Abstract
Long-term potentiation (LTP) shows memory-like consolidation and thus becomes increasingly resistant to disruption by low-frequency stimulation (LFS). However, it is known that nicotine application during LFS uniquely depotentiates consolidated LTP. Here, we investigated how nicotine contributes to the disruption of stabilized LTP in the hippocampal CA1 region. We found that nicotine-induced depotentiation is not due to masking LTP by inducing long-term depression and requires the activation of GluN2A-containing NMDARs. We further examined whether nicotine-induced depotentiation involves the reversal of LTP mechanisms. LTP causes phosphorylation of Ser-831 on GluA1 subunits of AMPARs that increases the single-channel conductance of AMPARs. This phosphorylation remained unchanged after depotentiation. LTP involves the insertion of new AMPARs into the synapse and the internalization of AMPARs is associated with dephosphorylation of Ser-845 on GluA1 and caspase-3 activity. Nicotine-induced depotentiation occurred without dephosphorylation of the Ser-845 and in the presence of a caspase-3 inhibitor. LTP is also accompanied by increased filamentous actin (F-actin), which controls spine size. Nicotine-induced depotentiation was prevented by jasplakinolide, which stabilizes F-actin, suggesting that nicotine depotentiates consolidated LTP by destabilizing F-actin. α7 nicotinic acetylcholine receptor (nAChR) antagonists mimicked the effect of nicotine and selective removal of hippocampal cholinergic input caused depotentiation in the absence of nicotine, suggesting that nicotine depotentiates consolidated LTP by inducing α7 nAChR desensitization. Our results demonstrate a new role for nicotinic cholinergic systems in protecting potentiated synapses from depotentiation by preventing GluN2A-NMDAR-mediated signaling for actin destabilization.
Keywords: Nicotine, hippocampus, LTP, depotentiation, ACh, α7 nicotinic acetylcholine receptor, GluN2A
1. Introduction
At first, memories are fragile, but through the process of consolidation become increasingly difficult to erase (reviewed in McGaugh, 2000, and Nadel et al., 2012). Although memory is expressed behaviorally, it is supported by changes in synaptic strength. An increase in synaptic strength is known as long-term potentiation (LTP), which is the leading cellular model for the initial encoding and subsequent stabilization of memory (Bliss and Collingridge, 1993). In hippocampal slices, LTP shows memory-like consolidation, which reaches completion within 30 min after LTP induction (Arai et al., 1990; Stäubli et al., 1998). If low-frequency stimulation (LFS) is applied immediately after LTP induction then LFS causes a complete reversal of LTP (Fujii et al., 1991; O'Dell and Kandel, 1994; Yang et al., 2008); however, the process of consolidation renders LFS ineffective when applied 30 min after LTP induction (Larson et al., 1993; Guan et al., 2006; Kramar and Lynch, 2003). Understanding the mechanisms that protect potentiated synapses from depotentiation could provide insight into memory consolidation.
The cellular and molecular processes that occur during consolidation have been investigated by inducing depotentiation with various stimulation paradigms, which activate different intracellular signaling cascades that are dependent on stimulation strength and synaptic states (Lee et al., 2000; Winder and Sweatt, 2001; Jouvenceau et al., 2003). Prolonged stimulation, which induces long-term depression (LTD) in naïve slices, can depotentiate consolidated LTP (LTD-DP) (Lee et al., 2000; Fujii and Sumikawa, 2001; Jouvenceau et al., 2003). While LFS depotentiates unconsolidated LTP (E-DP), but not consolidated LTP. However, LFS reverses consolidated LTP when applied in the presence of nicotine (Nic-DP) (Guan et al., 2006). Understanding the effect of nicotine could reveal a unique role of nicotinic cholinergic systems in LTP consolidation. Nic-DP is dependent on NMDAR activation (Guan et al., 2006) as in E-DP and LTD-DP (Huang et al., 2001; Li et al., 2007; Zhang et al., 2009). However, it remains to be determined whether all forms of depotentiation are mediated by the same NMDAR subtype and how nicotine interacts with the signaling pathway.
LTP induction increases phosphorylation, culminating in persistent increases in AMPAR-mediated currents, AMPAR surface expression and spine size (Benke et al., 1998; Hayashi et al., 2000; Yuste and Bonhoeffer, 2001; Malenka and Bear, 2004; Matsuzaki et al., 2004). All forms of depotentiation involve the NMDAR-dependent activation of protein phosphatases (O'Dell and Kandel, 1994; Huang et al., 2001; Jouvenceau et al., 2003; Guan et al., 2006), indicating the reversal of LTP mechanisms. Indeed, both E-DP and LTD-DP are associated with decreases in AMPAR-mediated currents, and E-DP is accompanied by reduced spine sizes (Yang et al., 2008; Kramár et al., 2006; Rex et al., 2009). However, depotentiation is not always associated with the reversal of LTP mechanisms and can lead to a separate form of depression (Delgado and O'Dell, 2005; Yamazaki et al., 2011).
In the current study, we investigated how nicotine contributes to the reversal of consolidated LTP at Schaffer collateral-CA1 synapses in the hippocampus. To this end, we examined whether Nic-DP involves LTD mechanisms, activation of a particular NMDAR subtype, reversal of LTP mechanisms, and desensitization of α7 nAChR subtype.
2. Materials and methods
All animal procedures were conducted in accordance with the National Institute of Health Guide for the care and use of laboratory animals and with protocols approved by the Institutional Animal Care and Use Committee at the University of California, Irvine.
2.1. Hippocampal Slice Preparation
Transverse hippocampal slices (375 μm) were prepared from P30-50 Sprague-Dawley male rats anesthetized with isoflurane. The brains were harvested and cut in ice-chilled cutting solution containing (in mM): NaCl 85, KCl 2.5, NaH2PO4 1.25, MgSO4 4, CaCl2 0.5, NaHCO3 24, sucrose 75 and glucose 25), and maintained at 30–32 °C in oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 124, KCl 5, NaH2PO4 1.25, MgSO4 2, CaCl2 2.5, NaHCO3 22, and glucose 10) for at least 1 hour before recording.
2.2. Electrophysiology
Slices were placed in a recording chamber, submerged and continuously superfused with oxygenated ACSF at 30 °C. A NiCr bipolar stimulating electrode was placed to stimulate the Schaffer collateral/commissural pathway. Field EPSPs (fEPSP) were recorded from the stratum radiatum of the CA1 region using glass electrodes filled with 2M NaCl. Stimuli were short current pulses (0.2 ms duration) delivered every 20 seconds. The strength of the stimulus was adjusted to elicit fEPSPs that were 30–50% of the maximum response. The intensity and duration of each stimulus pulse remained invariant thereafter. Recorded signals were amplified (A-M Systems, Sequim, WA, USA), digitized, stored on a computer and analyzed using NAC 2 software (Thetaburst Corp., Irvine, CA, USA). Baseline responses were recorded for at least 15 minutes to establish stable baseline conditions. LTP was then induced by theta-burst stimulation (TBS, 10 theta bursts with each burst containing 4 pulses at 100 Hz, individual bursts were separated by 200 ms); pulse duration was doubled during TBS. Depotentiation was induced either 6 or 30 minutes after LTP induction by low frequency stimulation (LFS, 5 Hz train for 1 min; three trains were used with the interval between trains set at 1 min). To evaluate the magnitude of LTP, the mean value for the slope of fEPSPs recorded at 20-30 min or 55-60 min after stimulation was calculated and expressed as a percentage of the mean value of the initial baseline slope of fEPSPs. The levels of LTP reversal were calculated by comparing the magnitudes of LTP between 20–30 min and 55–65 min (50-60 min in the case of slices used for western blotting) from each slice and expressed as follows: % LTP remaining = (% potentiation after LFS – 100) × 100/(% potentiation before LFS – 100).
2.3. Surgery
A selective lesion of cholinergic neurons was performed using the cholinergic toxin 192-IgG-saporin (Wiley et al., 1991). Rats (~P30) were anesthetized with ketamine (60 mg/kg) and Xylazine (10 mg/kg), placed in a stereotaxic instrument, and 0.4 μl 192-IgG-Saporin (192-SAP, Cat. # IT-01; Advanced Targeting Systems; 3 mg/ml) or phosphate-buffered saline (PBS) was infused into the medial septum by four 0.1 μl infusions (from bregma: P 0.5 mm, L ± 0.45 mm, V 7.3 or 6.0 mm). Animals were allowed to recover and maintained for at least 14 days before used in electrophysiology experiments. Hippocampal slices from PBS-injected and 192-SAP-injected rats were stained for acetylcholinesterase (AChE) after electrophysiological recording as described previously (Yamazaki et al., 2002).
