | p>0.8 | p>0.9 | p>0.2 | p>0.9 | p<0.02 | p>0.4 |
| Relative Circadian Amplitude | p<10−4 | p<0.05 | p<10−7 | p>0.9 | p>0.5 | p>0.1 |
| Hemicircadian Amplitude | p>0.4 | p>0.9 | p<0.003 | p>0.7 | p>0.2 | p<0.04 |
| Ultradian Amplitude | p>0.9 | p>0.9 | p>0.7 | p>0.9 | p>0.4 | p>0.8 |
| 12:24 Hr. Rhythm Ampl. | p>0.2 | p>0.9 | p<0.02 | p>0.8 | p>0.9 | p>0.3 |
| 3 Oscillator Fit (r) | p>0.9 | p<0.02 | p<0.0008 | p<0.08 | p<0.02 | p>0.9 |
| Cosinor Acrophase | p>0.6 | p>0.3 | p>0.7 | p>0.9 | p>0.9 | p>0.9 |
| Entrainment Error | p>0.9 | p>0.9 | p>0.9 | p>0.9 | p>0.9 | p>0.9 |
| Interdaily Stability | p<0.005 | p>0.9 | p>0.9 | p>0.3 | p<0.02 | p>0.9 |
| Intradaily Variability | p>0.9 | p<0.005 | p<0.04 | p<0.02 | p>0.1 | p>0.8 |
| Vulnerability Index | p<0.005 | p<0.002 | p<10−7 | p>0.9 | p<0.08 | p>0.8 |
For predictive modeling we selected diurnal skew and L5 as arousal and sleep duration measures that showed the greatest statistical difference between BD and ADHD or ADHD+Dep. Relative circadian amplitude and VI were selected as circadian and wavelet measures that differed significantly in BD subjects versus subjects in the three other groups. As these parameters were significantly influenced by age they were covaried to remove error variance attributable to age to improve the assessment of group differences, which are illustrated by scatter plots in Figure 1.
Figure 1.
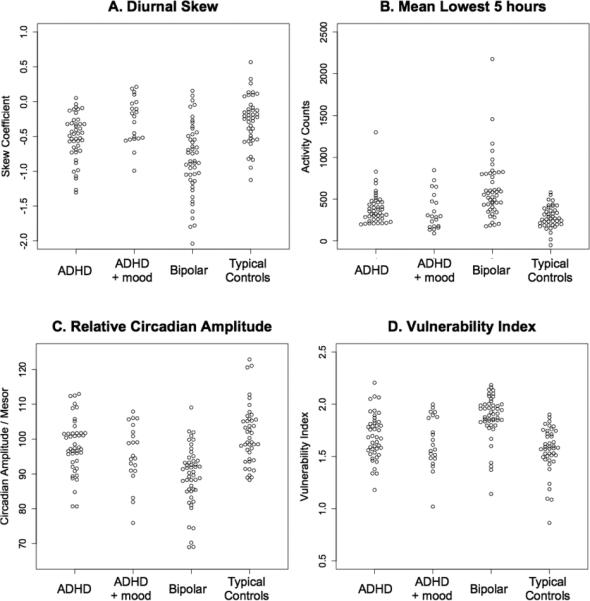
Scatter plots showing between group differences in age-covaried measures of (A) diurnal skew, (B) mean of lowest five hours, (C) relative circadian amplitude, and (D) Indic et al bipolar vulnerability index (Indic et al., 2011)
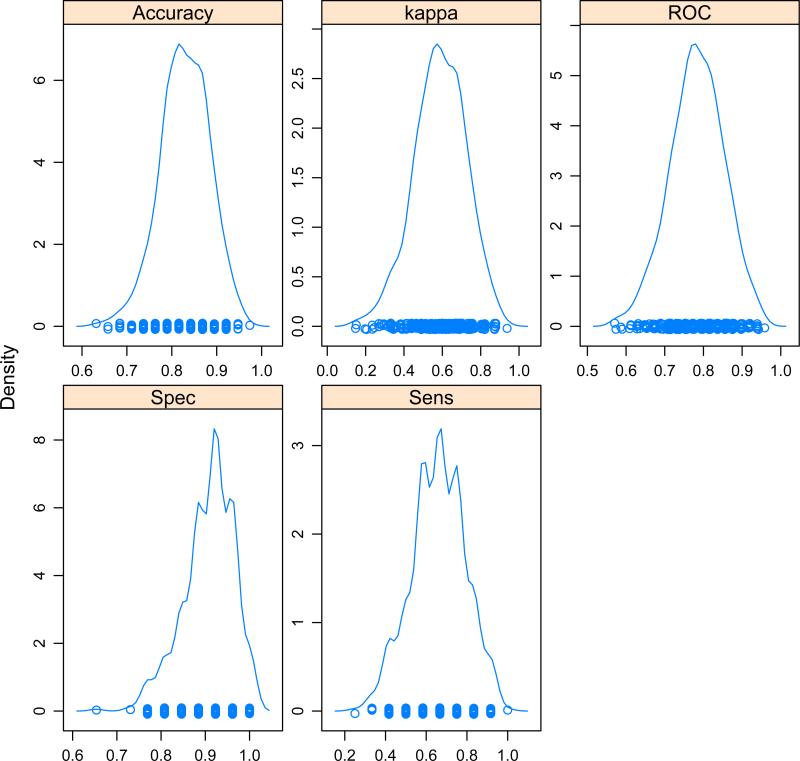
Predictive modeling showed that children with BD could be discriminated with considerable accuracy from typically developing controls on one hand, and from children with ADHD on the other hand, based solely on these four age-covaried activity parameters (see Table 3). The strongest results were obtained using SVM or MNR as artificial intelligence learning algorithms. Both of these machine-learning strategies provided average kappa coefficients for agreement with clinical assessment of 0.587. The distribution of accuracy, kappa and ROC-AUC measures across the 500 random splits are shown in Figure 2. Altogether, the aggregate predictive model correctly identified 33/48 subjects with BD and 99/107 non-BD subjects. All of the potential false positive cases were from the ADHD (n=5) or ADHD+Dep (n=3) groups, not controls.
