Abstract
Tumor cells inherit from their normal precursors an extensive stress response machinery that is critical for survival in response to challenges including oxidative stress, wounding and shear stress. Kruppel-like transcription factors, including KLF4 and KLF5, are rarely affected by genetic alteration during tumorigenesis, but compose key components of the stress response machinery in normal and tumor cells and interact with critical survival pathways, including RAS, p53, survivin and the BCL2 family of cell death regulators. Within tumor cells KLF4 and KLF5 play key roles in tumor cell fate, regulating cell proliferation, cell survival and the tumor initiating properties of cancer stem-like cells. These factors can be preferentially expressed in embryonic stem cells or cancer stem-like cells. Indeed, specific KLFs represent key components of a cross regulating pluripotency network in embryonic stem cells, and induce pluripotency when coexpressed in adult cells with other Yamanaka factors. Suggesting analogies between this pluripotency network and the cancer cell adaptive reprogramming that occurs in response to targeted therapy, recent studies link KLF4 and KLF5 to adaptive prosurvival signaling responses induced by HER2-targeted therapy. We review literature supporting KLFs as shared mechanisms in stress adaptation and cellular reprogramming and address the therapeutic implications.
Kruppel-like factors (KLFs) compose a family of 17 distinct zinc finger transcription factors that function in diverse cell types. A subset of these, including KLF2, KLF4 and KLF5, have been linked to pluripotency (1,2,3). Pluripotent stem cells (PSCs) can differentiate into any of three germ layers including ectoderm, mesoderm, and endoderm. In tumor cells, KLF4 and KLF5, the focus of this review, can exert profound pro-tumorigenic or anti-tumorigenic effects. Recent results implicate these two factors as mediators of adaptive responses following targeted therapy, and as prosurvival factors that can be preferentially expressed within cancer stem-like cells (4,5).
Identifying a cooperative relationship within tumor cells, KLF4 and KLF5 were shown to function in concert to promote tumorigenesis and drug resistance in HER2-positive breast cancer models, with potential effects on distant metastasis-free survival in patients (4). These results may mirror a cooperative function of KLF4 and 5 in embryonic stem cells (ESCs), where pluripotency is maintained by their conserved (i.e., convergent) and distinctive (i.e., divergent) signaling (3). In this review we contrast recent insights obtained in breast cancer to observations made in other contexts including embryonic stem cells (ESCs), normal adult tissues, and select pathological conditions.
The Role of “core” KLFs in Pluripotency: KLF2, KLF4 and KLF5
ESCs are derived from the inner cell mass of the blastocyst, and are propagated in vitro as self-renewing, undifferentiated, and rapidly proliferating cells, typically in the presence of the cytokine Leukemia Inhibitory Factor (LIF), which signals through Stat3 to suppress cell differentiation (2). The pluripotency of ESCs is maintained by a core transcription factor network in which extensive cross regulation sustains the various components. Oct4 and Sox2 compose an essential “hub”, with “outer circuit” factors including Nanog, Tbx3, Esrrb, and distinct KLFs (2). Outer circuit members, although individually more or less dispensable for pluripotency, cooperate in ESCs to maintain the naïve, undifferentiated state. In addition, pluripotent cells can be obtained by reprogramming of differentiated adult somatic cells to induced pluripotent stem cells (iPSCs), typically mediated by the exogenous quartet of OCT4, SOX2, KLF4 and c-MYC (i.e., OSKM). c-Myc is strictly dispensable for generation of iPSCs. Nevertheless, c-Myc promotes reprogramming, acting early in conjunction with KLF4 (6). The enhancing effect of c-MYC is attributed to its various effects on cell proliferation, metabolism, and the genome wide regulation of transcription pause release to enhance RNA Polymerase II mediated transcription (7,8). Later during reprogramming, KLF4 co-occupies many loci with OCT4 and SOX2, together comprising a “pioneer” activity that efficiently induces chromatin structural changes including decondensation, leading to gene transcription (6,9,10). Interestingly, like KLF4, c-Myc has been characterized as a direct target of LIF-Stat3 signaling in ESCs (2,7). Consequently, enforced expression of the four Yamanaka factors during generation of iPSCs substantially recapitulates the endogenous ESC pluripotency network.