2.4. Drugs Application
Nicotine (Sigma, Cat. # N3876), the GluN2A-NMDAR selective antagonist NVP-AAM077 (Novartis Pharma) and the GluN2B-NMDAR selective antagonist ifenprodil (Tocris, Cat. # 0545) were dissolved in ACSF and used at concentrations of 1 μM, 50 nM, and 3 μM respectively. Jasplakinolide (200 nM; Tocris, Cat. # 2792) was added immediately after TBS and remained present until the end of the experiment. The caspase-3 inhibitor Z-DEVD-FMK (R&D Systems, Cat. # FMK004) was dissolved in DMSO and added to the holding chamber at a concentration of 2 μM for at least two hours. Unless stated otherwise, drugs were bath-applied 10 min before and during LFS.
2.5. Western Blot Analysis
After the induction of LTP or depotentiation, CA1 regions were isolated and immediately placed in 1x SDS solution (1x Tris-Glycine SDS sample buffer + 5% 2-mercaptoethanol) and heated to 95°C. Each CA1 slice was then homogenized by pipette and frozen at −80°C. Homogenates were later thawed and centrifuged for 5 minutes. Samples were loaded onto SDS-PAGE gels (4-12% Bis-Tris gel) and run. The resulting gels were transferred onto PVDF membranes. Membranes were blocked for 1 hour in 5% BSA and 0.1% TBST then immersed in anti-phospho-GluA1-S831 (Upstate, Cat. # 06-772), anti-phospho-GluA1-S845 (Upstate, Cat. # 06-773), or anti-phospho-GluA1-S845 (Cell Signaling, Cat. # 8084). The appropriate secondary antibodies coupled to horseradish peroxidase were used, and immunoreactive bands were visualized using the Pierce Super-signal chemiluminescent substrate (Pierce) and analyzed using Kodak Image Station 400MM Pro with Molecular Imaging Software. Blots were then stripped using a solution of 2% glycogen with 1% SDS (pH 2.0), and reprobed for the total-GluA1 with anti-GluA1 antibody (Upstate, Cat. # 06-306). The relative amount of GluA1 phosphorylation was determined by calculating the ratio of signals (the phosphorylation site-specific-antibody signal/the total GluA1 signal). The ratio was then used for statistical analysis.
2.6. Statistics
Data are expressed as the mean ± SEM. Statistical analyses using one-way ANOVA and a Student's t-test were applied. The overall ANOVA was followed by post hoc Tuckey HSD test to identify which groups were significantly different.
3. Results
3.1. Nicotine-induced depotentiation involves the reversal of LTP mechanisms
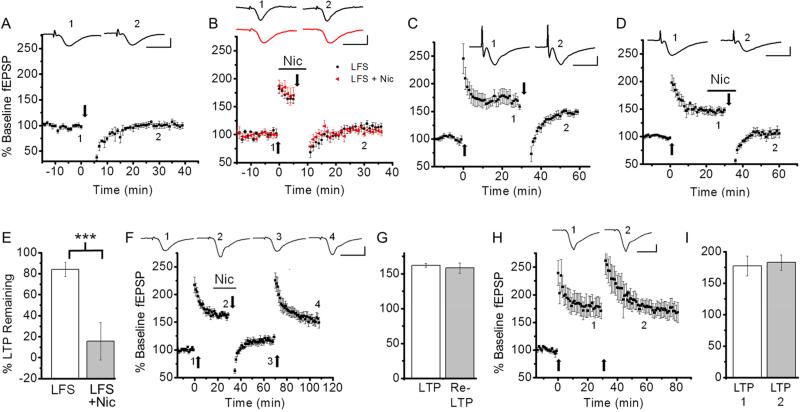
Depotentiation can occur either by inducing LTD or reversing LTP mechanisms. We first investigated whether nicotine contributes to depotentiation by inducing LTD. LFS applied to non-potentiated slices caused a transient reduction in fEPSP, which returned to baseline levels within 30 minutes (Fig. 1A; baseline, 96.2 ± 2.4% vs. 20-30 min after LFS, 100.9 ± 5.6%, n=6, p=0.53), confirming that LFS alone does not induce LTD. LFS delivered to potentiated slices prior to consolidation caused the reversal of LTP, and a similar magnitude of depotentiation occurred when LFS was applied in the presence of nicotine (1 μM) (Fig. 1B; % of baseline fEPSP slope; LFS alone, 113.4 ± 13.0%, n=5 vs. LFS + nicotine, 106.8 ± 4.3%, n=5, p=0.93). These results indicate that LFS delivered in the absence or presence of nicotine reverses LTP without inducing LTD. Thus, nicotine does not contribute to depotentiation by inducing LTD.
Fig. 1. Nicotine-induced depotentiation occurred by reversing LTP mechanisms.
A. Delivery of LFS did not result in LTD. B. Bath application of nicotine had no effect on LFS-induced reversal of unconsolidated LTP. Six minutes after LTP induction by TBS, LFS was applied in the absence or presence of nicotine. C. LFS had no effect on consolidated LTP. Thirty minutes after LTP induction, LFS was delivered. D. Nicotine application during LFS depotentiated consolidated LTP. E. Summary data comparing LTP remaining after LFS in the absence and presence of nicotine. F. After nicotine-induced depotentiation, reapplication of TBS caused repotentiation. G. Summary data comparing the magnitudes of the first LTP and the second LTP induced following depotentiation. H. Delivery of the first TBS induced a saturated LTP. The second TBS application had no further effect on the magnitude of LTP. I. Summary data comparing the magnitudes of the first LTP and the second LTP induced without depotentiation. In this figure and the following figures, traces above each graph are representative waveforms recorded at the time indicated by the number and scale bars are 10 ms and 1 mV. TBS and LFS were delivered at the time indicated by the upward and downward arrows, respectively, and the horizontal bar indicates bath application of nicotine (1 μM). ***P<0.001.
If synapses expressing LTP are depotentiated by the reversal of LTP mechanisms then they can be repotentiated by the same LTP mechanisms (O'Dell and Kandel, 1994). However, synapses depotentiated by other mechanisms cannot be repotentiated (Delgado and O'dell, 2005). Thus, we next examined whether Nic-DP involved the reversal of LTP mechanisms by monitoring the induction of repotentiation. As reported previously (Guan et al., 2006), application of nicotine (1 μM) during LFS reversed consolidated LTP (Fig. 1C-E; % of LTP remaining; LFS alone, 84.1 ± 6.6%, n=5 vs. LFS + nicotine, 22.0 ± 8.9%, n= 7, p<0.001). We then reapplied LTP-inducing TBS and found that repotentiation reached levels similar to the initial potentiation (Fig. 1F, G; % of baseline fEPSP slope; first LTP, 162.2 ± 2.9% vs. second LTP, 158.4 ± 7.4%, n=5, p=0.64). To ensure that the initial TBS induced a saturating level of potentiation, we delivered the second TBS after the first LTP without depotentiation. We found that the magnitudes of the first and the second LTP were very similar (Fig. 1H, I; % of baseline fEPSP slope; first LTP, 177.1 ± 15.7% vs. second LTP, 182.7 ± 12.3%, n=5, p=0.79), indicating that the first TBS induced a saturated level of LTP. These findings suggest that the second TBS-induced potentiation, following nicotine-induced reversal of the first LTP, occurs at the depotentiated synapses, but not synapses that had not experienced LTP and Nic-DP. Together, these findings provide support for the idea that Nic-DP is mediated by the reversal of LTP mechanisms.