Table 3.
Average fit statistics of predictive machine learning models in discriminating children with bipolar disorders from non-bipolar subjects using 4 age-covaried activity measures as predictors
| Method | Accuracy | Kappa | ROC Area | Specificity | Sensitivity |
|---|---|---|---|---|---|
| Random Forest | 0.798±0.059 | 0.514±0.143 | 0.748±0.073 | 0.885±0.062 | 0.612±0.137 |
| Neural Network | 0.813±0.061 | 0.562±0.145 | 0.779±0.045 | 0.872±0.066 | 0.685±0.138 |
| Partial Least Squares | 0.826±0.055 | 0.571±0.143 | 0.771±0.073 | 0.920±0.051 | 0.622±0.140 |
| Multinomial Regression | 0.829±0.055 | 0.587±0.136 | 0.783±0.070 | 0.907±0.057 | 0.659±0.132 |
| Support Vector Machine | 0.831±0.054 | 0.587±0.136 | 0.781±0.071 | 0.917±0.053 | 0.644±0.136 |
Figure 2.
Distribution of fit statistics for cross-validated predictive models from 500 random splits of the data with 75% of the data in each split used to train a multinomial regression model which was evaluated on the remaining 25%
Virtually identical predictions could be made using the selective combination of just two or three activity parameters. The best three-parameter combination consisted of diurnal skew, L5 and circadian amplitude (accuracy = 0.833±0.054, kappa 0.597±0.128, ROC area = 0.793±0.068). The best two-parameter combination consisted of diurnal skew and circadian amplitude (accuracy = 0.835±0.051, kappa 0.601±0.126, ROC area = 0.795±0.067).
Discussion
One of the strengths of the study is that current participants were medication-free or naïve, as activity levels may be significantly influenced by medications. Another key consideration is that activity data was collected during the school week in children attending school, where they are expected to periodically inhibit activity to low levels. Levels and patterns of activity can be quite different on weekends or vacations. Porrino et al (Porrino et al., 1983) found that hyperactivity boys were objectively hyperactive during lesson times but not during recess.