KLF2, KLF4 and KLF5 are coexpressed in ESCs and their combined knockdown in these cells leads to robust differentiation, as does withdrawal of LIF (1,2,11). Within ESCs the core KLFs appear to exert distinct effects (11). Chromatin immunoprecipitation analysis of KLF4 and 5 followed by next-generation sequencing has identified both shared and distinct target genes (3). Although KLF4 and 5 cooperate to suppress differentiation, each appears to preferentially inhibit differentiation of a specific lineage, respectively the endoderm and mesoderm. While the ability of exogenous KLFs to sustain the ESC phenotype in the absence of LIF is shared, KLF2 and 4 are much more effective compared to KLF5 (2,11). Similarly, for the reprogramming of somatic cells to iPSCs, KLF2 and 4 are far more efficient compared to KLF5 (11). Despite reports indicating that Klf5 promotes ESC pluripotency (1,3), others favor the view that Klf5 has no specific or critical role in pluripotency, but rather plays a general role in the early embryo (personal communication, Austin Smith, University of Cambridge) (2). Such a noncritical function of KLF5 in pluripotency does not preclude a critical role in adult stem cells. Indeed, conditional deletion of Klf5 within the Lgr5+ intestinal stem cells of mice conferred a selective disadvantage relative to residual Klf5+ stem cells, and completely prevented tumorigenesis driven by cotemporal, conditional activation of β-catenin in the same stem cells (12).
Conserved and Distinct Functions of KLF4 and KLF5
When mutually present in epithelial tissues, often times KLF4 and 5 localize to distinct compartments. In normal adult tissues KLF4 is commonly associated with post-mitotic, differentiated cells, whereas KLF5 is more highly expressed in proliferating cells (13,14). In tumor tissues this compartmentalization is often lost, and the two appear to be coexpressed in the same cells. In support of opposing functions of KLF4 and 5 in somatic tissues, each appears to regulate cell cycle genes such as p21Waf1/Cip1, p15Ink4B, p27Kip1, CCNB1, CCND1, and/or CCNE1, but often with opposite effects (13).
Despite these distinctions, several contexts appear to support convergent signaling. Conditional deletion of Klf4 or Klf5 in the surface ectoderm of the mouse eye reveal similar developmental deficits (15,16). Indeed, genetic ablation of either Klf in the eye results in dramatically reduced goblet cell number and function. Similarly in the gut mucosa, epithelial knockout of Klf4 or 5 resulted in goblet cell deficits (17,18).
Crucial Effects of KLF4 and KLF5 in the Stress Response
Early studies implicated KLF4 as a stress responsive gene, with induction by factors such as shear stress or wounding (19). Interactions between KLF4 and 5 have been especially well examined in the cardiovascular system. Although they are highly expressed in the embryonic vasculature, typically levels of these factors are low in normal adult vascular smooth muscle cells (SMCs) (20). However KLF4 and 5 are induced in SMCs in response to various noxious stimuli. Vascular injury models including shear stress, coronary atherosclerosis, vein graft hyperplasia, oxidized phospholipids, and/or ischemia can trigger an adaptive response in SMCs prompting dedifferentiation and proliferation (20,21). In conditional Klf4−/− mice the damage-related suppression of SMC differentiation markers is delayed compared to wildtype mice (21). Although the neointimal layers of these mice exhibit elevated cell growth in response to injury, conditional Klf4−/− mice are nevertheless more vulnerable to vascular insults, suggesting defects in repair due to impaired dedifferentiation. These results appear to mirror KLF4 deficient breast cancer cells, which proliferate somewhat more rapidly than control cells but also show increased cell death following stressors such as matrix deprivation (i.e., anoikis) or exposure to a targeted therapeutic (4,5,22).
On the other hand, KLF5 may mediate cardiovascular stress responses though enhanced proliferation. Mice heterozygous for Klf5 show reduced response to cardiovascular insults, such as arterial polyethylene cuffs, characterized by thinner medial and intimal layers (20). Moreover, KLF5 expression confers increased cell survival in atherosclerotic vascular lesions (23). Interestingly, KLF5 as well as KLF4 is induced by endothelin-1 in neonatal rat myocytes. Endothelins are the most potent known vasoconstrictors, have important roles in pressure regulation of the vasculature, and are implicated in cardiovascular disease (24). These results highlight roles for KLF4 and 5 in the injury response program of vascular SMCs.