3.2. Nicotine-induced depotentiation requires GluN2A-NMDAR activation
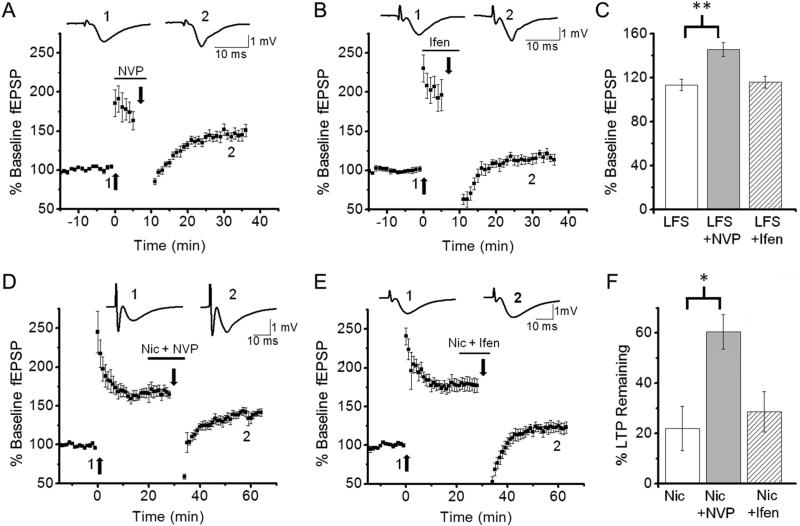
All forms of depotentiation are dependent on NMDAR activation (Huang et al., 2001; Massey et al., 2004; Zhu et al., 2005; Guan et al., 2006; Li et al., 2007; Zhang et al., 2009). GluN2A-NMDARs and GluN2B-NMDARs are the major subtypes of NMDAR expressed in the hippocampus (Monyer et al., 1994) and LTD-DP is known to require GluN2A-NMDAR activation (Massey et al., 2004; Liu et al., 2004). However, it is unknown whether E-DP and Nic-DP also require common GluN2A-NMDAR-mediated signaling. LFS-mediated NMDAR activation reverses unconsolidated LTP (i.e., E-DP), but LFS-mediated NMDAR activation alone is not sufficient for reversing consolidated LTP (Larson et al., 1993; Kramar and Lynch, 2003; Guan et al., 2006). To investigate whether NMDAR-mediated signaling for depotentiation is altered during LTP consolidation, we first investigated whether E-DP is induced in the presence of the GluN2A-selective antagonist NVP-AAM077 (NVP; 50 nM) or the GluN2B-selective antagonist ifenprodil (3 μM). We found that the induction of E-DP was prevented in the presence of NVP (Figs. 1B, 2A, C; % of baseline fEPSP slope; LFS alone, 113.4 ± 5.3%, n=5 vs. LFS + NVP, 145.4 ± 6.2%, n=5, p<0.01), but not in the presence of ifenprodil (Figs. 1B, 2B, C; % of baseline fEPSP slope; LFS alone, 113.4 ± 5.3%, n=5 vs. LFS + ifenprodil, 115.9 ± 5.5%, n=5, p=0.99). These results suggest that E-DP, like LTD-DP, depends on GluN2A-NMDAR activation.
Fig. 2. Depotentiation of unconsolidated and consolidated LTP required GluN2A-NMDAR activation.
A-C. The GluN2A-NMDAR selective antagonist NVP, but not the GluN2B-NMDAR selective antagonist ifenprodil, prevented LFS-induced depotentiation of unconsolidated LTP. LFS was delivered 6 minutes after LTP induction in the presence of NVP (A) or ifenprodil (B). C. Summary data comparing the effects of the antagonists on LFS-induced depotentiation of unconsolidated LTP. D-F. Nicotine-induced depotentiation of consolidated LTP was blocked by NVP (D), but not ifenprodil (E). LFS was delivered 30 minutes after LTP induction in the presence of nicotine + NVP (D) or nicotine + ifenprodil (E). F. Summary data comparing the effects of the antagonists on nicotine-induced depotentiation of consolidated LTP. *P<0.05, **P<0.01
Nicotine application during LFS causes depotentiation of consolidated LTP (i.e., Nic-DP) that is blocked by the NMDAR antagonist 2-amino-5-phosphonopentanoate, but not ifenprodil (Guan et al., 2006), indicating the requirement of either GluN2A-NMDAR signaling or combined signaling of GluN2A- and GluN2B-NMDARs. To determine the role of GluN2A-NMDAR activation in Nic-DP, we next investigated the effects of NVP on Nic-DP. We found that NVP blocked the nicotine-induced depotentiation (Figs. 1D, 2D, F; % of LTP remaining; LFS-nicotine alone, 22.0 ± 8.9%, n=7 vs. LFS-nicotine + NVP, 60.5 ± 6.8%, n=5, p<0.05). Additionally, we confirmed that ifenprodil had no significant effect on the reversal (Figs. 1D, 2E, F; % of LTP remaining; LFS-nicotine alone, 22.0 ± 8.9%, n=7 vs. LFS-nicotine + ifenprodil, 28.6 ± 8.1%, n=6, p=0.99). These results indicate that the requirement of GluN2A-NMDAR-mediated signaling is not altered during LTP consolidation, but this signaling alone becomes ineffective in the reversal of consolidated LTP, necessitating the effect of nicotine for depotentiation.
3.3. Nicotine-induced depotentiation occurs without dephosphorylation of Ser-831 on GluA1 of AMPARs
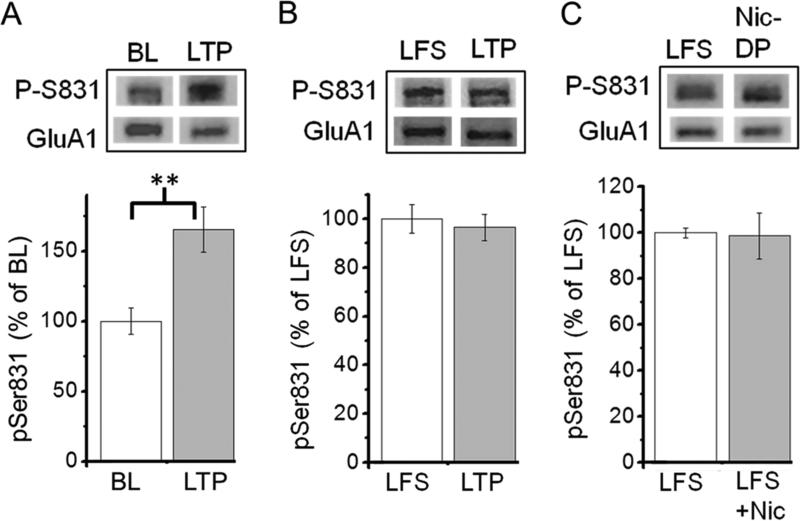
LTP induction is accompanied by an increase in the single-channel conductance of AMPARs, which is mediated by Ca2+/calmodulin-dependent protein kinase II (CaMKII)-dependent phosphorylation of Ser-831 on GluA1 of AMPARs. This is thought to be one of LTP expression mechanisms (Huang et al., 2001; Lu and Roche, 2011). Because both LTD-DP and E-DP are associated with dephosphorylation of Ser-831 (Lee et al., 2000; Huang et al., 2001), we next investigated whether Nic-DP is also mediated through dephosphorylation of Ser-831. To monitor changes in phosphorylation of Ser-831 after LTP and nicotine-induced depotentiation, we used western blot analysis with anti-GluA1 phospho Ser-831 antibody and anti-GluA1 antibody. In agreement with previous studies (Lee et al., 2000; Huang et al., 2001), we found that samples taken from potentiated CA1 slices showed a significant increase in Ser-831 phosphorylation as compared to CA1 slices that received baseline stimulation only (Fig. 3A; % change relative to baseline slices; baseline slices, 100 ± 9.4%, n=4 vs. LTP slices, 176.7 ±1 5.3%, n=4, p<0.01). Increased levels of Ser-831 phosphorylation in potentiated (LTP) slices were not altered following LFS, which did not induce depotentiation (Fig. 3B; % change relative to LFS slices; LFS slices, 100 ±5 .9%, n=4 vs. LTP slices, 96.5 ± 5.5%, n=5, p=0.68). We then compared levels of Ser-831 phosphorylation between slices that received LFS in the absence or presence of nicotine. We found that Nic-DP occurred without altering levels of Ser-831 phosphorylation (Fig. 3C; % change relative to LFS alone slices; LFS alone slices, 100 ± 2.1%, n=4 vs. LFS + nicotine, 98.7 ± 10%, n=5, p=0.92). These results strongly suggest that nicotine-induced depotentiation is not mediated by dephosphorylation of Ser-831.
Fig. 3. Nicotine-induced depotentiation was not accompanied by dephosphorylation of Ser-831 on GluA1 of AMPARs.
A. LTP induction increased the level of Ser-831 phosphorylation. Western blot analysis with anti-GluA1 phospho Ser-831 antibody and anti-GluA1 antibody was carried out with protein samples from CA1 slices, which received baseline stimulation only (BL) or in which LTP was induced (LTP). Representative phospho-Ser-831 (P-S831) and GluA1 bands are shown (top). Phosphorylation at Ser-831 was analyzed by normalizing the signal from phosphorylation site-specific antibody to the total amount of GluA1 measured using anti-GluA1 antibody. Summary data are presented (bottom). B. LFS delivered to potentiated slices did not alter the level of Ser-831 phosphorylation. Protein samples from LTP slices (LTP) and LFS delivered-LTP slices (LFS) are compared. C. Nicotine-induced depotentiation did not change the level of Ser-831 phosphorylation. LFS was delivered to slices in the absence or presence of nicotine after LTP induction. Protein samples from LFS alone slices (LFS) and LFS + nicotine slices (Nic-DP) are compared. **P<0.01.