A number of limitations need to be acknowledged. First, cases were recruited from a private outpatient clinic or by advertisement looking for “moody kids with sleep troubles” or “Attention-Deficit Hyperactivity Disorder?” Therefore the sample is not necessarily representative of the majority of pediatric BD cases. Moreover, most of the BD subjects were recruited from the private clinic (n = 35) producing a significant potential confound. However, the same actigraphs were used at both sites, eliminating the possibility that diagnostic differences were simply an artifact of different recording devices. We did not collect consistent measures of symptom severity across diagnoses and were unable to correlate actigraph measures and symptom scores. Finally, prepubertal and pubertal cases were combined in each group, as the number of subjects evaluated did not provide sufficient power to analyze separate age groups.
Activity measures
There were numerous activity differences between typically developing controls and both children with BD and children with ADHD without comorbid mood disorders. Overall, this study confirms prior reports of hyperactivity in subjects with mania or hypomania (Teicher, 1995). Among the manic symptoms, increased energy and goal-directed activity were reported in studies of adult (Merikangas et al., 2007) and pediatric BD (Kowatch et al., 2005). Hyperactivity is an observable behavior, easier to remember and to elicit in the anamnesis and therefore more useful than mood change when probing for a past history of (hypo)mania (Angst et al., 2003).
Interestingly, there were fewer differences between controls and children with ADHD+Dep. The presence of a comorbid mood disorder appeared to attenuate the severity of hyperactivity seen in ADHD, whereas the presence of ADHD appeared to attenuate the severity of circadian rhythm disturbances previously observed in children with mood disorders (Glod et al., 1997, Teicher et al., 1993). There were more significant differences between BD and ADHD+Dep disorders than between BD and ADHD without affective comorbidity. Hence, objective measures of activity may prove to be particularly helpful is sorting out cases with multiple overlapping clinical symptoms.
Sleep measures
Children with BD in the present study also had substantially reduced measures of total sleep time and increased nocturnal activity relative to controls and to children with ADHD. Impaired sleep, in this study, was a relatively specific characteristic or indicator of BD. The finding of decreased sleep duration and markedly increased nocturnal activity early in the course of bipolar illness in unmedicated children underscores its potential importance in differential diagnoses and possible utility as a primary target symptom.
Previous research supports the idea that sleep disturbances occur frequently in children with BD (Geller et al., 2002, Faedda et al., 2004, Kowatch et al., 2005, Staton, 2008, Lofthouse et al., 2007, Baroni et al., 2012, Lofthouse et al., 2008) and are more prevalent in BD than ADHD: Geller et al. (Geller et al., 2002) reported in a large study of children (90 BD, 90 ADHD, 90 controls) that sleep disorders were present in 39% of BD, 6% of ADHD and 1% of normal controls. This is in agreement with parents' reports that nearly all children with BD have sleep disturbances or parasomnias: 95.1% - 96.2% of interviewed families (Faedda et al., 2004) and 96.9% of parents of children with BD that completed a web-based survey incorporating the Children's Sleep Habits Questionnaire (Lofthouse et al., 2008). Finally, sleep disturbances occurred in 84.5% of 130 consecutively assessed 3-17 year old with BD diagnosed by semi-structured interview using modified DSM-IV criteria (Staton, 2008). Children with BP-I and BP-NOS experience comparable levels of impaired sleep, which were inversely related to their global assessment of functioning (Baroni et al., 2012). Although prior studies have reported subjective and objective difficulties with sleep in ADHD (e.g., bedtime resistance and increased sleep latency, reduced sleep efficiency, decreased daytime wakefulness) (Cortese et al., 2009, Singh and Zimmerman, 2015), no significant differences were observed between ADHD and controls on actigraphic measures of sleep duration, latency or efficiency. ADHD subjects were more active at night than controls, without interfering with actigraph-assessed sleep. Our actigraphic sleep findings in children with ADHD were consistent with some (Corkum et al., 2001, Wiggs et al., 2005) but not all (Hvolby et al., 2008) prior reports.