Stress-adaptive and Pro-survival Functions of the KLF4 and KLF5
In addition to their role in the vasculature, KLF4 and 5 mediate survival to noxious stimuli in other contexts. Early studies reported elevation of KLF4 levels in keratinocytes during wound healing, and the induction of KLF4 by heat stress is critical to cell viability (19,25,26). Subsequent studies demonstrated an important role for KLF4 in cellular survival following γ-radiation (27,28,29). In gut epithelium or gut-derived malignant cells, KLF4 attenuates radiation-induced damage and cell death (27,28). Mechanistically, the radioprotective effects of KLF4 are in part attributed to p53 signaling (27,29). The p53-dependent induction of KLF4 causes growth arrest through p21Waf1/Cip1, where the cessation of the cell cycle may allow for adequate DNA repair. Additionally, KLF4 also inhibits pro-apoptotic Bax expression in response to γ-radiation (27). More recently, KLF4 was found to be vital for protecting neurons and fibroblasts from oxidative damage (30,31). Taken together, KLF4 is capable of protecting cells from a variety of harmful stimuli and may be associated with a conserved cellular response to toxic insults.
Similar to KLF4, KLF5 participates in the adaptive response to external stressors in several organ systems. In the gut, KLF5 enhances protection and recovery to dextran sodium sulfate induced injury (32). Likewise, KLF5 is a key mediator in the colonic response to pathogenic bacterial infection. KLF5 promotes survival in the pulmonary blood vessels as well. In hypertensive settings KLF5 contributes to pulmonary artery SMC survival, and its expression correlates with increased levels of activated survivin in experimental models of congenital diaphragmatic hernia (33,34). In fact, KLF5 enhances anti-apoptotic activity through survivin in a variety of contexts. Alternatively, KLF5 can modulate other aspects of apoptotic signaling. In response to intracerebral hemorrhage, KLF5 protects against neuronal apoptosis by suppressing pro-apoptotic Bad activation (35).
As for KLF4, KLF5 function is heavily intertwined with that of p53. KLF5 and p53 show coordinate regulation at promoters for genes such as BIRC5 (encoding survivin), hypoxia inducible factor HIF1A, CDKN1A (encoding p21Waf1/CIP1) and NOTCH1 (36,37,38,39). In keratinocytes, p53 status provides a major contextual determinant for the output of KLF5 signaling. Here, mutation or loss of p53 switches KLF5 signaling so as to suppress cell proliferation, mediated by KLF5 induction of p21Waf1/Cip1 (39). p53 mutation or deficiency also renders NOTCH1 transcription to be dependent upon KLF5, consequently tumor cell invasion is associated with loss of KLF5 expression and with loss of KLF5-dependent induction of the NOTCH1, a potent tumor suppressor in this context (38). In summary, the consistent involvement of KLF4 and 5 in the stress response in multiple organ systems argue that these factors are conserved responders to the damage of cells and tissues.
Context-Dependent KLF Function in Cancer
Although elevated expression of KLF4 and 5 in tumor cells relative to normal tissue is quite common, genetic alterations within either gene in cancer is rather uncommon, arguing against roles as a classical oncogene or tumor suppressor. Instead, KLFs appear to be critical stress responsive plasticity and pro-survival factors not only in normal cells and tissues but also in their malignant derivatives. In short, based upon current literature tumor cells appear have acquired these critical regulators of plasticity and survival by inheritance rather than by somatic genetic alteration. Nevertheless, these factors appear formidable as components of the tumor cell stress response that mediates resistance of tumor cells to a wide variety of insults, including therapy.
Consistent with this view are the disparate and context dependent effects of KLF4 and 5 in cancer models. KLF5 has typically been implicated as protumorigenic, including breast, bladder, colon, lung, gastric, and HPV-associated cancers, and is often associated with the promotion of cell proliferation (29). On the other hand, KLF5 is linked to tumor suppressor activities in prostate cancer and esophageal squamous cell carcinoma. In the squamous cell carcinoma context it appears to function as a sort of surrogate for p53, inducing tumor suppressors such as p21Waf1/Cip1 and NOTCH1 whenever p53 is deficient (38,39).
Given its ability to suppress proliferation, to promote or suppress differentiation, and to impact cell survival, it is not surprising that KLF4 can elicit dual effects on malignant properties. For example, KLF4 has been reported to inhibit tumorigenesis and/or malignant properties in a variety of contexts, including neuroblastoma, leukemia, pancreatic, lung, and colon cancers (29). Conversely, adverse impact on clinical outcome and/or protumorigenic effects in functional assays have been reported for KLF4 in breast, skin, and others, possibly attributed to transcriptional regulation of miR-206 and miR-21 leading to RAS-ERK pathway activation, to prosurvival signaling and/or to the suppression of TP53 transcription (5,22,40). KLF4 appears especially apt to exert protumorigenic effects in cells containing dysregulation of Rb-dependent G1 cell cycle progression, as results from expression of DNA tumor virus proteins such as adenovirus E1a, from signaling of RAS to Cyclin D1, or from insufficiency of CDK inhibitors such as p21Waf1/Cip1 (29,40).