3.4. Nicotine-induced depotentiation does not involve AMPAR internalization via dephosphorylation of Ser-845 on GluA1 or caspase-3 activity
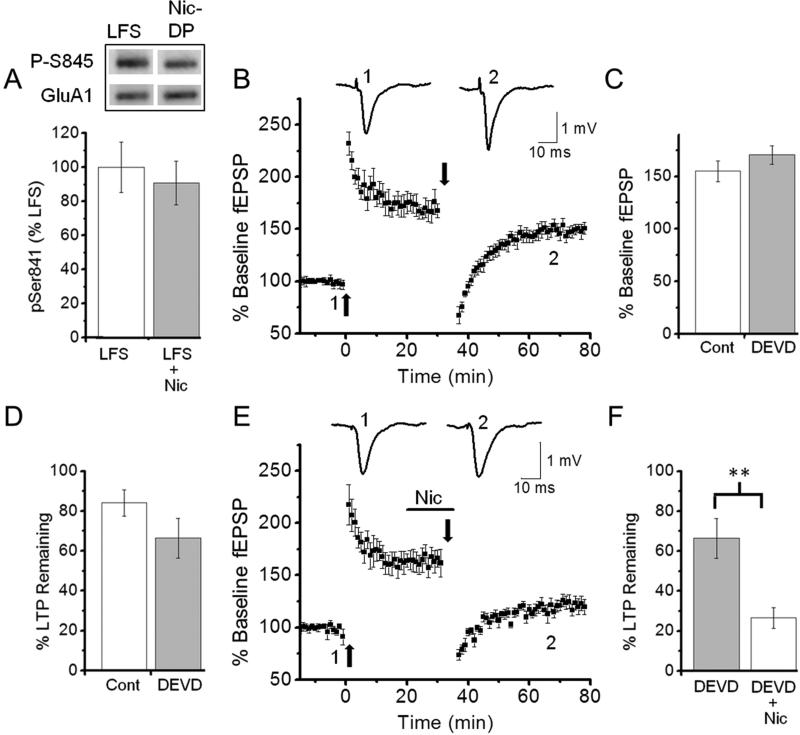
PKA-mediated phosphorylation of the GluA1 subunit at the Ser-845 site is involved in AMPAR trafficking (Oh et al., 2006; Man et al., 2007; He et al., 2011). Dephosphorylation of Ser-845 is associated with AMPAR internalization and is required for NMDA-dependent LTD (Lee et al., 2010; He et al., 2011). Although both LTD-DP and E-DP occur without altering phosphorylation levels of Ser-845 (Lee et al., 2000; Huang et al., 2001), we investigated the possibility that Nic-DP involves dephosphorylation of Ser-845 by western blot analysis with anti-GluA1 phospho Ser-845 antibody and anti-GluA1 antibody. We found that there was no difference in phosphorylation levels of Ser-845 between the slices that received LFS alone following LTP and those depotentiated by application of nicotine during LFS (Fig. 4A; % change related to LFS alone; LFS alone, 100 ± 14.8%, n=5 vs. LFS + nicotine, 91.0 ± 12.9%, n=5, p=0.66). These findings indicate that Nic-DP, like E-DP and LTD-DP, does not require dephosphorylation of Ser-845 on GluA1.
Fig. 4. Nicotine-induced depotentiation occurred without Ser-845 dephosphorylation on GluA1 or caspase-3 activity.
A. Nicotine-induced depotentiation did not change the level of Ser-845 phosphorylation. Western blot analysis with anti-GluA1 phospho Ser-845 antibody and anti-GluA1 antibody was carried out with protein samples from CA1 slices, which received LFS in the absence (LFS) or presence of nicotine (Nic-DP or LFS + Nic) after LTP. Representative phospho-Ser-845 and GluA1 bands are shown (top). Phosphorylation at Ser-845 was normalized by the total amount of GluA1 and summary data are presented (bottom). B-D. The caspase-3 inhibitor DEVD had no effect on LTP induction (B, C) and depotentiation (B, D). Hippocampal slices preincubated for at least 2 hours with DEVD (2 μM) were used. C. Summary data comparing the magnitudes of LTP induced in untreated and DEVD-treated slices. D. Summary data comparing LTP remaining between untreated and DEVD-treated slices 20-30 minutes after LFS. E, F. DEVD had no effect on nicotine-induced depotentiation. E. LFS was delivered in the presence of nicotine in DEVD-treated slices 30-35 minutes after LTP induction. F. Summary data comparing LTP remaining in DEVD-treated slices after LFS in the absence or presence of nicotine. **P<0.01
Internalization of AMPARs occurring during LTD requires caspase-3 activity, which indirectly activates LTD-promoting glycogen synthase kinase-3β by inactivating Akt1 (Peineau et al., 2007; Li et al., 2010). To determine whether Nic-DP utilizes the caspase-3-dependent process, we examined the effect of the irreversible caspase-3 inhibitor Z-DEVD-FMK (DEVD) on depotentiation. Preincubation of slices with DEVD did not affect LTP induction (Figs. 1C, 4B, C; % of baseline fEPSP slope; untreated, 156.7 ± 8.6%, n=5 vs. DEVD-treated, 170.3 ± 8.7%, n=5, p=0.58). Furthermore, DEVD-treated slices underwent the normal LTP consolidation process, becoming resistant to LFS-induced depotentiation (Figs. 1C, 4B, D; % of LTP remaining; LFS alone, 84.1 ± 6.6%, n=5 vs. LFS + DEVD, 66.4 ± 9.9%, n=5, p=0.18). In addition, the treatment did not block Nic-DP (Fig. 4B, E, F; % of LTP remaining; LFS + DEVD, 66.4 ± 9.9%, n=5 vs. LFS + DEVD + nicotine, 26.5 ± 5.3%, n=6, p<0.01). These results indicate that Nic-DP does not involve caspase-3-dependent processes.
3.5. Nicotine-induced depotentiation requires destabilization of F-actin
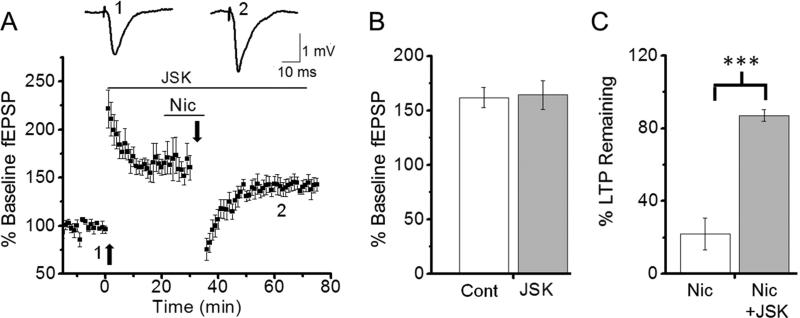
LTP is associated with the enlargement of dendritic spines (Yuste and Bonhoeffer, 2001; Yang et al., 2008; Bellot et al., 2014; Bosch et al., 2014), whereas spine shrinkage is considered a major factor leading to LTD and several forms of DP (Okamoto et al., 2004; Yang et al., 2008). Actin is the main cytoskeletal component of dendritic spines and exists in either a polymerized filamentous state (F-actin) or a depolymerized globular state (Lang et al., 2004). Increases in F-actin correlate with increased spine size, whereas decreasing F-actin causes spine shrinkage (Fukazawa et al., 2002; Zhou et al., 2004; Bosch et al., 2014). Because inhibiting actin depolymerization prevents LTD-DP and E-DP (Yang et al., 2008, Rex et al., 2009; Peng et al., 2010), we examined whether blocking actin depolymerization also prevents Nic-DP. We used jasplakinolide (JSK; 200 nM), which is known to stabilize F-actin, and found that JSK had no effect on LTP induction (Figs. 1D, 5A, B; % of baseline fEPSP slope; untreated, 161.8 ± 9.1%, n=5 vs. JSK-treated, 164.2 ± 13.3%, n=5, p=0.89). However, JSK-treated slices did not undergo Nic-DP (Figs. 1D, 5A, C; % of LTP remaining; LFS + nicotine, 22.0 ± 8.9%, n=7 vs. LFS + nicotine + JSK, 87.0 ± 3.2%, n=5, p<0.001). These results indicate that Nic-DP involves spine shrinkage.