Circadian regulation
Reduced relative circadian amplitude was a significant distinguishing feature between BD and other groups. This confirms previous studies indicating the importance of circadian dysregulation in affective disorders (Teicher, 1995, Wehr et al., 1980, Salvatore et al., 2008, Glod et al., 1997, Teicher et al., 1993, Teicher et al., 1997). While this parameter differed significantly between BD versus ADHD or controls, it is unlikely, by itself, to distinguish between unipolar and bipolar depression (Teicher, 1995, Teicher et al., 1993).
Bipolar vulnerability index
This study provides independent replication of the potential utility of Indic's bipolar vulnerability index (Indic et al., 2011), which appeared to discriminate BD from ADHD and healthy controls, but not ADHD from controls (Table 2). The bipolar vulnerability index is an empirically derived and non-intuitive measure calculated as the integrated area of shape coefficients of the gamma function fit to the distribution of Morlet wavelet coefficients at scales from 0.2 – 2 hours (Indic et al., 2011). Interestingly, the bipolar vulnerability index is calculated across the entire time series and includes both daytime and nighttime epochs. It correlated most strongly with sleep duration (r = −0.702) and mean nocturnal activity level (r = 0.657) suggesting that it may reflect differences in scaling behavior between daytime and nighttime activity across multiple time scales. The bipolar vulnerability index clearly warrants further scrutiny as a biomarker.
Predictive modeling
Based on the average sensitivity and specificity statistics for the 4-parameter model it appeared that a positive score was associated with a 18.7-fold (95% CI: 7.7 - 45.3) increase in odds of receiving a bipolar diagnosis. The false positive rate was low (8.4%) while the false negative rate was moderate (35.4%). Since there is no absolute gold standard we do not know if the model simply failed to detect 35% of the bipolar participants, or if the false negative cases constituted a subgroup that differed from the true positives in some important way. Perhaps these cases represent a less severely ill – good-prognosis cohort, with better chance of recovery. In this regard, 30% (n = 202/658) of subjects in the COBY Study recovered from their index episode and did not experience a recurrence during four years of follow-up (Birmaher et al., 2009). Longitudinal follow-up of true positive and false negative cases may prove enlightening. The superior fit of the SVM and PLS models strongly suggest that the different actigraphy measures contribute independently to the prediction. ANN and RFC would likely have provided superior fits if there were complex interactions between the measures (see online Appendix).
The kappa coefficients for agreement in diagnosis of BD between clinical evaluation using structured diagnostic interviews and predictive modeling of actigraphy measures is quite promising. Only 5 of 33 disorders assessed in the DSM-5 field trials showed superior between rater interclass reliability kappa coefficients (i.e., κ ≥ 0.6) with acceptable error variance: (i.e., PTSD, major neurocognitive disorder and complex somatic symptom disorder in adults and autistic spectrum disorder and ADHD in children (Regier et al., 2013)). BD in adults had a pooled interclass kappa of 0.56 (95% CI 0.45 – 0.67), BD in children had a single site kappa of 0.52 with an unacceptable margin of error (95% CI 0.13–0.80), and DMDD had a barely acceptable pooled kappa of 0.25 (95% CI 0.15–0.36).
The predictive model developed in the present study will need to be evaluated in an independent sample to provide a definitive assessment of reliability. However, we do not believe that biobehavioral markers or laboratory tests need be used or judged as stand alone diagnostic alternatives. What we envision are future diagnostic criteria that blend clinical signs, symptoms and laboratory measures. A good example is the American College of Rheumatology's diagnostic criteria for Systemic Lupus Erythematosus, which requires 4 of 11 criteria, 3 of which can be determined by blood tests. Adding objective measures to the criterion would likely enhance interrater reliability and possibly validity, but this remains to be determined.
Several features make actigraphy a potentially useful laboratory test for psychiatric research and practice. It is non-invasive, inexpensive, well tolerated, free of side effects, and provides accurate measures of sleep continuity and circadian regulation. Further, the technology is now being incorporated into a vast array of consumer products so that millions of individuals own or will soon own the necessary hardware. If these findings are confirmed, actigraphy might add an objective component and increase the accuracy and reliability of assessment of children for early onset bipolar disorder.
Supplementary Material
Key points.
Bipolar Disorder and ADHD are common psychiatric disorders that share several clinical features and often co-occur, complicating diagnosis and treatment.