KLF4, KLF5 and Therapeutic Resistance
Without precluding potential roles in adult stem cells, tissue maintenance and/or tumor pathogenesis, our studies and those of others appear to support a model in which KLF4 and 5 play a vital role in the response to therapy. Such functions are likely adapted from their role as stress response factors in normal tissues. Consequently, some of the drug resistance of cancer cells and CSCs, in part attributed to their ability to undergo adaptive reprogramming in response to a targeted therapy, may be “inherited” from the tumor precursor cell rather than selected for through clonal evolution during tumor development (41,42).
As noted above, KLF4 has a well established role in cellular survival following therapies such as γ-radiation (27,28,29). Moreover KLF4, in part via miR-206 signaling, promotes breast cancer stem cell features and conveys enhanced cell survival and tumorigenicity in mice, as represented by increased tumor initiation, anoikis resistance, and resistance to cytotoxic drugs (5). Similarly, KLF5 can confer resistance to chemotherapies by upregulation of survivin or HIF1α (36,43). Additionally, depletion of KLF5 conferred colon cancer cell sensitivity to DNA damaging agents through activated Pim1 (44).
Most studies have examined the function of KLF4 and 5 in relatively permissive contexts, without exposure to any therapeutic. Conversely, tumors in human patients are subjected to diverse stresses including anti-tumor immune responses, conventional chemotherapy, targeted therapy and radiotherapy. As stress response factors, examining how KLF4/KLF5 influence the cellular adaptation to noxious stimuli may be more informative to their clinical relevance.
In breast cancer, KLF4 and 5 expression have been repeatedly associated with poor patient outcome (4,45,46,47). When KLF4 and 5 transcript levels were examined in tandem in breast cancer, higher co-expression was correlated with a poor outcome selectively within the HER2-enriched subtype (4). In this study, endogenous KLF4 and 5 cooperated to suppress the therapeutic response to HER2-inhibition through the induction of anti-apoptotic factors MCL1 and BCL-XL, an example of convergent signaling. Consistent results were obtained in human and mouse models of breast cancer. The rapid induction of anti-apoptotic factors by KLF4 and 5 appears to represent a type of adaptive reprogramming, a process previously linked to the induction of receptor tyrosine kinases when tumor cells are treated with agents such as MEK inhibitor or lapatinib (41,42). Interestingly, the individual contribution of KLF4 or 5 paled in comparison to their combined output. Cooperativity in tumor cells was demonstrated for the endogenous factors, which combined to promote not only drug resistance but also the growth of xenografted tumors (4).
Potential OSKM-like networks in human tumor cells
In addition to KLF4 and 5, an extensive literature currently indicates that tumor cells can co-express each of the OSKM factors, indicating the potential for a cross regulating, adaptable pluripotency-like network that could mediate tumor cell plasticity. For example, we recently analyzed expression of OSKM in pancreatic tumor cells and cancer stem-like cells (CSCs) (48). Tumor cells expressed all four Yamanaka factors, and three of the four (i.e., OKM) were maintained within CSCs by the transcription factor GLI1, expression of which is driven by activated KRAS in this tumor type. This example provides a link between a common cancer genetic alteration, KRAS mutation, and an endogenous pluripotency factor-like program.
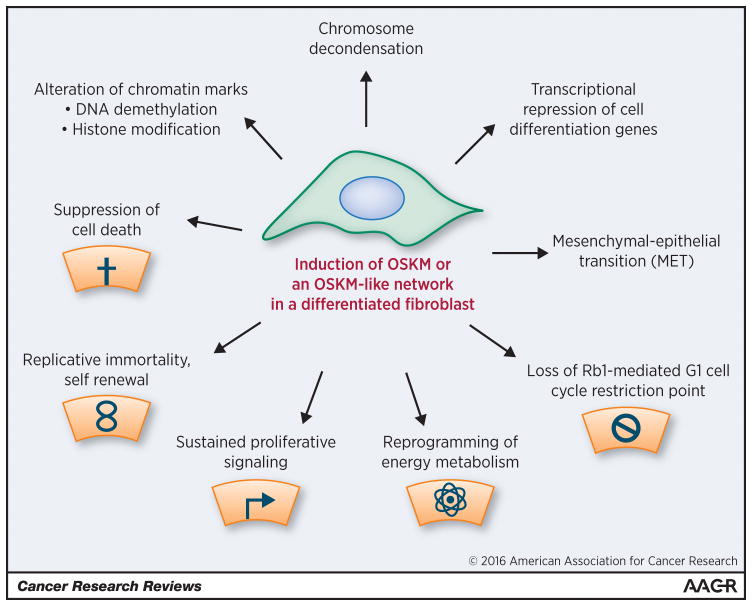
As reviewed elsewhere, there is remarkable overlap in the phenotype of PSCs and tumor cells, including multiple hallmarks of cancer that appear to be present within PSCs (Fig. 1) (2,6,9,49,50,51). Supporting the idea that tumor cells contain a pluripotency-like network capable of adaptive responses, we have observed upregulation of Nanog and c-MYC transcripts in breast cancer cells deficient in KLF4 and/or KLF5 (unpublished data). Providing some rationale that these results represent a compensatory response, KLF4 and Nanog show cross regulation in ESCs, and Nanog has been used with OCT4 and SOX2 to reprogram human cells (9). Also, c-MYC and KLF4 together compose a first wave of transcription factor binding during iPSC reprogramming (6).
