Abstract
Dysregulation of the p16-cyclin D1-CDK4/6-Rb pathway occurs frequently in melanoma; however, the therapeutic efficacy of CDK4/6 inhibition remains to be critically evaluated. We demonstrate that CDK4/6 inhibition inhibits melanoma progression through induction of senescence. Palbociclib, a specific CDK4/6 inhibitor, rapidly induces cell cycle within 24h and continued exposure for 8-days or longer induces senescence. The induction of senescence correlates with inhibition of mTOR and more specifically mTORC1 signaling. Vemurafenib, a specific BRAFV600E inhibitor, has significant clinical efficacy in BRAFV600E positive melanomas but its impact is hampered by a rapid acquisition of resistance. Strikingly, we found that vemurafenib-resistant tumors remain sensitive to palbociclib suggesting that initial treatment with vemurafenib followed by palbociclib with or without mTOR inhibitors might provide an avenue to overcome recurrence of vemurafenib resistant, metastatic disease. Taken together these results support palbociclib as a promising therapeutic for treatment of melanoma.
Keywords: CDK4, cyclin D, palbociclib, mTOR, senescence
Introduction
The most frequent mutation that occurs in melanoma occurs in Braf at amino acid 600 (BrafV600E). However BrafV600E alone elicits senescence (1,2). Multiple studies provide evidence that Ras-Raf-driven tumorigenesis is markedly enhanced in the context of dysregulated cyclin D1/CDK4 activity (3–7). Indeed, inactivation of p16INK4a, an allosteric inhibitor of CDK4/6, occurs in up to 70% of human melanomas (8). Additional genetic support for the importance of dysregulation of cyclin D1/CDK4 in melanoma stems from the demonstration that inactivation of p16INK4a or deletion of Fbxo4, the specificity component of the E3 ligase that directs cyclin D1 degradation, cooperates with BrafV600E to induce metastatic melanoma (9–13).
Vemurafenib, an ATP competitive small molecule BrafV600E inhibitor, has been approved by the US Food and Drug Administration (FDA), and has marked antitumor effects with BrafV600E melanoma, but not Braf WT melanoma (14). Initial treatment of vemurafenib results in tumor shrinkage lasting 3 to 6 months in clinical trials; unfortunately however, tumors relapse due to the acquired resistance to vemurafenib, and overall survival is only extended several months (15). Current efforts are focused on the development of second line therapies that have significant clinical impact on the resistant tumors.
Senescence, initially identified by Hayflick and Moorhead, is induced by a number of biological stresses such as DNA damage accumulation, acute oncogene activation, reactive oxygen species (ROS), and expression of INK4A/ARF locus in over passaged primary cells in a p53 dependent or independent manner (16). Critically, senescence is defined as a permanent cell cycle arrest and considered as one of the defense mechanisms against cancers (17).
PD0332991, commonly referred to as palbociclib, is a FDA-approved orally available, highly selective ATP competitive inhibitor of CDK4 (IC50: 0.011 µM) and CDK6 (IC50: 0.016 µM) (18) with clinical evidence of growth inhibitory activity. Palbociclib is being tested in more than seventeen clinical trials for malignancies such as mantle cell lymphoma, breast cancer, and colorectal cancer (18–21). However, the utility of the drug has not yet been evaluated in melanoma, a cancer that when metastatic remains largely intractable. We have investigated the efficacy of CDK4/6 inhibition in vemurafenib resistant melanoma and have begun to dissect the molecular mechanism of acquired resistance to palbociclib.
Materials and Methods
Cell culture and viral infection
Authenticated human melanoma cell lines (1205Lu, WM983, WM983BR, WM451Lu, WM239A, WM3918) were obtained from Meenhard Herlyn at The Wistar Institute in 2013 (22), and cultured in Tu2% media (80% MCDB153 medium supplemented with 20% Leibovitz’s L-15, 2% fetal bovine serum (FBS), and 1.68 mM CaCl2. Authenticated human embryonic kidney (HEC) 293T cells were purchased from American Type Cell Culture in 2014 and cultured in Dulbecco’s modified Eagle’s medium (DMED) supplemented with 10% FBS, 100 units/ml penicillin, and 100µg/ml streptomycin. For synchronization in G0/G1, G1/S, and G2/M phase, cells were incubated in medium containing 0.5% FBS for 48 hours at high density on the plates (contact inhibition) for G0/G1 phase; treated with 1µM hydroxyurea for G1/S phase, 40ng/mL nocodazole for G2/M phase respectively. For the cytotoxicity experiment for vemurafenib (PLX4032), melanoma cells were incubated with vemurafenib at 10µM for 2 days. For viral production, viral expression plasmids were transfected into 293T cells with Lipofectamine together with packaging plasmid. Virus supernatants harvested 48–72 hours after transfection were used to infect melanoma cells with polybrene (10µg/ml).
Flow cytometric analysis (FACS)
Cells were trypsinized and stained with 10 µg/ml propidium iodide (PI) containing 100 µg/ml RNaseA and 0.5% FBS followed by fixation with 70% ethanol. For the BrdU incorporation, cells were incubated in medium containing 10µM BrdU for 45min prior to harvest and then fixed with 70% ethanol, treated with 1.5N HCl, stained with anti-BrdU mouse monoclonal antibody and subsequently FITC-linked mouse IgG staining. Cytotoxicity for Palbociclib was assayed by Annexin V apoptosis detection kit (BD biosciences) according to the manufacturer’s instructions. Cell cycle distribution, BrdU positive cells, and Annexin V positive cells were quantified using a BD LSRFortessa (BD Biosciences).
Western analysis
Cells were lysed in EBC buffer (50 mM Tris [pH 8.0], 120 mM NaCl, 1 mM EDTA, 0.5% NP40) containing 1 µM phenylmethylsulfonyl fluoride, 20 U/ml aprotinin, 1µM leupeptin, 1 mM dithiothreitol, 0.1 mM NaF, 0.1 mM Sodium orthovanadate, and 10 mM β-glycerophosphate. Proteins were resolved by SDS-PAGE, transferred to a PVDF membrane, and immunoblotted with appropriate antibodies. pRb (S780), p19Ink4d (M-167), p62 (D-3), and MDM2 (C-18) were obtained from Santa Cruz. P-ERK1/2 (Thr202/Tyr204), ERK1/2, P-mTOR (Ser2448), mTOR, P-Akt (S478), Pan Akt (11E7), P-GSK3β (Ser9), P-S6K (Thr389), S6K (49D7), and P-4E-BP1 (Thr37/46) from Cell Signaling Technology. Raptor and Rictor were purchased from Bethyl labolatories. Cyclin D1 (Ab-3) was purchased from Millipore. LC3B and β actin (AC-15) were purchased from Sigma. Rb (G3-245) was obtained from BD Pharmingen. Signals were visualized using ECL (PerkinElmer).
Quantitative RT-PCR analysis
Total RNA was extracted using the RNeasy Micro Kit (Qiagen), and reverse transcribed using iScript™ cDNA Synthesis Kit (BIO-RAD) according to the manufacturer’s instructions. Quantitative RT-PCR was performed using SsoAdvanced™ Universal SYBR Green Supermix (BIO-RAD) and the data were normalized by GAPDH.
Immunohistocemistry and Immunohistofluorescence
Tissues were fixed in 4% paraformaldehyde, deparaffinized and rehydrated in gradient ethanol. Sections were blocked with 10% goat serum and incubated with the primary antibodies against the following: P-S6 (S235/236), P-Akt (S473), Ki67 and pRb (S780). For immunohistochemistry, sections were incubated with biotinylated rabbit antibodies and developed with ABC substrate kit (Vector) followed by DAB reaction (Vector), and counterstained with Hematoxylin (Thermo Scientific). For immunofluorescence, sections were incubated with Alex Fluor 488 or Alexa Fluor 546-conjugated goat anti-mouse/rabbit antibodies (Life technologies), and mounted with ProLong Gold antifade reagent with DAPI (Invitrogen).