Wrist-worn devices containing accelerometers (i.e., actigraphs) can record minute-to-minute levels of physical activity.
Children with bipolar disorder differed from typically developing children and children with ADHD (with or without comorbid depression) on measures of sleep, circadian rhythmicity and amplitude fluctuations.
Children with ADHD differed from typically developing controls on measures of daytime hyperactivity but not in measures of sleep or circadian rhythmicity.
Predictive modeling indicated that bipolar and non-bipolar children could be distinguished with high accuracy (kappa ca 0.6) using either 2, 3 or 4 measures with strength of the circadian rhythm and intensity of daytime activity the most critical.
Acknowledgments
Author G.L.F. receives royalties from New Harbinger Publications Inc. M.H.T. has received consulting fees and royalties from BioBehavioral Diagnostic Company (BioBDx) through a licensing agreement with McLean Hospital. BioBDx was recently acquired by Pearson. M.H.T. holds nine patents related to the diagnosis of ADHD and other psychiatric disorders, and six patents related to the treatment of ADHD or depression. None of these involve actigraphy. K.O. holds one patent with M.H.T., related to the diagnosis of ADHD. The other authors reported no biomedical financial interests or potential conflicts of interest. This research was supported, in part, by National Institute of Mental Health Challenge Grant 1RC1-MH089743 (M.H.T., G.L.F.).
The authors would like to thank Elizabeth Bolger and Hannah C. McCormack for recruitment and study coordination, Alaptagin Khan for subject assessment and Gordana Vitaliano for medical coverage. A poster with preliminary data was presented at the 57th Annual Meeting of the Academy of Child and Adolescent Psychiatry in New York, NY and the 68th Annual Meeting of the Society of Biological Psychiatry, San Francisco, CA.
Footnotes
Conflict of interest statement: See Acknowledgments for disclosures.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Activity measures.
Appendix S2. Supervised machine learning algorithms.
Appendix S3. Representative actigraph plots.
References
- ACEBO C, SADEH A, SEIFER R, TZISCHINSKY O, WOLFSON AR, HAFER A, CARSKADON MA. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22:95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- AMERICAN PSYCHIATRIC ASSOCIATION . Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- ANGST J, GAMMA A, BENAZZI F, AJDACIC V, EICH D, ROSSLER W. Toward a re-definition of subthreshold bipolarity: epidemiology and proposed criteria for bipolar-II, minor bipolar disorders and hypomania. J Affect Disord. 2003;73:133–146. doi: 10.1016/s0165-0327(02)00322-1. [DOI] [PubMed] [Google Scholar]
- ARNS M, KENEMANS JL. Neurofeedback in ADHD and insomnia: vigilance stabilization through sleep spindles and circadian networks. Neurosci Biobehav Rev. 2014;44:183–194. doi: 10.1016/j.neubiorev.2012.10.006. [DOI] [PubMed] [Google Scholar]
- AXELSON DA, BIRMAHER B, STROBER MA, GOLDSTEIN BI, HA W, GILL MK, GOLDSTEIN TR, YEN S, HOWER H, HUNT JI, LIAO F, IYENGAR S, DICKSTEIN D, KIM E, RYAN ND, FRANKEL E, KELLER MB. Course of subthreshold bipolar disorder in youth: diagnostic progression from bipolar disorder not otherwise specified. J Am Acad Child Adolesc Psychiatry. 2011;50:1001–1016. e1003. doi: 10.1016/j.jaac.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAETHGE C, GLOVINSKY I, BALDESSARINI RJ. Manic-depressive illness in children: an early twentieth-century view by Theodor Ziehen (1862-1950). Introduction. Hist Psychiatry. 2004;15:201–226. doi: 10.1177/0957154X04044083. [DOI] [PubMed] [Google Scholar]