Fig. 5. Inhibition of actin depolymerization blocked nicotine-induced depotentiation of consolidated LTP.
A-C. Jasplakinolide (JSK) had no effect on LTP induction (A, B), but prevented nicotine-induced depotentiation of consolidated LTP (A, C). A. TBS and LFS were delivered in the presence of JSK (200 nM) as indicated by the horizontal bar. Nicotine (1 μM) was also present during LFS. B. Summary data comparing the magnitudes of LTP induced in slices in the absence and presence of JSK. C. Summary data comparing LTP remaining in unexposed and JSK-exposed slices after LFS in the presence of nicotine. ***P<0.001
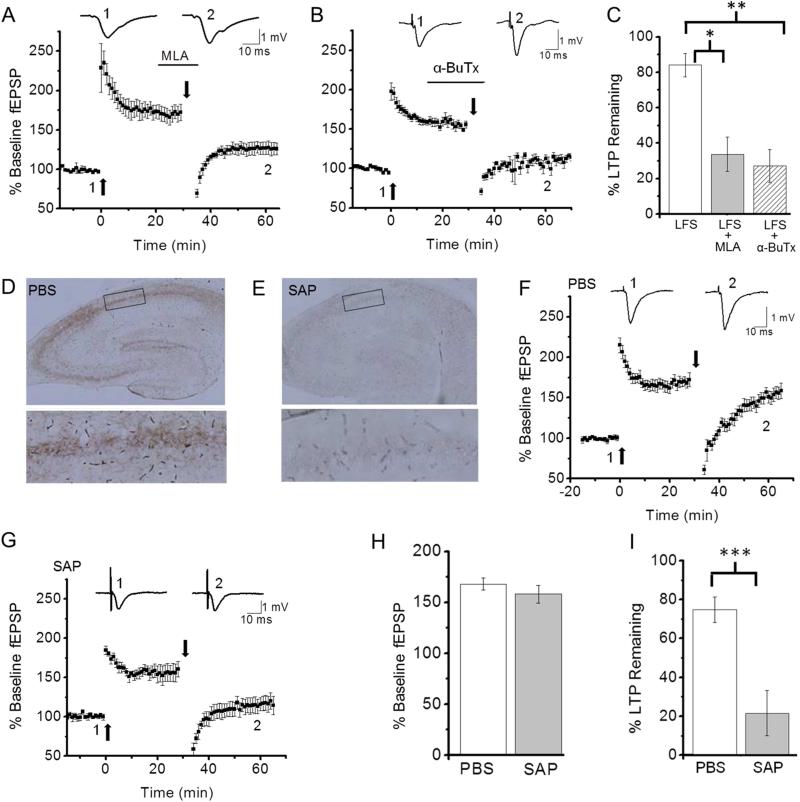
3.6. Nicotine and blockade of α7 nAChR activation depotentiate consolidated LTP
Bath application of nicotine (1 μM) used in current experiments has been shown to desensitize α7 nAChRs (McQuiston and Madison, 1999; Alkondon et al., 2000; Quick and Lester, 2002). We have previously shown that the α7 nAChR selective antagonist methyllycaconitine (MLA; 10-100 nM) causes depotentiation of consolidated LTP, suggesting that the effect of nicotine is mediated by desensitization of α7 nAChRs (Guan et al., 2006). To further explore this possibility, we carried out additional experiments. We first confirmed that MLA (20 nM) reversed consolidated LTP (Figs. 1C, 6A, C; % of LTP remaining; LFS alone, 84.1 ± 6.6%, n=5 vs. LFS + MLA, 33.7 ± 9.7%, n=6, p<0.05). In addition, we found that another α7 nAChR selective antagonist, α-bungarotoxin (α-BuTX; 100 nM), caused depotentiation (Figs. 1C, 6B, C; % of LTP remaining; LFS alone, 84.1 ± 6.6%, n=5 vs. LFS + α-BuTX, 27.3 ± 9.3%, n=5, p<0.01). These results suggest that ACh is released during LFS to prevent depotentiation via α7 nAChR activation. If this is the case, in slices lacking hippocampal cholinergic input LTP should remain vulnerable to disruption 30 minutes after induction. Thus, we tested this possibility. The medial septum, which provides up to 90% of cholinergic input to the hippocampus (Duter et al., 1995), was targeted with the selective cholinergic immunotoxin 192-SAP or PBS (control). Removal of cholinergic afferents was confirmed by the loss of staining for the cholinergic axonal marker AChE in hippocampal slices (Fig. 6D, E). Following the lesion of cholinergic projections to the hippocampus, we examined whether LFS alone induces the reversal of consolidated LTP. We found that similar magnitudes of LTP were induced in hippocampi from PBS-injected and 192-SAP-infused rats (Fig. 6F-H; % of baseline fEPSP slope; PBS, 167.9 ± 5.9%, n=9 vs. 192-SAP, 158.1 ± 8.8%, n=5, p=0.35). However, LFS delivered 30 min after LTP induction reversed LTP in the 192-SAP-infused hippocampi, but not the PBS-injected control hippocampi (Fig. 6F, G, I; % of LTP remaining; PBS, 74.7 ± 6.5%, n=9 vs. 192-SAP, 21.6 ± 11.7%, n=5, p<0.001). Taken together, our findings suggest that ACh released during LFS protects potentiated synapses from depotentiation via α7 nAChR activation and nicotine prevents endogenous ACh signals by inducing α7 nAChR desensitization.
Fig. 6. Blockage of α7 nAChR activation reversed consolidated LTP.
A-C. α7 nAChR-selective antagonists mimicked the effect of nicotine. A. Application of MLA (20 nM) during LFS depotentiated consolidated LTP. B. Application of α-bungarotoxin (α-BuTX; 100 nM) during LFS reversed consolidated LTP. C. Summary data of the effect of α7 nAChR-selective antagonists on depotentiation of consolidated LTP. D, E. Representative images of AChE staining in the hippocampus from PBS (D) or 192-SAP (E) infused rats. Enlarged images of AChE staining in the hippocampus region indicated by the black box in D (top) and E (top) are shown in D (bottom) and E (bottom). F. Reversal of consolidated LTP did not occur in the hippocampus from PBS-infused rats as in the hippocampus from naïve rats. G. LTP and depotentiation of consolidated LTP were induced in the hippocampus from 192-SAP-infused rats. H. Summary data comparing the magnitudes of LTP induced in slices from PBS- and 192-SAP (SAP)-infused rats. I. Summary data comparing the effect of LFS on consolidated LTP in hippocampal slices from PBS- and 192-SAP-infused rats. *P<0.05, **P<0.01, ***P<0.001
4. Discussion
ACh is an important modulator of many forms of synaptic plasticity and memory (Deiana et al., 2011; Kenney et al, 2011). In the current study, we used nicotine to identify a new role for ACh in protecting potentiated synapses from depotentiation during theta pattern stimulation in the hippocampal CA1 region. In hippocampal slices, nicotine at 1 or 10 μM, but not 0.1 μM, facilitates LTP induction, and 1 μM nicotine has no effect on baseline fEPSPs (Fujii et al., 1999; Nakauchi et al., 2007). Furthermore, 1 μM, but not 0.1 μM, nicotine facilitates LTD and LTP reversal (Fujii and Sumikawa, 2001; Guan et al., 2006; unpublished results). Based on these findings, we used 1 μM nicotine in the current study. We have previously shown that nicotine (1 μM) and MLA block cholinergic synaptic currents elicited by LTP-inducing stimulation in hippocampal slices, suggesting that 1 μM nicotine desensitizes α7 nAChRs (Yamazaki et al., 2005). Others have also previously demonstrated that bath application of nicotine (0.1-5 μM) desensitizes α7 nAChR-mediated currents in hippocampal slices (Frazier et al., 1998a; McQuiston and Madison, 1999; Alkondon et al., 2000). However, it is also known that the agonist effect of nicotine on α7 nAChRs can potentiate fEPSPs in the hippocampus (Mann and Greenfield, 2003). The finding might be explained if the application of nicotine causes a brief activation of α7 nAChRs before receptor desensitization and this brief activation is sufficient to trigger intracellular signaling for fEPSP potentiation. Thus, nicotine acts not only as an agonist, but also as a desensitizing agent at α7 nAChR. Our current findings suggest that the role for ACh in protecting potentiated synapses from depotentiation during theta pattern stimulation is mediated through α7 nAChR activation, and nicotine prevents this signaling via inducing α7 nAChR desensitization.