Senescence assays
Cells, 2.5×103/60mm plate, were treated with 1µM palbociclib with fresh palbociclib added daily for 8 days. Cells were cultured for an additional 14 days following removal of drug, in complete growth media and colonies were visualized by Giemsa stain. For SA-βGal, cultured melanoma cells or frozen tissue samples in Tissue-Tek OCT compounds (Sakura Finetek, Torrance, CA) were fixed and incubated with X-gal staining solution (Sigma) according to manufacturer’s instructions. For ELISA, melanoma cells were cultured with or without palbociclib. SASP expression such as IL-6 (D6050), IL-8 (D8000C), CXCL1/GROα (DGR00) in supernatants were analyzed with ELISA kits (R&D) according to manufacture’s protocol.
Xenograft
2×106 melanoma cells were subcutaneously injected into 6-weeks-old SCID mice with matrigel. Mice were treated with DMSO (control) or 90mg/kg of palbociclib every day when tumors developed 2mm after injection. Tumor volumes were measured by caliper every 2 or 3 days and calculated by the following formula V = (Length × width × height)/2. Mice were sacrificed and the tumor size was measured when tumors reached 10 mm. Care of experimental animals was in accordance with institutional guidelines.
Results
CDK4/6 inhibition induces senescence in melanoma-derived cells
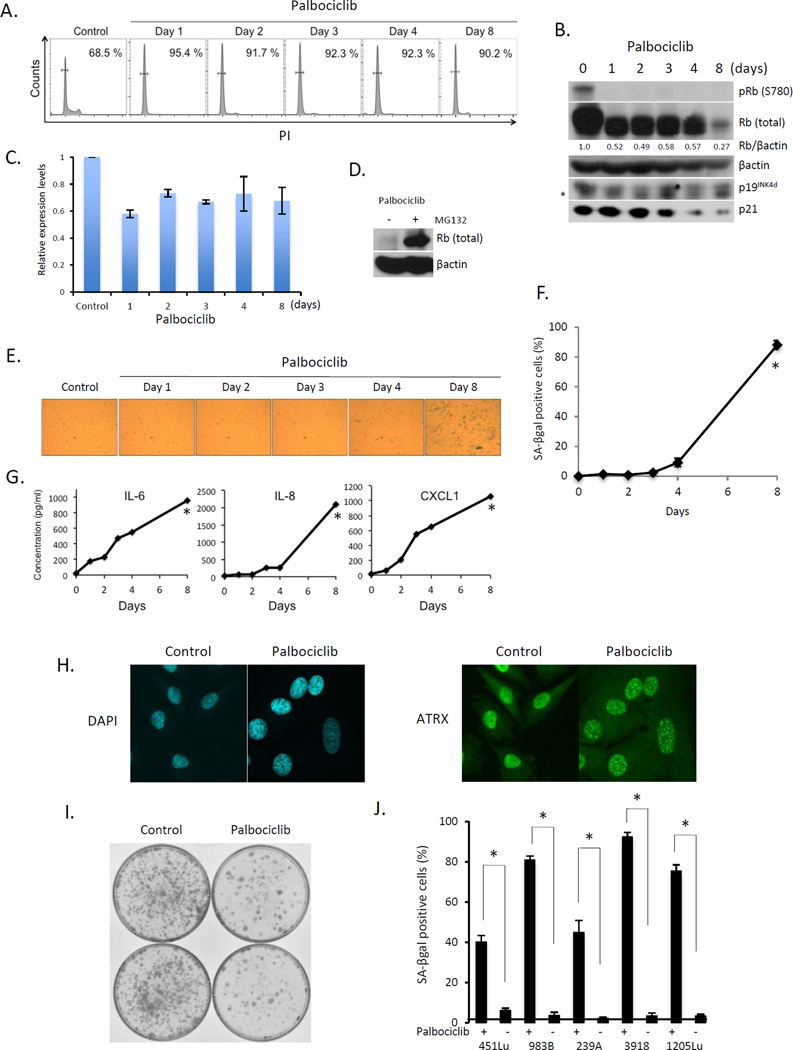
The dysregulation of p16-CDK4-cyclin D1-Rb pathway in a majority of melanomas suggests that small molecule inhibition of the cyclin D1/CDK4 kinase might be an effective therapeutic strategy for limiting melanoma progression. To test this, we treated 1205Lu melanoma cells with palbociclib, a specific CDK4/6 inhibitor (18). Palbociclib treatment triggered G0/G1 arrest that was associated with dephosphorylation of Rb at serine-780 (S780), with no increase in p19INK4d or p21 noted (Fig. 1A, B). Palbociclib-dependent reduction in Rb levels have been observed previously and while it has been associated with reduced stability, the mechanism remains unclear (23). QPCR analysis revealed an approximate 30–40% reduction in Rb expression (Fig. 1C). Rb expression could be maintained by proteasome inhibitor treatment revealing coordinate regulation of both expression and protein stability (Fig. 1D). While CDK4/6 inhibition is widely known to trigger reversible quiescence, prolonged inhibition of cyclin D1-CDK4/6 has also been associated with senescence or permanent growth arrest (19,24,25). To address the acquisition of a senescence, 1205Lu cells were exposed to palbociclib for varying intervals and senescence was addressed through several distinct criteria: 1) senescence-associated β-galactosidase activity (SA-βgal); 2) senescence-associated secretory phenotype (SASP) such as IL-6, IL-8, and CXCL1; 3) senescence-associated heterochromatic foci (SAHF) formation and 4) ATR-X foci formation (23). An increase in all senescence-associated marks was noted at day 4 with maximal induction noted by day 8 by all criteria (Fig 1E–H).
Fig. 1. Palbociclib treatment induces senescence in melanoma-derived cells.
(A) FACS analysis of 1205Lu cells treated with palbociclib. (B) Western analysis of the samples from (A). * indicates nonspecific band. (C) QPCR analysis of samples from (A). (D) Western analysis of 1205Lu cells with or without MG132 following palbociclib exposure for 8days. (E) SA-βgal staining of 1205 Lu cells following palbociclib exposure for the indicated intervals. (F) Quantification from (E) (*p < 0.001). (G) Cytokine secretion following treatment of 1205Lu cells with palbociclib (*p < 0.001). (H) DAPI staining for SAHF (left panel) and ATRX staining (right panel) in 1205Lu cells +/− palbociclib for 8 days. (I) Clonogenic assay of 1205Lu cells treated +/− palbociclib for 8 days. (J) Quantification of SA-βgal positive WM451Lu, WM983B, WM239A, WM3918, and 1205Lu cells following treatment with palbociclib for 8 days (*p < 0.001).
Because senescence is defined as permanent or irreversible cell cycle arrest, whereas quiescence is a reversible cell cycle exit, we examined reversibility of palbociclib-induced senescence using a clonogenic cell survival assay. Consistent with senescence, 8 days of palbociclib reduced colony formation by 80% (Fig. 1I). We exposed several additional melanoma cell lines to extended palbociclib treatment and observed markers of senescence including SA-βGal positivity (Fig 1J).