Fig. 1. Alterations induced during reprogramming of adult fibroblasts to iPSCs.
The various properties attributed to PSCs (i.e., ESCs and/or iPSCs) have been reviewed (2,6,9,49,50,51). In their undifferentiated state, PSCs mirror several of the hallmarks of cancer previously highlighted by Hanahan and Weinberg (gray icons) (55). iPSC reprogramming is mediated by delivery of exogenous Yamanaka factors (OSKM) to normal cells or, alternatively, by a wide variety of distinct treatment regimens (OSKM-like) that ultimately impact upon endogenous pluripotency factor expression (i.e., OSKM, Nanog, others), epigenetic marks and/or chromosome compaction (52). As OSKM enforces the epithelial phenotype (MET), the iPSC model may be particularly analogous to epithelial-derived solid tumors (carcinomas). For more effective therapies and for phenotypic studies in the laboratory it may be important to target the broader pluripotency network, because few individual components of this network are essential, and network cross regulation enables effective adaptation and ongoing self-renewal (2).
Given the expression of multiple hallmarks of cancer within PSCs (Fig. 1), what factors might distinguish the epigenetically determined PSC phenotype from the invasive and metastatic phenotype of genetically defective tumor cells? One distinguishing trait of PSCs is low RAS-MEK-ERK signaling (2). RAS-ERK activation induces differentiation in PSCs, whereas MEK inhibitor helps to maintain self-renewal. Another distinguishing trait is that PSCs are epithelial, whereas carcinoma cells derive from epithelia but typically show some loss of epithelial features, and may express epithelial and/or mesenchymal markers. Given the considerable phenotypic overlap between PSCs and tumor cells (Fig. 1), it may be revealing of the cancer phenotype to further investigate the potential role of pluripotency OSKM or similarly acting, OSKM-like networks in adaptive processes such as reprogramming induced by drug exposure (6,52).
Therapeutic Targeting of the KLF-mediated Adaptive Response
Although KLF4 and 5 have multiple effectors to impact cell survival, both KLFs regulate components of the intrinsic pathway of apoptosis, highlighting an attractive node to target therapeutically. Currently under clinical investigation are small molecule inhibitors that target the KLF-regulated BCL2 family of anti-apoptotic factors. These may be useful agents to attenuate the KLF-mediated adaptive responses (4). Obatoclax (GX15-070) and Navitoclax (ABT-263) are BH3-mimetics which disrupt BCL2 family member protein-protein interactions, thereby enabling enhanced pro-apoptotic signaling. Where Obatoclax broadly inhibits all the anti-apoptotic BCL2 family members with a reduced potency, Navitoclax has a high affinity for BCL-2, -XL, and –W (53). Combined, these drugs are involved in 42 active/completed Phase I/II clinical trials for various malignancies, however their efficacy in breast cancer patients has yet to be explored. Although these drugs are generally well-tolerated in patients, preliminary data suggests they have limited utility as single-agents, advocating consideration for combination therapy instead.
High-throughput screening identified the small molecule ML264 as a selective inhibitor of KLF5 expression, with recent documentation of efficacy in a mouse model (54). A promising avenue of research includes the use of iPSC and ESC technology to screen for other inhibitors of core pluripotency factors. Small molecules that impact chromatin modifiers and chromatin remodeling enzymes have great promise to impact both regenerative medicine and cancer therapeutics. Consequently the careful dissection of pluripotency factor biology and signaling, including the Kruppel-like factors, their regulators, and their effectors holds substantial promise.