All forms of depotentiation require phosphatase activity (O'Dell and Kandel, 1994; Lee et al., 2000; Huang et al., 2001; Jouvenceau et al., 2003; Guan et al., 2006; Yang et al., 2008), suggesting the reversal of phosphorylation as a mechanism of depotentiation. Ca2+ entry through NMDARs during LTP-inducing TBS activates CaMKII to increase single-channel conductance of AMPARs via Ser-831 phosphorylation of GluA1. Indeed, both LFS prior to LTP consolidation (E-DP) and prolonged LTD-inducing stimulation (LTD-DP) lead to the reversal of Ser-831 phosphorylation (Huang et al., 2001; Lee et al., 2000). However, in the present study, we found that application of LFS after LTP consolidation no longer induces the reversal of Ser-831 phosphorylation. Furthermore, the presence of nicotine during LFS induces the reversal of consolidated LTP (Nic-DP), but does not cause the reversal of Ser-831 phosphorylation. These results indicate that LFS-induced signaling that shifts a balance between kinase and phosphatase activity towards dephosphorylation of Ser-831 becomes ineffective after consolidation. However, it remains unknown what changes occur during LTP consolidation that prevent LFS from causing Ser-831 dephosphorylation. The current study shows that nicotine-induced depotentiation does not require dephosphorylation of Ser-831 phosphorylated by LTP. However, because our previous study demonstrates that phosphatase inhibitors prevent nicotine-induced depotentiation (Guan et al., 2006), the results suggest that nicotine-induced depotentiation requires dephosphorylation of phosphoproteins other than AMPARs. It is possible that LTP-induced phosphorylation of signaling proteins increases actin polymerization and this is reversed by phosphatase activity.
LTD induction requires NMDAR-dependent activation of the phosphatases PP2B (calcineurin) and PP1 (Malenka and Bear, 2004) and involves internalization of AMPARs from synapses (Malenka and Bear, 2004; Collingridge et al., 2004; Shepherd and Huganir, 2007). Dephosphorylation of Ser-845 and caspase-3 activity are involved in AMPAR internalization during LTD (Oh et al., 2006; Man et al., 2007; Li et al., 2007; Lee et al., 2010; He et al., 2011). However, our results show that Nic-DP, like E-DP and LTD-DP, does not require dephosphorylation at Ser-845 of GluA1 and caspase-3 activity. The control mechanism of AMPAR trafficking is complex and previous studies indicate that AMPAR trafficking additionally involves MAPK (Liang et al., 2008) and Rap-JNK (Zhu et al., 2005; Yang et al., 2011) pathways. Inhibitors of p38 MAPK, Rap2, or JNK all impair either E-DP or LTD-DP (Zhu et al., 2005; Liang et al., 2008; Yang et al., 2011). The involvement of these signaling pathways in Nic-DP was not investigated and remains unknown. However, because our findings suggest that Nic-DP is mainly mediated by actin destabilization, the contribution of AMPAR internalization via MAPK or Rap-JNK pathway to Nic-DP, if any, would be small. Both nicotine and MLA facilitate LTD induction (Nakauchi and Sumikawa, 2014), suggesting that LTD-inducing stimulation activates α7 nAChRs that normally suppresses LTD induction. However, despite similar roles of α7 nAChR activation in LTD and Nic-DP, our current study demonstrated that Nic-DP does not involve nicotine-dependent LTD induction.
LTP is accompanied by increased levels of F-actin and enlarged spines (Yuste and Bonhoeffer, 2001; Yang et al., 2008; Bellot et al., 2014; Bosch et al., 2014). LFS applied within minutes after LTP induction induces E-DP and leads to decreased levels of F-actin and spine shrinkage (Kramár et al., 2006; Yang et al., 2008). However, the same LFS applied after LTP consolidation neither induces depotentiation nor affects spine size (Yang et al., 2008; Rex et al., 2009). It is unknown what changes occur during consolidation that prevent LFS from causing depotentiation and spine shrinkage. F-actin increased following LTP induction becomes gradually stabilized over the same period that LTP consolidates (Yuste and Bonhoeffer, 2001; Kramár et al., 2006; Chen et al., 2007; Lynch et al., 2007). Thus, it has been suggested that the increase in spine size accompanying LTP takes time to stabilize and this stabilization is responsible for LTP consolidation (Lynch et al., 2007; Bosch et al., 2014). Indeed, blocking actin polymerization eliminates consolidation of LTP (Rex et al., 2009; Ramachandran and Frey, 2009), whereas inhibiting actin depolymerization prevents LFS-induced E-DP (Rex et al., 2009). These findings indicate that LFS-mediated signaling for actin depolymerization is interrupted after consolidation. LFS-mediated NMDAR activation reverses unconsolidated LTP, but is unable to reverse consolidated LTP (Larson et al., 1993; Kramar and Lynch, 2003; Guan et al., 2006). These results indicate that the NMDAR-mediated signaling is interrupted after LTP consolidation. Our current study demonstrates that delivery of LFS in the presence of nicotine induces the reversal of consolidated LTP by inducing actin depolymerization. Furthermore, our results demonstrate that this nicotine-induced depotentiation of consolidated LTP requires NMDAR activation (Guan et al., 2006). These results suggest that NMDAR-mediated signaling for actin depolymerization is not altered after consolidation, but consolidation recruits nicotinic signaling that controls actin depolymerization by interacting with NMDAR-mediated signaling. The removal of ACh eliminates consolidation of LTP, and our results suggest that the effect of nicotine mimics the removal of ACh by preventing the ACh signaling via α7 nAChR desensitization. Thus, changes that occur during consolidation might involve the recruitment of ACh-mediated signaling to interrupt NMDAR-mediated signaling for actin depolymerization.
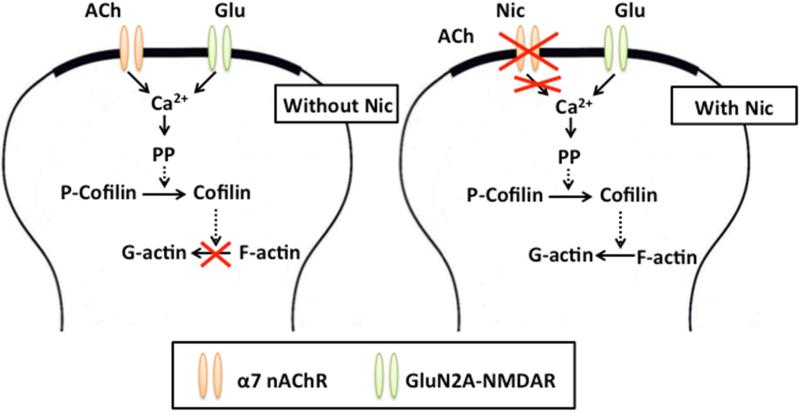
LTP-inducing TBS drives phosphorylation of the actin-depolymerizing protein cofilin via the signaling pathway (NMDAR-RhoA-ROCK-LIMK-cofilin) that inactivates cofilin and thus promotes actin polymerization (Rex et al., 2009; Dityatev et al., 2010). Actin depolymerization and spine shrinkage require not only activation of NMDARs and phosphatase PP2B, but also activation of cofilin via dephosphorylation (Zhou et al., 2004). Thus, Nic-DP likely involves cofilin dephosphorylation (Fig. 7). However, how the ACh signaling for preventing actin depolymerization is recruited during consolidation and its activation prevents NMDAR-mediated cofilin dephosphorylation remains to be explored.
Fig. 7. Possible pathways involved in nicotine-induced depotentiation of consolidated LTP.