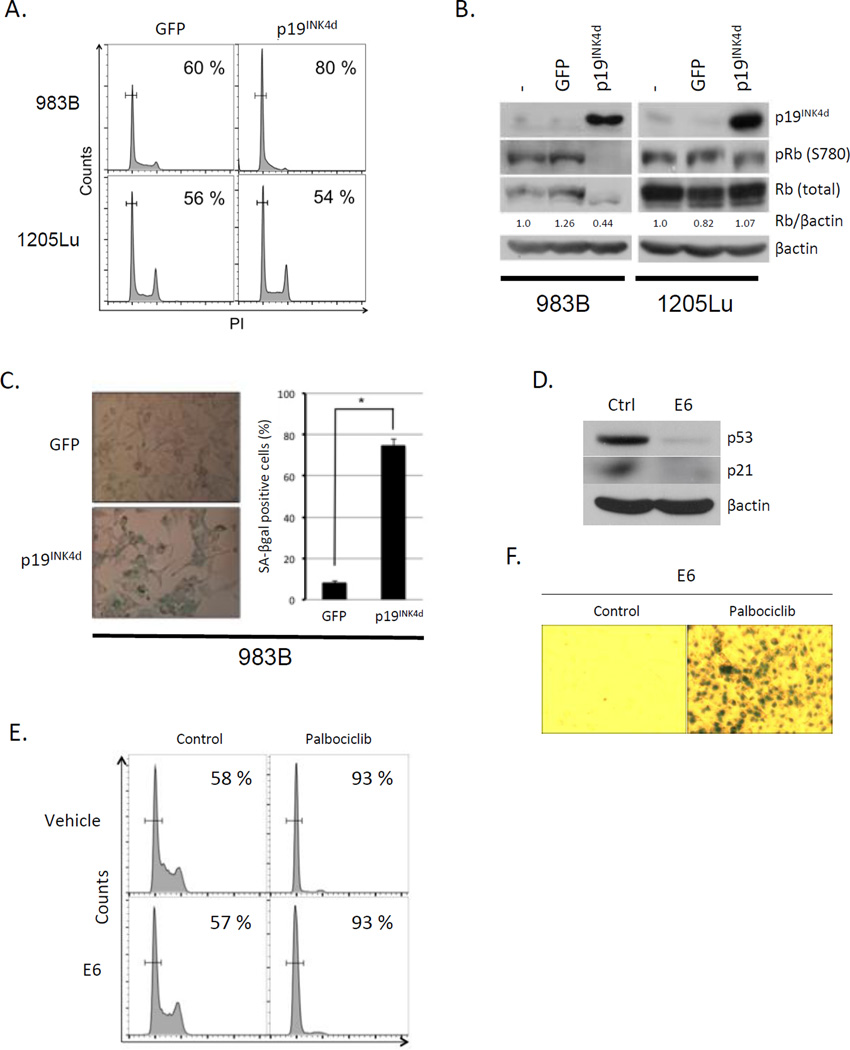
To independently evaluate the impact of CDK4/6 inhibition, we utilized an INK4 family protein, p19INK4d that binds and specifically inhibits CDK4/6 in Rb positive cells (26,27). We utilized two distinct melanoma cell lines. 1205Lu, which in addition to harboring a BRAFV600E mutation, also has a CDK4-R24C mutation; the R24C mutation falls within the INK4 binding interface making this CDK4 allele resistant to INK4 mediated inhibition (28,29). We also used 983B cells that express wild type CDK4 and CDK6, as well as BRAFV600E. Expression of p19INK4d induced G0 arrest and Rb dephosphorylation in 983B within the first 36h (Fig 2A–B), while 1205Lu cells were refractory. Consistent with persistent CDK4/6 inhibition inducing a switch from quiescence (G0) to senescence, we noted a striking increase in SA-βgal positivity 8-days post infection of 983B cells (Fig 2C). To assess whether senescence depends on p53-p21 signaling, we expressed the HPV E6 to inactivate p53 (Fig. 2D). Palbociclib treatment triggered G0/G1 arrest at 1-day and senescence by 8-days in cells expressing E6 (Fig. 2E, F). This is consistent with the observation that p21 is not induced by palbociclib exposure (Fig. 1B).
Fig. 2. p19INK4d introduction induces senescence in melanoma cells.
(A) FACS analysis of 983B cells (upper panel) and 1205Lu (bottom panel) cells expressing GFP (left panel) or p19INK4d (right panel). (B) Western analysis of the samples from (A). (C) SA-βgal staining of 983B cells expressing GFP (top) or p19INK4d (bottom panel). Quantification of SA-βgal positive cells (right panel) (*p < 0.001). (D) Western analysis of 1205Lu cells stably expressing E6. (E) FACS analysis of 1205Lu cells infected with control vector (top panel) or E6 (bottom panel) +/− palbociclib for 1 day. (F) SA-βgal staining of 1205 Lu cells expressing E6 following palbociclib exposure.
Palbociclib-induced senescence abrogates vemurafenib-dependent cytotoxicity
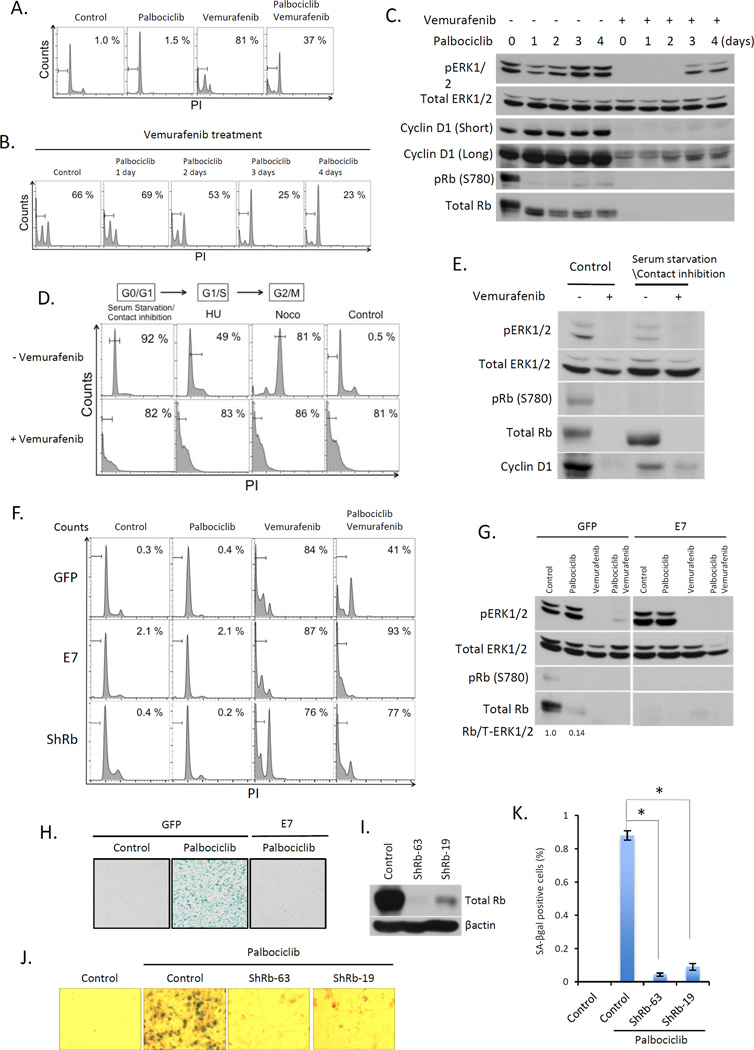
To assess the effect of combining vemurafenib with palbociclib 1205Lu cells were treated with vemurafenib, palbociclib or both. Vemurafenib treatment resulted in 80% of cells undergoing apoptosis, while palbociclib treatment promoted only cell cycle arrest (Fig 3A). Strikingly, palbociclib substantially inhibited vemurafenib dependent cytotoxicity, resulting in a shift from cell death to G0 arrest (Fig. 3A, B). With increasing time of exposure to palbociclib, cells exhibited significantly reduced sensitivity to vemurafenib-dependent killing (Fig 3B) suggesting acquisition of senescence-conferred resistance. To further test this hypothesis, 1205Lu cells were arrested by serum starvation/contact inhibition (G0/quiescence, (Fig. 3E), hydroxyurea (G1/S) or nocodazole (G2/M) and then exposed to vemurafenib for 48 hours. Vemurafenib efficiently triggered apoptosis under all conditions (Fig. 3D) demonstrating that cell cycle arrest per se did not prevent vemurafenib dependent cytotoxicity. Immunoblot analysis revealed that palbociclib rapidly reduced Rb phosphorylation; strikingly vemurafenib treatment was associated with reduced Erk activation only in cells exposed to palbociclib for 2 days. Palbociclib treatment for 3 or more days reduced sensitivity to vemurafenib (Fig 3C).
Fig. 3. Palbociclib-induced senescence abrogates vemurafenib-dependent cytotoxicity in melanoma.