- BARONI A, HERNANDEZ M, GRANT MC, FAEDDA GL. Sleep Disturbances in Pediatric Bipolar Disorder: A Comparison between Bipolar I and Bipolar NOS. Front Psychiatry. 2012;3:22. doi: 10.3389/fpsyt.2012.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRMAHER B, AXELSON D, GOLDSTEIN B, STROBER M, GILL MK, HUNT J, HOUCK P, HA W, IYENGAR S, KIM E, YEN S, HOWER H, ESPOSITO-SMYTHERS C, GOLDSTEIN T, RYAN N, KELLER M. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166:795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORKUM P, TANNOCK R, MOLDOFSKY H, HOGG-JOHNSON S, HUMPHRIES T. Actigraphy and parental ratings of sleep in children with attention-deficit/hyperactivity disorder (ADHD). Sleep. 2001;24:303–312. doi: 10.1093/sleep/24.3.303. [DOI] [PubMed] [Google Scholar]
- CORTESE S, FARAONE SV, KONOFAL E, LECENDREUX M. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J Am Acad Child Adolesc Psychiatry. 2009;48:894–908. doi: 10.1097/CHI.0b013e3181ac09c9. [DOI] [PubMed] [Google Scholar]
- FAEDDA GL, BALDESSARINI RJ, GLOVINSKY IP, AUSTIN NB. Pediatric bipolar disorder: phenomenology and course of illness. Bipolar Disord. 2004;6:305–313. doi: 10.1111/j.1399-5618.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- FAEDDA GL, BALDESSARINI RJ, SUPPES T, TONDO L, BECKER I, LIPSCHITZ DS. Pediatric-onset bipolar disorder: a neglected clinical and public health problem. Harv Rev Psychiatry. 1995;3:171–195. doi: 10.3109/10673229509017185. [DOI] [PubMed] [Google Scholar]
- GELLER B, ZIMERMAN B, WILLIAMS M, DELBELLO MP, FRAZIER J, BERINGER L. Phenomenology of prepubertal and early adolescent bipolar disorder: examples of elated mood, grandiose behaviors, decreased need for sleep, racing thoughts and hypersexuality. J Child Adolesc Psychopharmacol. 2002;12:3–9. doi: 10.1089/10445460252943524. [DOI] [PubMed] [Google Scholar]
- GLOD CA, TEICHER MH. Relationship between early abuse, posttraumatic stress disorder, and activity levels in prepubertal children. J Am Acad Child Adolesc Psychiatry. 1996;35:1384–1393. doi: 10.1097/00004583-199610000-00026. [DOI] [PubMed] [Google Scholar]
- GLOD CA, TEICHER MH, POLCARI A, MCGREENERY CE, ITO Y. Circadian rest-activity disturbances in children with seasonal affective disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:188–195. doi: 10.1097/00004583-199702000-00009. [DOI] [PubMed] [Google Scholar]
- HALBERG F, PANOFSKY H. Thermovariance spectra: method and clinical illustration. Exp Med Surg. 1961;19:284–309. [PubMed] [Google Scholar]
- HOCHBERG Y, BENJAMINI Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- HVOLBY A, JORGENSEN J, BILENBERG N. Actigraphic and parental reports of sleep difficulties in children with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2008;162:323–329. doi: 10.1001/archpedi.162.4.323. [DOI] [PubMed] [Google Scholar]
- INDIC P, SALVATORE P, MAGGINI C, GHIDINI S, FERRARO G, BALDESSARINI RJ, MURRAY G. Scaling behavior of human locomotor activity amplitude: association with bipolar disorder. PLoS One. 2011;6:e20650. doi: 10.1371/journal.pone.0020650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEAN-LOUIS G, KRIPKE DF, COLE RJ, ASSMUS JD, LANGER RD. Sleep detection with an accelerometer actigraph: comparisons with polysomnography. Physiol Behav. 2001;72:21–28. doi: 10.1016/s0031-9384(00)00355-3. [DOI] [PubMed] [Google Scholar]