GluN2A-NMDARs are activated by glutamate (Glu) released during LFS. (Left) Activation of α7 nAChRs by ACh released during LFS blocks GluN2A-NMDAR-mediated destabilization of F-actin, preventing depotentiation of consolidated LTP. (Right) Nicotine (Nic) desensitizes α7 nAChRs, preventing ACh-induced receptor activation during LFS. In the absence of α7 nAChR-mediated signaling, GluN2A-NMDAR activation destabilizes F-actin, inducing depotentiation of consolidated LTP. The broken arrows indicate that several steps are involved in the signaling process. PP, protein phosphatases; P-Cofilin, phosphorylated cofilin
Before LTP consolidation, GluN2A-NMDAR-mediated signaling alone causes depotentiation, but this signaling alone is not sufficient for the reversal of consolidated LTP, requiring the removal of α7 nAChR-mediated signaling. However, the location of α7 nAChRs involved in LTP stabilization remains unknown. α7 nAChRs are localized at dendritic spines of CA1 pyramidal cells (Fabian-Fine et al., 2001). Thus, a straightforward explanation of the finding is that α7 nAChR-mediated signaling in pyramidal cells affects GluN2A-NMDAR-mediated signaling for the reversal of consolidated LTP (Fig. 7). Because both receptors are highly Ca2+ permeable (Fucile, 2004; Monyer et a., 1994), this could be mediated by generating different Ca2+ signals that could stimulate different downstream signaling pathways. Indeed, it is known that α7 nAChR activation prolongs the NMDAR-mediated Ca2+ transients (Gu and Yakel, 2011). However, there are alternative possibilities. α7 nAChRs are abundantly expressed on GABAergic interneurons in the hippocampal CA1 region (Frazier et al., 1998a; McQuiston & Madison, 1999; Sudweeks & Yakel, 2000; Alkondon & Albuquerque, 2001), where they can be activated by endogenously released ACh (Alkondon et al., 1998; Frazier et al., 1998b; Yamazaki et al., 2005). Activation of α7 nAChRs on feedforward interneurons in the stratum radiatum negatively contributes to NMDAR activation in pyramidal cells by increasing inhibition of these cells (Ji et al., 2001; Yamazaki et al., 2005; Wanaverbecq et al., 2007; Zhang & Berg, 2007). This would decrease GluN2A-NMDAR-mediated signaling, thereby preventing the reversal of consolidated LTP. Alternatively, increased release of GABA or glutamate by the activation of presynaptic α7 nAChRs (McGehee and Role, 1996) could affect depolarization of pyramidal cells during LFS. This would decrease or increase GluN2A-NMDAR-mediated Ca2+ influx, disturbing normal signaling that leads to depotentiation.
An important question that arose from this study is how α7 nAChR-mediated signaling becomes effective in preventing LTP reversal only ~30 min after LTP induction. Previous work showed that on chick ciliary ganglion neurons the activity of α7 nAChRs is positively regulated by actin stabilization and PP2B, but is negatively controlled by CaMKII (Liu and Berg, 1999; Shoop et al., 2000). Because LTP-inducing stimulation increases CaMKII activity, increased CaMKII activity might dominantly and negatively control α7 nAChR activity immediately after LTP induction, although DP-inducing LFS can activate PP2B. However, increased actin stabilization following LTP and LFS-induced PP2B activation ~30 min after LTP induction might relieve the negative effect of CaMKII on α7 nAChR activity. This might explain why α7 nAChR-mediated signaling becomes effective in preventing LTP reversal only ~30 min after LTP induction. However, this possibility remains to be tested.
A recent study suggests a possible role for depotentiation in forgetting (Navabi et al., 2014). Elevated levels of ACh occur after memory induction and blocking the effect of hippocampal ACh during memory consolidation inhibits later performance (Marrosu et al., 1995; Orsetti et al., 1996; Barros et al., 2004; Deiana et al., 2011). Our findings implicate actin instability as a possible mechanism for why ACh is required during memory consolidation. The lack of ACh could allow for spine instability and depotentiation, which is then expressed behaviorally as a lack of memory. A similar mechanism could operate by the loss of cholinergic projections to the hippocampus found in Alzheimer's disease patients to affect memory formation.
Nicotine and blockade of α7 nAChR activation depotentiate consolidated LTP
Nicotine-induced depotentiation requires GluN2A-NMDAR activation
Nicotine-induced depotentiation requires destabilization of F-actin
Acknowledgements
This work was supported by NIDA Grants DA025269, DA025676, and DA026458.
Abbreviations
- AChE
acetylcholinesterase
- ACSF
artificial cerebrospinal fluid
- AMPAR
α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor
- α-BuTX
α-bungarotoxin
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- DP
depotentiation
- F-actin
filamentous actin
- fEPSPs
field excitatory postsynaptic potentials
- JSK
jasplakinolide
- LFS
low frequency stimulation
- LTD
long-term depression
- LTP
long-term potentiation
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptors
- NMDAR
N-methyl-D-aspartate receptor
- PBS
phosphate-buffered saline
- PP
protein phosphatase
- TBS
theta burst stimulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkondon M, Pereira EF, Albuquerque EX. alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. doi: 10.1016/s0006-8993(98)00880-4. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira E, Almeida L, Randall W, Albuquerque E. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology. 2000;39:2726–2739. doi: 10.1016/s0028-3908(00)00156-8. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Arai A, Larson J, Lynch G. Anoxia reveals a vulnerable period in the development of long-term potentiation. Brain Research. 1990;511:353–357. doi: 10.1016/0006-8993(90)90184-d. [DOI] [PubMed] [Google Scholar]
- Barros M, Ramirez M, Reis E. Participation of hippocampal nicotinic receptors in acquisition, consolidation and retrieval of memory for one trial inhibitory avoidance in rats. Neuroscience. 2004;126:651–656. doi: 10.1016/j.neuroscience.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Bellot A, Guivernau B, Tajes M, Bosch-Morató M, Valls-Comamala V, Muñoz FJ. The structure and function of actin cytoskeleton in mature glutamatergic dendritic spines. Brain Research. 2014;1573:1–16. doi: 10.1016/j.brainres.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Benke TA, Lüthi A, Isaac JTR, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M, Hayashi Y. Structural and Molecular Remodeling of Dendritic Spine Substructures during Long-Term Potentiation. Neuron. 2014;82:444–459. doi: 10.1016/j.neuron.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in Synaptic Morphology Accompany Actin Signaling during LTP. The Journal of Neuroscience. 2007;27:5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Deiana S, Platt B, Riedel G. The cholinergic system and spatial learning. Behavioural Brain Research. 2011;221:389–411. doi: 10.1016/j.bbr.2010.11.036. [DOI] [PubMed] [Google Scholar]
- Delgado JY, O'dell TJ. Long-term potentiation persists in an occult state following mGluR-dependent depotentiation. Neuropharmacology. 2005;48:936–948. doi: 10.1016/j.neuropharm.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010;11:735–746. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- Dutar P, Bassant M, Senut M, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiological reviews. 1995;75:393–427. doi: 10.1152/physrev.1995.75.2.393. [DOI] [PubMed] [Google Scholar]
- Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998a;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci. 1998b;18:8228–8235. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35:1–8. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Fujii S, Saito K, Miyakawa H, Ito K, Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Research. 1991;555:112–122. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Morita N, Sumikawa K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res. 1999;846:137–143. doi: 10.1016/s0006-8993(99)01982-4. [DOI] [PubMed] [Google Scholar]
- Fujii S, Sumikawa K. Nicotine accelerates reversal of long-term potentiation and enhances long-term depression in the rat hippocampal CA1 region. Brain research. 2001;894:340–346. doi: 10.1016/s0006-8993(01)02058-3. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP Is Accompanied by Enhanced F-Actin Content within the Dendritic Spine that Is Essential for Late LTP Maintenance In Vivo. Neuron. 2002;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71:155–165. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Nakauchi S, Sumikawa K. Nicotine reverses consolidated long-term potentiation in the hippocampal CA1 region. Brain research. 2006;1078:80–91. doi: 10.1016/j.brainres.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- He K, Lee A, Song L, Kanold PO, Lee H-K. AMPA receptor subunit GluR1 (GluA1) serine-845 site is involved in synaptic depression but not in spine shrinkage associated with chemical long-term depression. Journal of Neurophysiology. 2011;105:1897–1907. doi: 10.1152/jn.00913.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Liang Y, Hsu K. Characterization of the mechanism underlying the reversal of long term potentiation by low frequency stimulation at hippocampal CA1 synapses. The Journal of biological chemistry. 2001;276:48108–48117. doi: 10.1074/jbc.M106388200. [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Jouvenceau A, Billard J, Haditsch U, Mansuy IM, Dutar P. Different phosphatase-dependent mechanisms mediate long-term depression and depotentiation of long-term potentiation in mouse hippocampal CA1 area. European Journal of Neuroscience. 2003;18:1279–1285. doi: 10.1046/j.1460-9568.2003.02831.x. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Adoff MD, Wilkinson DS, Gould TJ. The effects of acute, chronic, and withdrawal from chronic nicotine on novel and spatial object recognition in male C57BL/6J mice. Psychopharmacology. 2011;217:353–365. doi: 10.1007/s00213-011-2283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Lynch G. Developmental and regional differences in the consolidation of long-term potentiation. Neuroscience. 2003;118:387–398. doi: 10.1016/s0306-4522(02)00916-8. [DOI] [PubMed] [Google Scholar]