(A) FACS of 1205Lu cells treated with palbociclib for 4 days, vemurafenib for 2 days, or pre-treated with palbociclib for 4 days followed by vemurafenib+palbociclib for 2 days. (B) FACS of 1205Lu cells pre-treated with palbociclib for 1, 2, 3, or 4 days and then treated with vemurafenib+palbociclib for 2 days. (C) Western analysis of the samples from (B). (D) 1205Lu cells were arrested at G0/G1 phase by serum starvation and contact inhibition for 2 days, at G1/S treated with Hydroxyurea (HU) or G2/M with Nocodazole (Noco). FACS of synchronized cells treated +/− vemurafenib for 2 days. (E) Western analysis of the samples from asynchronous or cells synchronized at G0/G1 phase followed by treatment +/− vemurafenib. (F) FACS of 1205Lu cells infected with GFP (top panels), E7 (middle panel), or ShRb1 (bottom panel), treated with either vemurafenib (2 days), palbociclib (4 days) or palbociclib (4 days_ followed by vemurafenib+palbociclib (2 days). (G) Immunoblot of samples from (F). (H) SA-βgal staining of 1205 Lu cells infected with GFP or E7 following palbociclib exposure. (I) Western analysis of total Rb in 1205Lu cells infected with ShRb1 (clone 63 and 19). (J) SA-βgal staining of cells from (I). (K) Quantification of SA-βgal positive cells from (J) (*p < 0.001).
To evaluate Rb function in palbociclib-induced senescence, E7 was introduced into 1205Lu cells. E7 expression inhibited palbociclib-induced senescence (Fig. 3F–H). E7 also re-sensitized cells to vemurafenib-dependent cytotoxicity (Fig. 3F). Since E7 interacts with p107 and p130, we utilized two distinct shRNAs to knockdown Rb. Rb knockdown cells were refractory to palbociclib-induced senescence (Fig. 3I–K), and were resensitized to vemurafenib-induced cell death (Fig. 3F), consistent with CDK4/6-dependent senescence being both Rb-dependent and antagonizing vemurafenib-dependent cell death (30).
Palbociclib induces senescence in vemurafenib-resistant melanoma
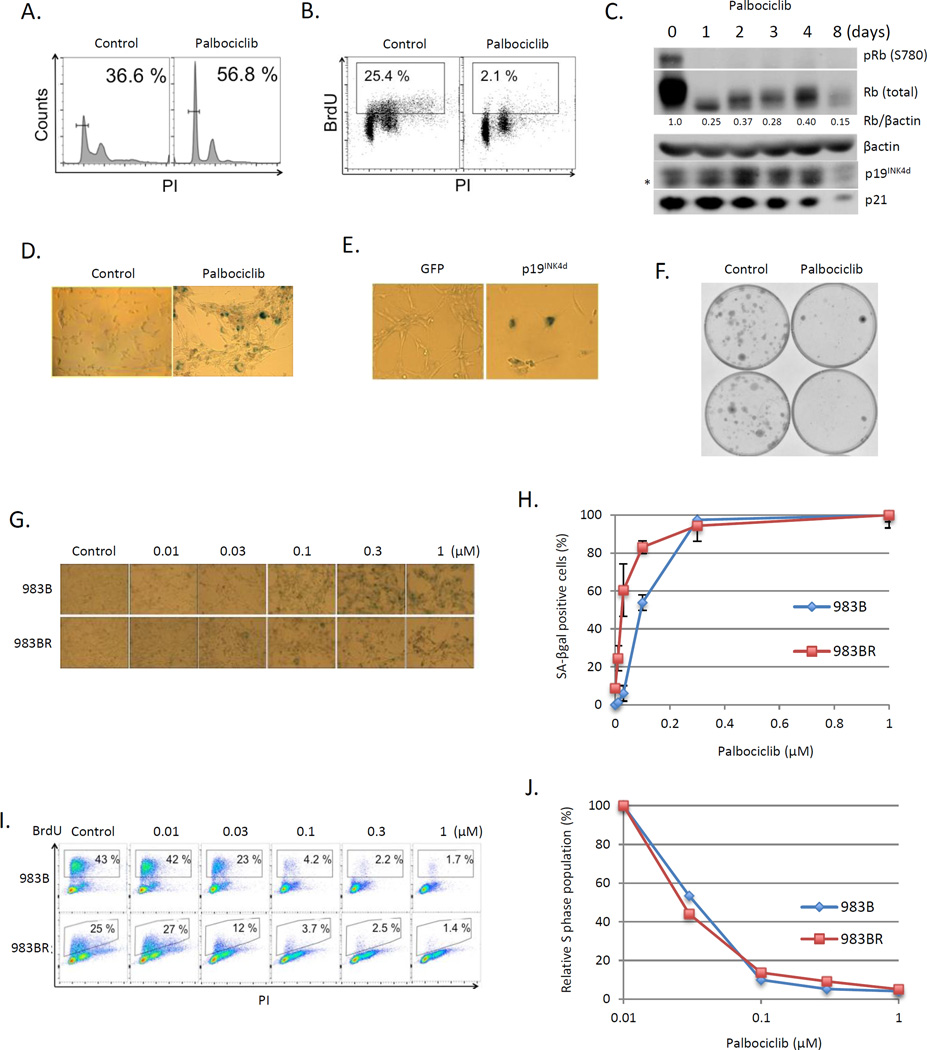
983BR is a vemurafenib-resistant cell line derived from 983B cultured under conditions of increasing concentrations of vemurafenib to acquire the resistance (Fig. S1). Palbociclib induced G0 arrest in both parental 983B (Fig. 1J) and 983BR cells (Fig. 4A), with markedly reduced BrdU incorporation (Fig. 4B). Arrest was associated with dephosphorylation of Rb, with no increase in CDK inhibitors such as p19INK4d and p21 (Fig. 4C; Fig. 1B). Eight days of palbociclib or p19INK4d introduction resulted in greater than 80% of cells staining positively for SA-βGal (Fig 4D–E). Palbociclib treatment for 8-days also inhibited long-term colony formation (Fig. 4F). Analysis of the kinetics of senescence induction revealed that 983BR exhibited a trend towards increased palbociclib-induced senescence (Fig. 4G, H). No difference was noted in the sensitivity of 983B versus 983BR to cell cycle arrest (Fig. 4I, J).
Fig. 4. Palbociclib efficacy in vemurafenib resistant melanoma cells.
(A) FACS analysis of 983BR vemurafenib resistant melanoma cells treated with palbociclib for 8 days. (B) 983BR cells were subjected to BrdU incorporation for 45min post palbociclib treatment for 8 days. BrdU and DNA content were determined by anti-BrdU (Y axis) and PI (X axis) respectively, and analyzed by FACS. (C) Western blot analysis of 983BR cells treated with palbociclib for 1, 2, 3, 4 and 8 days. * indicates nonspecific background. (D) SA-βgal staining of 983BR cells treated with or without palbociclib for 8 days. (E) SA-βgal staining of 983BR cells infected with retrovirus encoding GFP or p19INK4d. (F) Conogenic assay of 983BR cells treated with or without palbociclib for 8 days. (G) SA-βgal staining of 983B (vemurafenib sensitive) cells and 983BR (vemurafenib resistant) cells following palbociclib treatment at different concentrations (0.01, 0.03, 0.1, 0.3 and 1µM) for 8 days. (H) Quantification of SA-βgal positive cells from (G). (I) 983B cells and 983BR cells were subjected to BrdU incorporation for 45min post-palbociclib treatment (0.01, 0.03, 0.1, 0.3 and 1µM) for 24 hours. BrdU and DNA content determined by FACS. (J) Quantification of BrdU positive cells from (I).