Summary
The convergence of developmental biology and cancer has been evident for decades and continues unabated. Kruppel-like factors represent yet another example of this convergence, and it seems likely that there will be conserved roles of KLFs across several fields of research broadly related to epigenetics and cellular reprogramming, including PSC biology, CSC biology and the cancer cell adaptive reprogramming response that follows treatment with a variety of small molecules. Current research efforts seem likely to generate new candidate therapeutics that are highly relevant across disciplines. To achieve timely progress in the regenerative medicine and cancer therapeutic arenas, it will be critical for researchers to integrate results from disparate areas of research and to adapt and reprogram accordingly.
Acknowledgments
This work was supported by grants NCI RO1 CA127405 (to J.M.R.) and the Jo and Ben Statler Chair in Breast Cancer Research. We regret that due to space limitations it was not possible to cite all of the relevant literature.
References
- 1.Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- 2.Nichols J, Smith A. Pluripotency in the embryo and in culture. Cold Spring Harb Perspect Biol. 2012;4:a008128. doi: 10.1101/cshperspect.a008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aksoy I, Giudice V, Delahaye E, Wianny F, Aubry M, Mure M, et al. Klf4 and Klf5 differentially inhibit mesoderm and endoderm differentiation in embryonic stem cells. Nat Commun. 2014;5:3719. doi: 10.1038/ncomms4719. [DOI] [PubMed] [Google Scholar]
- 4.Farrugia MK, Sharma SB, Lin CC, McLaughlin SL, Vanderbilt DB, Ammer AG, et al. Regulation of anti-apoptotic signaling by Kruppel-like factors 4 and 5 mediates lapatinib resistance in breast cancer. Cell Death Dis. 2015;6:e1699. doi: 10.1038/cddis.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin CC, Sharma SB, Farrugia MK, McLaughlin SL, Ice RJ, Loskutov YV, et al. Kruppel-like factor 4 signals through microRNA-206 to promote tumor initiation and cell survival. Oncogenesis. 2015;4:e155. doi: 10.1038/oncsis.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apostolou E, Hochedlinger K. Chromatin dynamics during cellular reprogramming. Nature. 2013;502:462–471. doi: 10.1038/nature12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knoepfler PS. Why myc? An unexpected ingredient in the stem cell cocktail. Cell Stem Cell. 2008;2:18–21. doi: 10.1016/j.stem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Yeo JC, Ng HH. The transcriptional regulation of pluripotency. Cell Res. 2013;23:20–32. doi: 10.1038/cr.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plath K, Lowry WE. Progress in understanding reprogramming to the induced pluripotent state. Nat Rev Genet. 2011;12:253–265. doi: 10.1038/nrg2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaspar-Maia A, Alajem A, Meshorer E, Ramalho-Santos M. Open chromatin in pluripotency and reprogramming. Nat Rev Mol Cell Biol. 2011;12:36–47. doi: 10.1038/nrm3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourillot PY, Savatier P. Kruppel-like transcription factors and control of pluripotency. BMC Biol. 2010;8:125. doi: 10.1186/1741-7007-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakaya T, Ogawa S, Manabe I, Tanaka M, Sanada M, Sato T, et al. KLF5 regulates the integrity and oncogenicity of intestinal stem cells. Cancer Res. 2014;74:2882–2891. doi: 10.1158/0008-5472.CAN-13-2574. [DOI] [PubMed] [Google Scholar]
- 13.Ghaleb AM, Nandan MO, Chanchevalap S, Dalton WB, Hisamuddin IM, Yang VW. Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15:92–96. doi: 10.1038/sj.cr.7290271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Couble ML, Mouterfi N, Magloire H, Chen Z, Bleicher F. Spatial and temporal expression of KLF4 and KLF5 during murine tooth development. Arch Oral Biol. 2009;54:403–411. doi: 10.1016/j.archoralbio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Swamynathan SK, Katz JP, Kaestner KH, Ashery-Padan R, Crawford MA, Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol Cell Biol. 2007;27:182–194. doi: 10.1128/MCB.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenchegowda D, Swamynathan S, Gupta D, Wan H, Whitsett J, Swamynathan SK. Conditional disruption of mouse Klf5 results in defective eyelids with malformed meibomian glands, abnormal cornea and loss of conjunctival goblet cells. Dev Biol. 2011;356:5–18. doi: 10.1016/j.ydbio.