- KAPLAN KA, TALBOT LS, GRUBER J, HARVEY AG. Evaluating sleep in bipolar disorder: comparison between actigraphy, polysomnography, and sleep diary. Bipolar Disord. 2012;14:870–879. doi: 10.1111/bdi.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMAN J, BIRMAHER B, BRENT D, RAO U, RYAN ND. Kiddie-SADS PL. Western Psychiatric Institute and Clinic. University of Pittsburgh Medical Centre; 1996. [Google Scholar]
- KOWATCH RA, YOUNGSTROM EA, DANIELYAN A, FINDLING RL. Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disord. 2005;7:483–496. doi: 10.1111/j.1399-5618.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- KUHN M. Building predictive models in R using the caret package. Journal of Statistical Software. 2008;28:1–26. [Google Scholar]
- KUPFER DJ, WEISS BL, FOSTER G, DETRE TP, MCPARTLAND R. Psychomotor activity in affective states. Arch Gen Psychiatry. 1974;30:765–768. doi: 10.1001/archpsyc.1974.01760120029005. [DOI] [PubMed] [Google Scholar]
- LEVERICH GS, POST RM, KECK PE, JR., ALTSHULER LL, FRYE MA, KUPKA RW, NOLEN WA, SUPPES T, MCELROY SL, GRUNZE H, DENICOFF K, MORAVEC MK, LUCKENBAUGH D. The poor prognosis of childhood-onset bipolar disorder. J Pediatr. 2007;150:485–490. doi: 10.1016/j.jpeds.2006.10.070. [DOI] [PubMed] [Google Scholar]
- LOFTHOUSE N, FRISTAD M, SPLAINGARD M, KELLEHER K. Parent and child reports of sleep problems associated with early-onset bipolar spectrum disorders. J Fam Psychol. 2007;21:114–123. doi: 10.1037/0893-3200.21.1.114. [DOI] [PubMed] [Google Scholar]
- LOFTHOUSE N, FRISTAD M, SPLAINGARD M, KELLEHER K, HAYES J, RESKO S. Web survey of sleep problems associated with early-onset bipolar spectrum disorders. J Pediatr Psychol. 2008;33:349–357. doi: 10.1093/jpepsy/jsm126. [DOI] [PubMed] [Google Scholar]
- MEHL RC, O'BRIEN LM, JONES JH, DREISBACH JK, MERVIS CB, GOZAL D. Correlates of sleep and pediatric bipolar disorder. Sleep. 2006;29:193–197. doi: 10.1093/sleep/29.2.193. [DOI] [PubMed] [Google Scholar]
- MERIKANGAS KR, AKISKAL HS, ANGST J, GREENBERG PE, HIRSCHFELD RM, PETUKHOVA M, KESSLER RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY CJL, LOPEZ AD. The global burden of disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Harvard University Press; Cambridge, MA: 1996. [Google Scholar]
- NIMH . Arousal and Regulatory Systems: Workshop Proceedings. Rockville, MD: 2012. http://www.nimh.nih.gov/research-priorities/rdoc/arousal-and-regulatory-systems-workshop-proceedings.shtml. [Google Scholar]
- ORVASCHEL H, PUIG-ANTICH J. Schedule for Affective Disorder and Schizophrenia for School-Age Children, Epidemiologic Version, Fifth Revision. Nova Southeastern University; Fort Lauderdale, FL: 1994. [Google Scholar]
- PERLIS RH, DENNEHY EB, MIKLOWITZ DJ, DELBELLO MP, OSTACHER M, CALABRESE JR, AMETRANO RM, WISNIEWSKI SR, BOWDEN CL, THASE ME, NIERENBERG AA, SACHS G. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord. 2009;11:391–400. doi: 10.1111/j.1399-5618.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRINO LJ, RAPOPORT JL, BEHAR D, SCEERY W, ISMOND DR, BUNNEY WE., JR. A naturalistic assessment of the motor activity of hyperactive boys. I. Comparison with normal controls. Arch Gen Psychiatry. 1983;40:681–687. doi: 10.1001/archpsyc.1983.04390010091012. [DOI] [PubMed] [Google Scholar]
- REGIER DA, NARROW WE, CLARKE DE, KRAEMER HC, KURAMOTO SJ, KUHL EA, KUPFER DJ. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170:59–70. doi: 10.1176/appi.ajp.2012.12070999. [DOI] [PubMed] [Google Scholar]