Palbociclib induces senescence in vivo
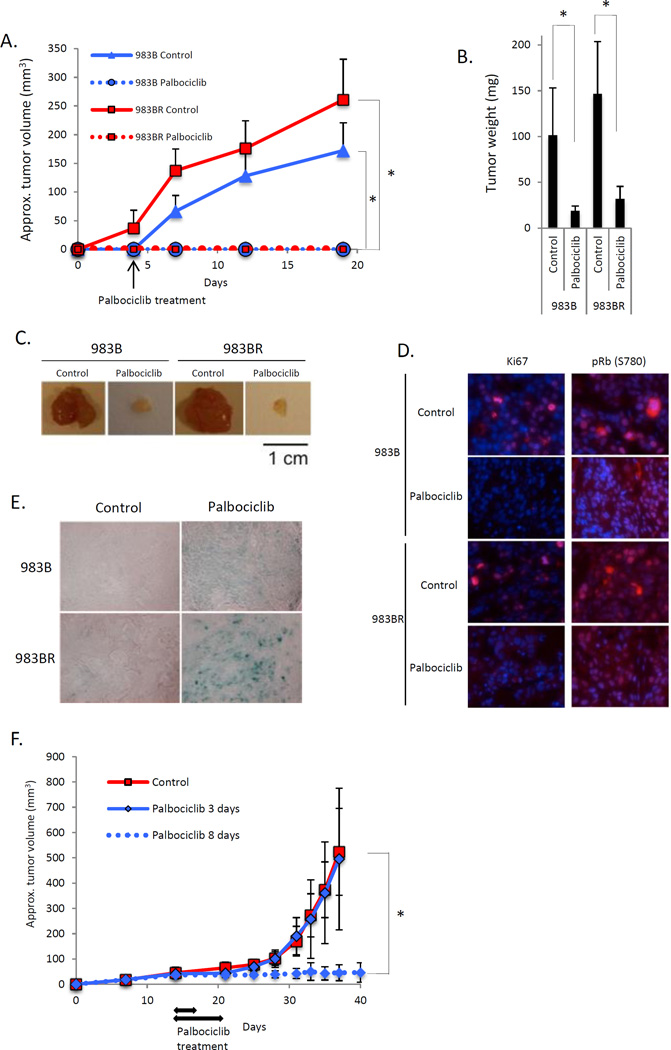
To address the ability of palbociclib to inhibit melanoma progression in vivo, 983B or 983BR cells were injected into SCID mice. Palbociclib efficiently inhibited growth of 983B and 983BR cells (Fig. 5A, B, C). Palbociclib reduced Ki67, Rb phosphorylation (Fig. 5D) and residual exhibited strong SA-βgal positivity (Fig. 5E). Analysis of palbociclib action in vitro suggested that 8 days of exposure was sufficient to induce senescence. To determine whether 8 days was sufficient for senescence in vivo, we conducted a second trial wherein tumor-burdened mice were administered palbociclib daily for 3 or 8 days. Tumors in the 3-day treatment group developed as fast as controls, while mice treated for 8 days exhibited stable disease (Fig. 5F). At later time points, some tumors escaped and re-initiated growth. Cell lines derived from these tumors were found to be resistant to palbociclib suggesting acquired resistance (see figure 6 below).
Fig. 5. Palbociclib induces senescence in melanoma in vivo.
2×106 of 983B (vemurafenib sensitive) cells and 983BR (vemurafenib resistant) cells were subcutaneously injected into 6-weeks-old SCID mice. Mice were treated with palbociclib every day after 3 days post injection for 14 days. (A) Tumor volume (*p < 0.001). (B) Tumor weight (*p < 0.001). (C) Representative images of the tumors from (B). (D) Immunofluorescence using sections from tumors treated with either vehicle or palbociclib. (E) SA-βgal staining of SubQ-injected 983B and 983BR cells treated with either vehicle or palbociclib. (F) 2×106 of 983BR (vemurafenib resistant) cells were subcutaneously injected into 6-weeks-old SCID mice. Treatments were initiated when tumors reached 2mm3 for either 3 or 8 days as indicated with arrows. Tumor volumes were assessed every 2–3 days (*p < 0.001).
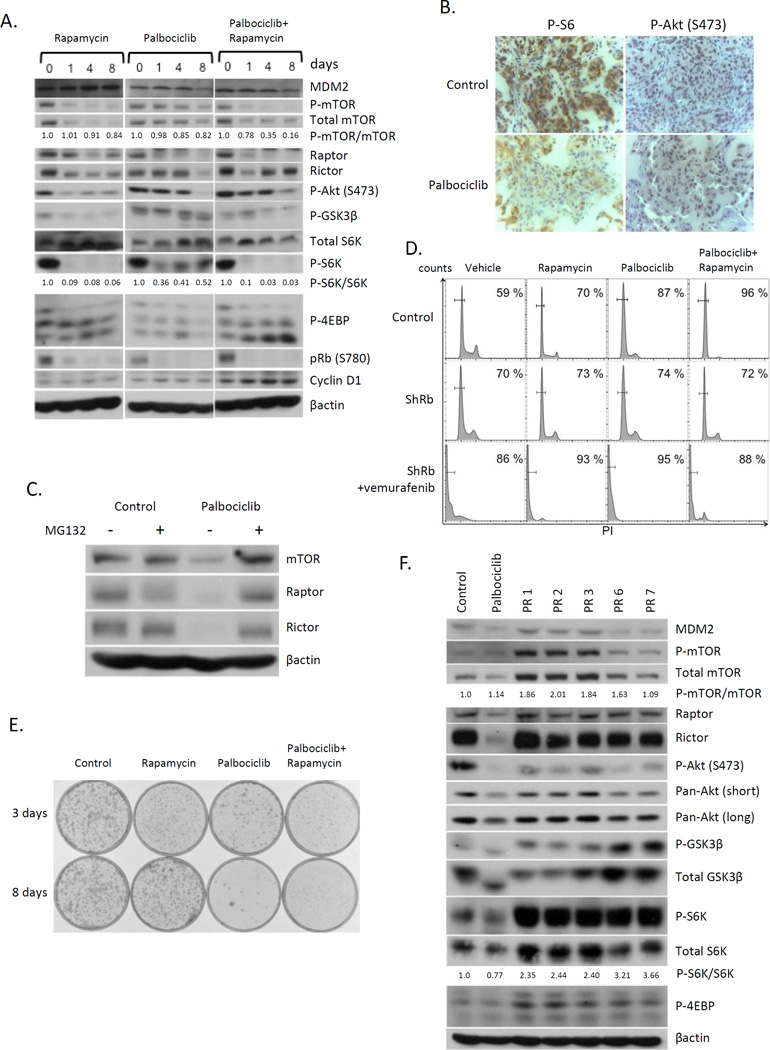
Fig. 6. Inhibition of mTOR signaling pathway is critical for palbociclib-induced senescence in melanoma.
(A) Western blot analysis of 1205Lu cells treated with rapamycin or palbociclib or both for 1, 4, or 8 days. (B) Immunohistochemistry of tumors derived +/− palbociclib. (C) Western analysis of 1205Lu cells +/− palbociclib,+/− MG132. (D) FACS analysis of 1205Lu cells infected with GFP (top panels) or ShRb1 (middle and bottom panel) treated with either rapamycin or palbociclib, or both followed by vemurafenib+palbociclib for 2 days (bottom panel). (E) Clonogenic assay of 1205Lu cells treated with rapamycin, palbociclib, or Rapamycin+palbociclib for 3 days (top panel) or 8 days (bottom panel). (F) Immunoblot of palbociclib-resistant 1205Lu cells.
Inhibition of mTOR signaling pathway is critical for palbociclib-induced senescence in melanoma
Recent work provided evidence that activation of mTOR signaling can bypass senescence in mouse models of melanoma (31). Highlighting a role for mTor decreased mTOR phosphorylation occurred by 8 days of palbociclib (Fig 6A). Decreased phosphorylation of Akt, GSK3β, S6K and 4EBP1, downstream of targets of mTOR, was observed demonstrating mTor inhibition (Fig 6A). In contrast, Rapamycin reduced mTORC1 signaling within the first 24h. Palbociclib with rapamycin accelerated downregulation of mTOR signaling pathway. Based upon this data we considered whether inhibition of mTOR signaling was critical for the transition for senescence. We first assessed whether palbociclib treatment also correlated with mTOR inhibition in vivo. Consistently, immunohistochemistry of palbociclib-treated tumors revealed reduced P-S6K and P-Akt (S473) (Fig. 6B). The downregulation of mTOR signaling is associated with reduced protein stability of mTOR, Raptor, and Rictor (Fig. 6C) following palbociclib treatment as MG132, 26S rescued expression levels while their mRNA levels were unchanged (Fig. S2A).