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandan MO, Ghaleb AM, Liu Y, Bialkowska AB, McConnell BB, Shroyer KR, et al. Inducible intestine-specific deletion of Kruppel-like factor 5 is characterized by a regenerative response in adult mouse colon. Dev Biol. 2014;387:191–202. doi: 10.1016/j.ydbio.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu CM, Russell CG, et al. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2001;98:8955–8960. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki T, Aizawa K, Matsumura T, Nagai R. Vascular implications of the Kruppel-like family of transcription factors. Arterioscler Thromb Vasc Biol. 2005;25:1135–1141. doi: 10.1161/01.ATV.0000165656.65359.23. [DOI] [PubMed] [Google Scholar]
- 21.Prosdocimo DA, Sabeh MK, Jain MK. Kruppel-like factors in muscle health and disease. Trends Cardiovasc Med. 2015;25:278–287. doi: 10.1016/j.tcm.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma SB, Lin CC, Farrugia MK, McLaughlin SL, Ellis EJ, Brundage KM, et al. microRNAs-206 and -21 cooperate to promote RAS-ERK signaling by suppressing the translation of RASA1 and SPRED1. Mol Cell Biol. 2014;34:4143–4164. doi: 10.1128/MCB.00480-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki T, Nishi T, Nagino T, Sasaki K, Aizawa K, Kada N, et al. Functional interaction between the transcription factor Kruppel-like factor 5 and poly(ADP-ribose) polymerase-1 in cardiovascular apoptosis. J Biol Chem. 2007;282:9895–9901. doi: 10.1074/jbc.M608098200. [DOI] [PubMed] [Google Scholar]
- 24.Sugden PH. An overview of endothelin signaling in the cardiac myocyte. J Mol Cell Cardiol. 2003;35:871–886. doi: 10.1016/s0022-2828(03)00153-6. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen TX, Leethanakul C, Patel V, Mitola D, Lund LR, Dano K, et al. Laser capture microdissection-based in vivo genomic profiling of wound keratinocytes identifies similarities and differences to squamous cell carcinoma. Oncogene. 2003;22:3964–3976. doi: 10.1038/sj.onc.1206614. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Wang J, Yi Y, Zhang H, Liu J, Liu M, et al. Induction of KLF4 in response to heat stress. Cell Stress Chaperones. 2006;11:379–389. doi: 10.1379/CSC-210.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghaleb AM, Katz JP, Kaestner KH, Du JX, Yang VW. Kruppel-like factor 4 exhibits antiapoptotic activity following gamma-radiation-induced DNA damage. Oncogene. 2007;26:2365–2373. doi: 10.1038/sj.onc.1210022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talmasov D, Xinjun Z, Yu B, Nandan MO, Bialkowska AB, Elkarim E, et al. Kruppel-like factor 4 is a radioprotective factor for the intestine following gamma-radiation-induced gut injury in mice. Am J Physiol Gastrointest Liver Physiol. 2015;308:G121–G138. doi: 10.1152/ajpgi.00080.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su C, Sun F, Cunningham RL, Rybalchenko N, Singh M. ERK5/KLF4 signaling as a common mediator of the neuroprotective effects of both nerve growth factor and hydrogen peroxide preconditioning. Age (Dordr ) 2014;36:9685. doi: 10.1007/s11357-014-9685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu C, La RS, Hagos EG. Oxidative DNA damage causes premature senescence in mouse embryonic fibroblasts deficient for Kruppel-like factor 4. Mol Carcinog. 2014 doi: 10.1002/mc.22161. [DOI] [PubMed] [Google Scholar]
- 32.McConnell BB, Kim SS, Bialkowska AB, Yu K, Sitaraman SV, Yang VW. Kruppel-like factor 5 protects against dextran sulfate sodium-induced colonic injury in mice by promoting epithelial repair. Gastroenterology. 2011;140:540–549. doi: 10.1053/j.gastro.2010.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Courboulin A, Tremblay VL, Barrier M, Meloche J, Jacob MH, Chapolard M, et al. Kruppel-like factor 5 contributes to pulmonary artery smooth muscle proliferation and resistance to apoptosis in human pulmonary arterial hypertension. Respir Res. 2011;12:128. doi: 10.1186/1465-9921-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofmann AD, Takahashi T, Duess JW, Gosemann JH, Puri P. Increased pulmonary vascular expression of Kruppel-like factor 5 and activated survivin in experimental congenital diaphragmatic hernia. Pediatr Surg Int. 2014;30:1191–1197. doi: 10.1007/s00383-014-3606-7. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Yuan D, Nie X, Shen J, Yan Y, Zhang D, et al. BTEB2 prevents neuronal apoptosis via promoting bad phosphorylation in rat intracerebral hemorrhage model. J Mol Neurosci. 