- SADEH A, LAVIE P, SCHER A, TIROSH E, EPSTEIN R. Actigraphic home-monitoring sleep-disturbed and control infants and young children: a new method for pediatric assessment of sleep-wake patterns. Pediatrics. 1991;87:494–499. [PubMed] [Google Scholar]
- SALVATORE P, GHIDINI S, ZITA G, DE PANFILIS C, LAMBERTINO S, MAGGINI C, BALDESSARINI RJ. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disord. 2008;10:256–265. doi: 10.1111/j.1399-5618.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- SINGH K, ZIMMERMAN AW. Sleep in Autism Spectrum Disorder and Attention Deficit Hyperactivity Disorder. Semin Pediatr Neurol. 2015;22:113–125. doi: 10.1016/j.spen.2015.03.006. [DOI] [PubMed] [Google Scholar]
- SNITSELAAR MA, SMITS MG. Sleep, melatonin, and circadian rhythmicity in attention deficit hyperactivity disorder. In: SRINIVASAN V, BRZEZINSKI A, OTER S, SHILLCUTT SD, editors. Melatonin and Melatonergic Drugs in Clinical Practice. Springer; India: 2014. [Google Scholar]
- STATON D. The impairment of pediatric bipolar sleep: hypotheses regarding a core defect and phenotype-specific sleep disturbances. J Affect Disord. 2008;108:199–206. doi: 10.1016/j.jad.2007.10.007. [DOI] [PubMed] [Google Scholar]
- TEICHER MH. Actigraphy and motion analysis: new tools for psychiatry. Harv Rev Psychiatry. 1995;3:18–35. doi: 10.3109/10673229509017161. [DOI] [PubMed] [Google Scholar]
- TEICHER MH, BARBER NI. COSIFIT: an interactive program for simultaneous multioscillator cosinor analysis of time-series data. Comput Biomed Res. 1990;23:283–295. doi: 10.1016/0010-4809(90)90022-5. [DOI] [PubMed] [Google Scholar]
- TEICHER MH, GLOD CA, HARPER D, MAGNUS E, BRASHER C, WREN F, PAHLAVAN K. Locomotor activity in depressed children and adolescents: I. Circadian dysregulation. J Am Acad Child Adolesc Psychiatry. 1993;32:760–769. doi: 10.1097/00004583-199307000-00009. [DOI] [PubMed] [Google Scholar]
- TEICHER MH, GLOD CA, MAGNUS E, HARPER D, BENSON G, KRUEGER K, MCGREENERY CE. Circadian rest-activity disturbances in seasonal affective disorder. Arch Gen Psychiatry. 1997;54:124–130. doi: 10.1001/archpsyc.1997.01830140034007. [DOI] [PubMed] [Google Scholar]
- VAN DER HEIJDEN KB, SMITS MG, VAN SOMEREN EJ, GUNNING WB. Idiopathic chronic sleep onset insomnia in attention-deficit/hyperactivity disorder: a circadian rhythm sleep disorder. Chronobiol Int. 2005;22:559–570. doi: 10.1081/CBI-200062410. [DOI] [PubMed] [Google Scholar]
- VAN DER HEIJDEN KB, SMITS MG, VAN SOMEREN EJ, RIDDERINKHOF KR, GUNNING WB. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. J Am Acad Child Adolesc Psychiatry. 2007;46:233–241. doi: 10.1097/01.chi.0000246055.76167.0d. [DOI] [PubMed] [Google Scholar]
- VAN METER AR, MOREIRA AL, YOUNGSTROM EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72:1250–1256. doi: 10.4088/JCP.10m06290. [DOI] [PubMed] [Google Scholar]
- WEHR TA, MUSCETTOLA G, GOODWIN FK. Urinary 3-methoxy-4-hydroxyphenylglycol circadian rhythm. Early timing (phase-advance) in manic-depressives compared with normal subjects. Arch Gen Psychiatry. 1980;37:257–263. doi: 10.1001/archpsyc.1980.01780160027002. [DOI] [PubMed] [Google Scholar]
- WIGGS L, MONTGOMERY P, STORES G. Actigraphic and parent reports of sleep patterns and sleep disorders in children with subtypes of attention-deficit hyperactivity disorder. Sleep. 2005;28:1437–1445. doi: 10.1093/sleep/28.11.1437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.