We subsequently evaluated cell cycle progression following single versus simultaneous exposure for 8 days. Both rapamycin and palbociclib induced G0 arrest with palbociclib exhibiting better potency (Fig. 6D). Combining rapamycin with palbociclib induced G0 arrest in greater than 95% of cells, with no discernable apoptosis; Rb knockdown inhibited palbociclib-induced senescence and resensitized the cells vemurafenib dependent cytotoxicity demonstrating Rb-dependence (Fig. 6D and Fig. 3F). Importantly, clonogenic assays revealed that the combination of palbociclib plus rapamycin synergistically inhibited colony formation relative to either compound alone (Fig. 6E), with 3 days of the combination being as effective as palbociclib only treatment for 8-days in vemurafenib resistant melanoma cells (Fig. S2B), indicating that mTOR signaling inhibition cooperates with palbociclib to induce senescence on melanoma.
Between 10–20% of cells treated with palbociclib escape senescence suggesting either intrinsic or acquired resistance to palbociclib-induced senescence. We therefore conducted additional experiments to address the potential contribution of mTOR signaling to resistance to palbociclib-induced senescence. We isolated palbociclib resistant melanoma cells by long-term culture in increasing concentrations of drug (PR1-3) or by isolation of single colonies that grew out in cell survival assays following exposure to palbociclib (PR6-7); their resistance to palbociclib-induced cell cycle arrest was confirmed by FACS (Fig. S2C). We subsequently examined mTOR signaling in palbociclib resistant clones. Intriguingly, mTOR signaling pathway was completely recovered and refractory to palbociclib treatment in the palbociclib resistant cells (PR1, 2, 3, 6, and 7) compared to untreated cells or palbociclib sensitive cells treated with palbociclib for 8 days (Fig. 6F). We note specifically that Raptor and Rictor protein levels are lost in naïve cells, but recovered in resistant clones; however their mRNA levels remain stable in the palbociclib resistant cells (PR1, 2, 3, 6, and 7; Fig. S2A). Collectively, our data suggest that inhibition of mTOR signaling following long-term CDK4/6 inhibition contributes to senescence and that acquisition of palbociclib resistance results in re-activation of mTORC1 signaling via induction of Raptor.
Over expression of mTOR signaling overrides palbociclib-induced senescence in melanoma
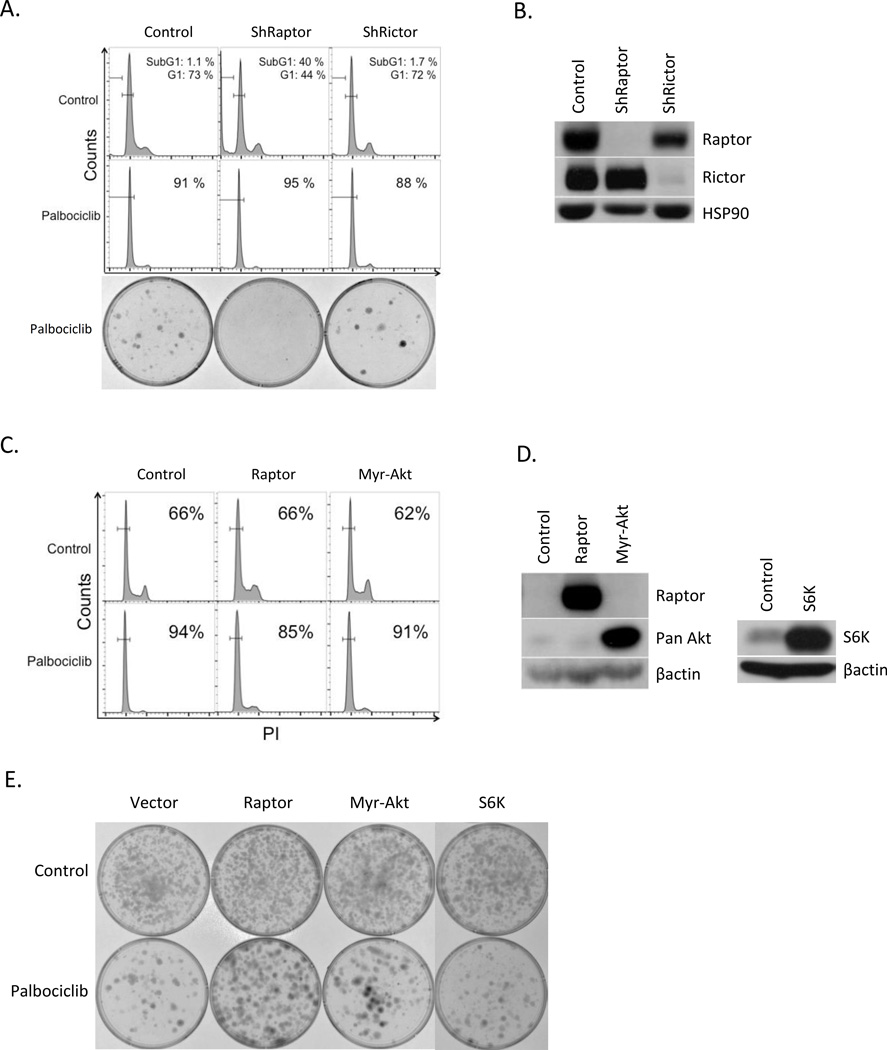
mTOR is a serine/threonine kinase that serves as the common catalytic component of two distinct complexes commonly referred to as mTORC1 and mTORC2 (32–34). Although these complexes share a number of components, they are distinguished by certain unique components including, Raptor for mTORC1 complex and Rictor for mTORC2 complex. While mTORC1 complex is a master regulator of cell proliferation and metabolism through phosphorylation of S6K and 4EBP1, mTORC2 has a more direct impact on cell proliferation, survival and metabolism through the regulation of AKT (34). Because palbociclib treatment induces Raptor and Rictor loss (and resistant cell re-acquire their expression), we chose to interrogate the contribution of mTORC1 and mTORC2 signaling pathways in context of palbociclib-induced senescence in melanoma. Raptor or Rictor were knocked down in 1205Lu melanoma cells by with validated shRNAs (Fig. 7B; (35)). Raptor knockdown was associated with cell cycle arrest while Rictor knockdown had little impact (Fig. 7A; top panels). Concurrent palbociclib treatment with Raptor knockdown for 24h induced complete G0 arrest. Extended palbociclib treatment of Raptor knockdown cells for 8 days eliminated colony outgrowth compared with palbociclib alone where approximately 20% of cells exhibited resistance to senescence and the combination of palbociclib and shRictor was similar to palbociclib alone (Fig. 7A; bottom panels). Similar results were obtained in 983BR (vemurafenib resistant) cells as well as 1205Lu cells, suggesting that mTORC1 and mTORC2 complexes, especially mTORC1 complex are critical for cell viability and proliferation in melanoma cells, and contribute to palbociclib-induced senescence.
Fig. 7. Increased Raptor abrogates palbociclib-induced senescence in melanoma.
(A) 1205Lu cells were infected with control (1st column), shRaptor (2nd column) or shRictor (3rd column panel) and treated with or without palbociclib for 8 days. FACS analysis (top panel) and clonogenic assay treated with palbociclib for 8 days (bottom panel). (B) Western analysis of cells from (A). (C) Cell cycle analysis of 1205Lu cells infected with control (1st column), Raptor (2nd column) or Myr-Akt (3rd column panel) with or without palbociclib exposure for 8 days. (D) Western analysis of the samples from (C) (left panel) and cells expressing S6K (right panel) (E) Clonogenic assay of the cells from (D) treated with or without palbociclib for 8 days.
We next determined if we could bypass palbociclib-dependent senescence by driving mTorc1 by Raptor overexpression or as a control myristoylated Akt (Myr-Akt) (Fig. 7D). Palbociclib treatment for 1 day induced G0 arrest in control cells. Cells overexpressing Raptor were refractory to palbociclib, while Myr-Akt overexpression had no impact (Fig. 7C). In addition, clonogenic assays revealed that Raptor expression inhibited palbociclib-induced senescence; in contrast overexpression of Akt or S6K, had little impact on senescence (Fig. 7E). Similar results were obtained in 983BR vemurafenib resistant cells (Fig. S3). These results indicate that inhibition of mTORC1 is essential for palbociclib-induced senescence
Discussion
Multiple genetic alterations are reported to directly contribute to the genesis of metastatic melanoma. Among these, BRAF is the most frequently mutated gene in melanoma where the BRAFV600E mutation is observed in approximately 66% of melanoma (36). Vemurafenib is a specific BRAFV600E inhibitor (14); it has significant initial clinical impact on BRAFV600E positive tumors, but has limited long-term potential due to the rapid acquisition of resistance. Thus, the development secondary treatment strategies for treatment of vemurafenib-resistant melanoma are of unquestionable importance. Based upon results described here, we propose the use of vemurafenib to reduce tumor volume/bulk followed by treatment with a CDK4/6 inhibitor to induce senescence in the residual tumor cells that are resistant to vemurafenib.