2015;55:206–216. doi: 10.1007/s12031-014-0305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ, No YR, Dang DT, Dang LH, Yang VW, Shim H, et al. Regulation of hypoxia-inducible factor 1α (HIF-1α) by lysophosphatidic acid is dependent on interplay between p53 and Kruppel-like factor 5. J Biol Chem. 2013;288:25244–25253. doi: 10.1074/jbc.M113.489708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong JT, Chen C. Essential role of KLF5 transcription factor in cell proliferation and differentiation and its implications for human diseases. Cell Mol Life Sci. 2009;66:2691–2706. doi: 10.1007/s00018-009-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, Nakagawa H, Tetreault MP, Billig J, Victor N, Goyal A, et al. Loss of transcription factor KLF5 in the context of p53 ablation drives invasive progression of human squamous cell cancer. Cancer Res. 2011;71:6475–6484. doi: 10.1158/0008-5472.CAN-11-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Tarapore RS, Jarmel MH, Tetreault MP, Katz JP. p53 mutation alters the effect of the esophageal tumor suppressor KLF5 on keratinocyte proliferation. Cell Cycle. 2012;11:4033–4039. doi: 10.4161/cc.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- 41.Duncan JS, Whittle MC, Nakamura K, Abell AN, Midland AA, Zawistowski JS, et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012;149:307–321. doi: 10.1016/j.cell.2012.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stuhlmiller TJ, Miller SM, Zawistowski JS, Nakamura K, Beltran AS, Duncan JS, et al. Inhibition of Lapatinib-Induced Kinome Reprogramming in ERBB2-Positive Breast Cancer by Targeting BET Family Bromodomains. Cell Rep. 2015;11:390–404. doi: 10.1016/j.celrep.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong Z, Yang L, Lai D. KLF5 strengthens drug resistance of ovarian cancer stem-like cells by regulating survivin expression. Cell Prolif. 2013;46:425–435. doi: 10.1111/cpr.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Hamza MS, Leong HS, Lim CB, Pan YF, Cheung E, et al. Kruppel-like factor 5 modulates p53-independent apoptosis through Pim1 survival kinase in cancer cells. Oncogene. 2008;27:1–8. doi: 10.1038/sj.onc.1210625. [DOI] [PubMed] [Google Scholar]
- 45.Pandya AY, Talley LI, Frost AR, Fitzgerald TJ, Trivedi V, Chakravarthy M, et al. Nuclear localization of KLF4 is associated with an aggressive phenotype in early-stage breast cancer. Clin Cancer Res. 2004;10:2709–2719. doi: 10.1158/1078-0432.ccr-03-0484. [DOI] [PubMed] [Google Scholar]
- 46.Tong D, Czerwenka K, Heinze G, Ryffel M, Schuster E, Witt A, et al. Expression of KLF5 is a prognostic factor for disease-free survival and overall survival in patients with breast cancer. Clin Cancer Res. 2006;12:2442–2448. doi: 10.1158/1078-0432.CCR-05-0964. [DOI] [PubMed] [Google Scholar]
- 47.Kamalakaran S, Varadan V, Giercksky Russnes HE, Levy D, Kendall J, Janevski A, et al. DNA methylation patterns in luminal breast cancers differ from non-luminal subtypes and can identify relapse risk independent of other clinical variables. Mol Oncol. 2011;5:77–92. doi: 10.1016/j.molonc.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng W, Vanderbilt DB, Lin CC, Martin KH, Brundage KM, Ruppert JM. SOX9 inhibits βTrCP-mediated protein degradation to promote nuclear GLI1 expression and cancer stem cell properties. J Cell Sci. 2015;128:1123–1138. doi: 10.1242/jcs.162164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh AM, Dalton S. The cell cycle and Myc intersect with mechanisms that regulate pluripotency and reprogramming. Cell Stem Cell. 2009;5:141–149. doi: 10.1016/j.stem.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 51.Niwa H. The pluripotency transcription factor network at work in reprogramming. Curr Opin Genet Dev. 2014;28:25–31. doi: 10.1016/j.gde.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Theunissen TW, Jaenisch R. Molecular control of induced pluripotency. Cell Stem Cell. 2014;14:720–734. doi: 10.1016/j.stem.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goard CA, Schimmer AD. An evidence-based review of obatoclax mesylate in the treatment of hematological malignancies. Core Evid. 2013;8:15–26. doi: 10.2147/CE.S42568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruiz de Sabando A, Wang C, He Y, Garcia-Barros M, Kim J, Shroyer KR, et al. ML264 - a novel small molecule that potently inhibits the growth of colorectal cancer. Mol Cancer Ther. 2015 doi: 10.1158/1535-7163.MCT-15-0600. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]