The CDK4-Rb pathway is disrupted in a majority of melanomas and mechanisms include cyclin D amplification (37); activating mutations in CDK4 (29); inactivation of Fbxo4, an E3 ligase that regulates degradation of cyclin D1 and thereby functions as a tumor suppressor (13); and deletion of gene encoding p16Ink4a (8). Based upon these and additional observations, the cyclin D1-CDK4/6-Rb axis is considered a major driver of melanomagenesis. Importantly, the observation that Rb a direct substrate for the cyclin D1/CDK4/6 kinase, is generally wild type in melanoma (>95%) suggests that continued CDK4/6 function will be required for ongoing melanoma cell proliferation and as such inhibition of CDK4/6 should result in Rb-dependent cell cycle arrest. Surprisingly, the therapeutic efficacy of the CDK4/6 inhibition in melanoma remains to be examined. Our work demonstrates that melanoma derived cells are indeed responsive to CDK4/6 inhibition. Treatment of cells with either palbociclib or enforced expression of an Ink4 family member induces rapid Rb-dependent G0 arrest. Prolonged CDK4/6 inhibition induced a switch from quiescence to senescence, “geroconversion” (Fig. 1). Geroconversion was noted to be time-dependent, was maximal at 8 days, and exhibited key characteristics of senescence, of which “permanent growth arrest” is the most clinically relevant. In a preclinical model, we noted that palbociclib treatment for 8 days was as effective as continuous exposure with regard to tumor progression. While the potential translation of this concept to the clinic remains to be carefully evaluated, xenograft tumors were characterized by reduced phospho-Rb, reduced proliferation and stained positively for SAβ-Gal consistent with on target effects of palbociclib. The robust impact of 8 days of palbociclib treatment could have important clinical implications. Clinically, reduced time of treatment will reduce side toxicities associated with CDK inhibition as well as reduce patient cost. More importantly, given the prevalence of acquired resistance to precision medicine, perhaps reduced time of patient exposure could also reduce acquisition of resistance.
Although palbociclib recently received FDA approval (38), there is little data on tumor-intrinsic or acquired resistance. However, intrinsic resistance could be predicted based upon Rb status (39). Importantly, Rb is rarely lost in melanoma. We did note that inactivation of Rb through knockdown or expression of HPV-E7 in melanoma cells, (vemurafenib sensitive or resistant), confers complete resistance to either palbociclib treatment or Ink4a expression. With regard to intrinsic and acquired resistance, resistance of a small number of clones that grew out during clonogenic cell survival assays was observed. Consistently, we also noted mice treated with palbociclib eventually succumbed to melanoma several weeks after drug removal (data not shown). Cultured cells from either source revealed resistance to palbociclib-induced growth arrest and senescence. Whether the resistance of cell lines is “acquired” or represents an intrinsic property of a certain population of cells remains to be established. We do note that resistant cell lines are both stably resistant and 100% of cells are resistant; thus they do not appear to represent outgrowth from a small fraction of stem/repopulating cells that give rise once again to 80% sensitive and 20% resistant. With regard to the mechanism of resistance, two events are worth noting. First, Rb loss was observed in some resistant cell lines (PR1, 2, and 3; Fig. S4A) generated in culture while Raptor overexpression and constitutive mTOR signaling was observed in all resistant cell lines (Fig. 6F) suggesting that mTOR signaling directly contributes to resistance. In addition, Rb suppression is not stable (Fig. S4B) while resistance to palbociclib and Raptor overexpression are both stably maintained in palbociclib resistant cells supporting a hypothesis where reduced mTOR/mTORC1 signaling with palbociclib is necessary for senescence.
While molecular mechanism whereby inhibition of CDK4/6 impacts Raptor and mTORC1 signaling remains to be further investigated, we observed that concurrent treatment of melanoma cells with palbociclib and rapamycin (or Raptor knockdown) was much more effective in triggering permanent growth arrest. Raptor was also strongly induced in palbociclib resistant clones suggesting that mTOR inhibition is critical for senescence induction with palbociclib. Finally, concurrent palbociclib+rapamycin increased melanoma sensitivity to palbociclib and coordinated CDK4/6 and mTOR inhibition both increased palbociclib-dependent senescence in 983BR cells (Fig. S2B), suggesting that perhaps reducing the acquisition of cellular resistance. Interestingly, rapamycin was suggested to suppress palbociclib-induced senescence in MEL10 cells (40). The basis of this result is unclear as all melanoma-derived cell lines tested herein exhibited increased sensitivity. Importantly, there is likely to be tissue specificity that will determine the impact of combination therapy. Indeed, we noted that rapamycin exposure opposed palbociclib-induced senescence in esophageal cancer cell lines (Fig. S5). Collectively, these data reveal the clinically relevant concept that concomitant treatment of palbociclib and rapamycin has a durable effect on senescence and inhibits acquired resistance to palbociclib in melanoma.
CDK4/6 inhibition is showing significant therapeutic promise in ER(+)/ HER2(−) advanced breast cancer (41); we suggest it should also have significant clinical value in vemurafenib-resistant metastatic melanoma. The ability to induce permanent growth arrest would reduce time of patient exposure to drug, which may in turn reduce side toxicities and potentially acquired resistance. The development of a treatment strategy however requires detailed molecular understanding of how CDK4/6 inhibition generates a senescent phenotype and in turn when resistance emerges, the precise underlying mechanism.
In this regard, one might first consider known substrates for the CDK4/6 kinase, which include Rb, p107, p130, FOXM1 (19), PRMT5 (42), and GCN5 (43) and how any of these intersect with mTOR signaling, given that mTORC1 suppression is necessary for senescence. How and whether any of these molecules contribute mTOR/mTORC1 signaling/inhibition following CDK4/6 inhibition remains to be established. Importantly, our data support a model where CDK4/6 inhibition triggers reduced expression of mTOR and the mTORC1 specific protein Raptor at the post-translational level.
A second clinically important consideration in developing a treatment strategy for the use of palbociclib emerges from the data on the concurrent use of vemurafenib and palbociclib treatment. Surprisingly concurrent treatment is not synergistic; in contrast palbociclib inhibits the cytotoxicity vemurafenib. Such an impact in vivo would prevent the efficacious debulking impact of vemurafenib treatment. At a mechanistic level, this is reflected in a failure of vemurafenib to inhibit ERK/MAPK activation. This result is not a simple matter of cell cycle arrest, as vemurafenib was effective in cells arrested via growth factor restriction (G0), nocodazole (G2/M) and hydroxyurea (G1/S). Rather, resistance to vemurafenib correlates directly with geroconversion. From a clinical point of view, our data support the concept of palbociclib as a second line therapy that could limit melanoma patient relapse following tumor de-bulking with a BRAF inhibitor such as vemurafenib (Fig. S6). Finally, concurrent use of palbociclib with an mTOR inhibitor may have increased efficacy in the induction of bona fide senescence and also limit the acquisition of palbociclib resistance.
Supplementary Material
Acknowledgments
We thank Martine F. Roussel for providing p19INK4d expression vector; Meenhard Herlyn and Patricia Brafford for providing melanoma cell lines.
Funding: CA133154 (JAD)
Footnotes
Competing interest: The authors declare no competing financial interests.
References and Notes
- 1.Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, Abdel-Malek ZA, et al. Cellular senescence in naevi and immortalisation in melanoma: a role for p16? British journal of cancer. 2006;95(4):496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436(7051):720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 3.Kannan K, Sharpless NE, Xu J, O'Hagan RC, Bosenberg M, Chin L. Components of the Rb pathway are critical targets of UV mutagenesis in a murine melanoma model. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(3):1221–1225. doi: 10.1073/pnas.0336397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackermann J, Frutschi M, Kaloulis K, McKee T, Trumpp A, Beermann F. Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer research. 2005;65(10):4005–4011. doi: 10.1158/0008-5472.CAN-04-2970. [DOI] [PubMed] [Google Scholar]
- 5.Dhomen N, Reis-Filho JS, da Rocha Dias S, Hayward R, Savage K, Delmas V, et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer cell. 2009;15(4):294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Goel VK, Ibrahim N, Jiang G, Singhal M, Fee S, Flotte T, et al. Melanocytic nevus-like hyperplasia and melanoma in transgenic BRAFV600E mice. Oncogene. 2009;28(23):2289–2298. doi: 10.1038/onc.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson B, Konrad Muller H, Handoko HY, Khosrotehrani K, Beermann F, Hacker E, et al. Differential roles of the pRb and Arf/p53 pathways in murine naevus and melanoma genesis. Pigment cell & melanoma research. 2010;23(6):771–780. doi: 10.1111/j.1755-148X.2010.00752.x. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim N, Haluska FG. Molecular pathogenesis of cutaneous melanocytic neoplasms. Annual review of pathology. 2009;4:551–579. doi: 10.1146/annurev.pathol.3.121806.151541. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson A, Tuominen R, Grafstrom E, Hansson J, Egyhazi S. High frequency of p16(INK4A) promoter methylation in NRAS-mutated cutaneous melanoma. The Journal of investigative dermatology. 2010;130(12):2809–2817. doi: 10.1038/jid.2010.216. [DOI] [PubMed] [Google Scholar]
- 10.Freedberg DE, Rigas SH, Russak J, Gai W, Kaplow M, Osman I, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. Journal of the National Cancer Institute. 2008;100(11):784–795. doi: 10.1093/jnci/djn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanki A, Li W, Colman M, Karim RZ, Thompson JF, Scolyer RA. Reduced expression of p16 and p27 is correlated with tumour progression in cutaneous melanoma. Pathology. 2007;39(6):551–557. doi: 10.1080/00313020701684409. [DOI] [PubMed] [Google Scholar]
- 12.Tsao H, Chin L, Garraway LA, Fisher DE. Melanoma: from mutations to medicine. Genes & development. 2012;26(11):1131–1155. doi: 10.1101/gad.191999.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee EK, Lian Z, D'Andrea K, Letrero R, Sheng W, Liu S, et al. The FBXO4 tumor suppressor functions as a barrier to BRAFV600E-dependent metastatic melanoma. Molecular and cellular biology. 2013;33(22):4422–4433. doi: 10.1128/MCB.00706-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim G, McKee AE, Ning YM, Hazarika M, Theoret M, Johnson JR, et al. FDA approval summary: vemurafenib for treatment of unresectable or metastatic melanoma with the BRAFV600E mutation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(19):4994–5000. doi: 10.1158/1078-0432.CCR-14-0776. [DOI] [PubMed] [Google Scholar]
- 15.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nature reviews Molecular cell biology. 2014;15(7):482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 17.Acosta JC, Gil J. Senescence: a new weapon for cancer therapy. Trends in cell biology. 2012;22(4):211–219. doi: 10.1016/j.tcb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Molecular cancer therapeutics. 2004;3(11):1427–1438. [PubMed] [Google Scholar]
- 19.Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer cell. 2011;20(5):620–634. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast cancer research : BCR. 2009;11(5):R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi KS, Rathos MJ, Joshi RD, Sivakumar M, Mascarenhas M, Kamble S, et al. In vitro antitumor properties of a novel cyclin-dependent kinase inhibitor, P276-00. Molecular cancer therapeutics. 2007;6(3):918–925. doi: 10.1158/1535-7163.MCT-06-0613. [DOI] [PubMed] [Google Scholar]
- 22.Greshock J, Feng B, Nogueira C, Ivanova E, Perna I, Nathanson K, et al. A comparison of DNA copy number profiling platforms. Cancer research. 2007;67(21):10173–10180. doi: 10.1158/0008-5472.CAN-07-2102. [DOI] [PubMed] [Google Scholar]
- 23.Kovatcheva M, Liu DD, Dickson MA, Klein ME, O'Connor R, Wilder FO, et al. MDM2 turnover and expression of ATRX determine the choice between quiescence and senescence in response to CDK4 inhibition. Oncotarget. 2015;6(10):8226–8243. doi: 10.18632/oncotarget.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rader J, Russell MR, Hart LS, Nakazawa MS, Belcastro LT, Martinez D, et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(22):6173–6182. doi: 10.1158/1078-0432.CCR-13-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leontieva OV, Blagosklonny MV. CDK4/6-inhibiting drug substitutes for p21 and p16 in senescence: duration of cell cycle arrest and MTOR activity determine geroconversion. Cell cycle (Georgetown, Tex) 2013;12(18):3063–3069. doi: 10.4161/cc.26130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherr CJ. The INK4a/ARF network in tumour suppression. Nature reviews Molecular cell biology. 2001;2(10):731–737. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]
- 27.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127(2):265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Wolfel T, Hauer M, Schneider J, Serrano M, Wolfel C, Klehmann-Hieb E, et al. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995;269(5228):1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- 29.Zuo L, Weger J, Yang Q, Goldstein AM, Tucker MA, Walker GJ, et al. Germline mutations in the p16INK4a binding domain of CDK4 in familial melanoma. Nat Genet. 1996;12(1):97–99. doi: 10.1038/ng0196-97. [DOI] [PubMed] [Google Scholar]
- 30.Chicas A, Wang X, Zhang C, McCurrach M, Zhao Z, Mert O, et al. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer cell. 2010;17(4):376–387. doi: 10.1016/j.ccr.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damsky W, Micevic G, Meeth K, Muthusamy V, Curley DP, Santhanakrishnan M, et al. mTORC1 Activation Blocks Braf(V600E)-Induced Growth Arrest but Is Insufficient for Melanoma Formation. Cancer cell. 2015;27(1):41–56. doi: 10.1016/j.ccell.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110(2):163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 33.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Current biology : CB. 2004;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 34.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science (New York, NY) 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 36.Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer research. 2002;62(23):6997–7000. [PubMed] [Google Scholar]
- 37.Sauter ER, Yeo UC, von Stemm A, Zhu W, Litwin S, Tichansky DS, et al. Cyclin D1 is a candidate oncogene in cutaneous melanoma. Cancer research. 2002;62(11):3200–3206. [PubMed] [Google Scholar]
- 38.Sherman RE, Li J, Shapley S, Robb M, Woodcock J. Expediting drug development--the FDA's new "breakthrough therapy" designation. The New England journal of medicine. 2013;369(20):1877–1880. doi: 10.1056/NEJMp1311439. [DOI] [PubMed] [Google Scholar]
- 39.Rocca A, Farolfi A, Bravaccini S, Schirone A, Amadori D. Palbociclib (PD 0332991) : targeting the cell cycle machinery in breast cancer. Expert opinion on pharmacotherapy. 2014;15(3):407–420. doi: 10.1517/14656566.2014.870555. [DOI] [PubMed] [Google Scholar]
- 40.Leontieva OV, Demidenko ZN, Blagosklonny MV. MEK drives cyclin D1 hyperelevation during geroconversion. Cell death and differentiation. 2013;20(9):1241–1249. doi: 10.1038/cdd.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. The New England journal of medicine. 2015;373(3):209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 42.Aggarwal P, Vaites LP, Kim JK, Mellert H, Gurung B, Nakagawa H, et al. Nuclear cyclin D1/CDK4 kinase regulates CUL4 expression and triggers neoplastic growth via activation of the PRMT5 methyltransferase. Cancer cell. 2010;18(4):329–340. doi: 10.1016/j.ccr.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee Y, Dominy JE, Choi YJ, Jurczak M, Tolliday N, Camporez JP, et al. Cyclin D1-Cdk4 controls glucose metabolism independently of cell cycle progression. Nature. 2014;510(7506):547–551. doi: 10.1038/nature13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.