- Kramár EAA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Barco A, Zablow L, Kandel ER, Siegelbaum SA, Zakharenko SS. Transient expansion of synaptically connected dendritic spines upon induction of hippocampal long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16665–16670. doi: 10.1073/pnas.0407581101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Xiao P, Lynch G. Reversal of LTP by theta frequency stimulation. Brain Research. 1993;600:97–102. doi: 10.1016/0006-8993(93)90406-d. [DOI] [PubMed] [Google Scholar]
- Lee H-K, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lee H-KK, Takamiya K, He K, Song L, Huganir RL. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. Journal of neurophysiology. 2010;103:479–489. doi: 10.1152/jn.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Huang F-SS, Abbas A-KK, Wigstrom Holger. Role of NMDA receptor subtypes in different forms of NMDA-dependent synaptic plasticity. BMC neuroscience. 2007;8:55. doi: 10.1186/1471-2202-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jo J, Jia J-M, Lo S-C, Whitcomb DJ, Jiao S, Cho K, Sheng M. Caspase-3 Activation via Mitochondria Is Required for Long-Term Depression and AMPA Receptor Internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y-C, Huang C-C, Hsu K-S. A role of p38 mitogen-activated protein kinase in adenosine A1 receptor-mediated synaptic depotentiation in area CA1 of the rat hippocampus. Molecular Brain. 2008;1:13. doi: 10.1186/1756-6606-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Qs, Berg DK. Actin filaments and the opposing actions of CaM kinase II and calcineurin in regulating alpha7-containing nicotinic receptors on chick ciliary ganglion neurons. J. Neurosci. 1999;19:10280–10288. doi: 10.1523/JNEUROSCI.19-23-10280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Lu W, Roche KW. Posttranslational regulation of AMPA receptor trafficking and function. Current opinion in neurobiology. 2012;22:470–479. doi: 10.1016/j.conb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Gall CM. LTP consolidation: substrates, explanatory power, and functional significance. Neuropharmacology. 2007;52:12–23. doi: 10.1016/j.neuropharm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: An Embarrassment of Riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Man H-YY, Sekine-Aizawa Y, Huganir RL. Regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Greenfield SA. Novel modulatory mechanisms revealed by the sustained application of nicotine in the guinea-pig hippocampus in vitro. J Physiol. 2003;551:539–550. doi: 10.1113/jphysiol.2003.045492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrosu F, Portas C, Mascia M, Casu M, Fà M, Giagheddu M, Imperato A, Gessa G. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671:329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential Roles of NR2A and NR2B-Containing NMDA Receptors in Cortical Long-Term Potentiation and Long-Term Depression. The Journal of Neuroscience. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory–a Century of Consolidation. (2000). Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Presynaptic ionotropic receptors. Curr. Opin. Neurobiol. 1996;6:342–349. doi: 10.1016/s0959-4388(96)80118-8. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J. Neurosci. 1999;19:2887–2896. doi: 10.1523/JNEUROSCI.19-08-02887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nadel L, Hupbach A, Gomez R, Newman-Smith K. Memory formation, consolidation and transformation. Neuroscience Biobehavioral Reviews. 2012;36:1640–1645. doi: 10.1016/j.neubiorev.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Nakauchi S, Brennan RJ, Boulter J, Sumikawa K. Nicotine gates long-term potentiation in the hippocampal CA1 region via the activation of alpha2* nicotinic ACh receptors. Eur J Neurosci. 2007;25:2666–2681. doi: 10.1111/j.1460-9568.2007.05513.x. [DOI] [PubMed] [Google Scholar]
- Nakauchi S, Sumikawa K. Endogenous ACh suppresses LTD induction and nicotine relieves the suppression via different nicotinic ACh receptor subtypes in the mouse hippocampus. Life Sci. 2014;111:62–68. doi: 10.1016/j.lfs.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, Fox R, Proulx C, Lin John., Tsien R, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell T, Kandel E. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learning & memory. 1994;1:129–139. [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic Membrane Trafficking Regulated by GluR1 Serine 845 Phosphorylation Primes AMPA Receptors for Long-term Potentiation. Journal of Biological Chemistry. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Okamoto K-I, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nature Neuroscience. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- Orsetti M, Casamenti F, Pepeu G. Enhanced acetylcholine release in the hippocampus and cortex during acquisition of an operant behavior. Brain research. 1996;724:89–96. doi: 10.1016/0006-8993(96)00292-2. [DOI] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Peng Y, Zhao J, Gu Q-HH, Chen R-QQ, Xu Z, Yan J-ZZ, Wang S-HH, Liu S-YY, Chen Z, Lu W. Distinct trafficking and expression mechanisms underlie LTP and LTD of NMDA receptor-mediated synaptic responses. Hippocampus. 2010;20:646–658. doi: 10.1002/hipo.20654. [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester RAJ. Desensitization of neuronal nicotinic receptors. Journal of Neurobiology. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Ramachandran B, Frey JU. Interfering with the Actin Network and Its Effect on Long-Term Potentiation and Synaptic Tagging in Hippocampal CA1 Neurons in Slices In Vitro. The Journal of Neuroscience. 2009;29:12167–12173. doi: 10.1523/JNEUROSCI.2045-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. The Journal of cell biology. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Shoop RD, Yamada N, Berg DK. Cytoskeletal links of neuronal acetylcholine receptors containing alpha 7 subunits. J. Neurosci. 2000;20:4021–4029. doi: 10.1523/JNEUROSCI.20-11-04021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Chun D, Lynch G. Time-dependent reversal of long-term potentiation by an integrin antagonist. The Journal of neuroscience. 1998;18:3460–3469. doi: 10.1523/JNEUROSCI.18-09-03460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527(Pt 3):515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanaverbecq N, Semyanov A, Pavlov I, Walker MC, Kullmann DM. Cholinergic axons modulate GABAergic signaling among hippocampal interneurons via postsynaptic alpha 7 nicotinic receptors. J Neurosci. 2007;27:5683–5693. doi: 10.1523/JNEUROSCI.1732-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley RG, Oeltmann TN, Lappi DA. Immunolesioning: selective destruction of neurons using immunotoxin to rat NGF receptor. Brain Res. 1991;562:149–153. doi: 10.1016/0006-8993(91)91199-b. [DOI] [PubMed] [Google Scholar]
- Winder DG, Sweatt JD. Roles of serine/threonine phosphatases in hippocampel synaptic plasticity. Nature Reviews Neuroscience. 2001;2:461–474. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Hamaue N, Sumikawa K. Nicotine compensates for the loss of cholinergic function to enhance long-term potentiation induction. Brain Res. 2002;946:148–152. doi: 10.1016/s0006-8993(02)02935-9. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Jia Y, Hamaue N, Sumikawa K. Nicotine-induced switch in the nicotinic cholinergic mechanisms of facilitation of long-term potentiation induction. Eur J Neurosci. 2005;22:845–860. doi: 10.1111/j.1460-9568.2005.04259.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Sugihara T, Goto J-I, Chida K, Fujiwara H, Kaneko K, Fujii S, Mikoshiba K. Role of inositol 1, 4, 5-trisphosphate receptors in the postsynaptic expression of guinea pig hippocampal mossy fiber depotentiation. Brain research. 2011;1387:19–28. doi: 10.1016/j.brainres.2011.02.088. [DOI] [PubMed] [Google Scholar]
- Yang H, Courtney MJ, Martinsson P, Manahan-Vaughan D. Hippocampal long-term depression is enhanced, depotentiation is inhibited and long-term potentiation is unaffected by the application of a selective c-Jun N-terminal kinase inhibitor to freely behaving rats. European Journal of Neuroscience. 2011;33:1647–1655. doi: 10.1111/j.1460-9568.2011.07661.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Frerking M, Zhou Q. Spine expansion and stabilization associated with long-term potentiation. The Journal of neuroscience. 2008;28:5740–5751. doi: 10.1523/JNEUROSCI.3998-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neuroscience. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Zhang J, Berg DK. Reversible inhibition of GABAA receptors by alpha7-containing nicotinic receptors on the vertebrate postsynaptic neurons. J Physiol. 2007;579:753–763. doi: 10.1113/jphysiol.2006.124578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Meng K, Li Y, Han T. NR2A-containing NMDA receptors are required for L-LTP induction and depotentiation in CA1 region of hippocampal slices. European Journal of Neuroscience. 2009;29:2137–2144. doi: 10.1111/j.1460-9568.2009.06783.x. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Pak D, Qin Y, McCormack SG, Kim MJ, Baumgart JP, Velamoor V, Auberson YP, Osten P, Aelst L. van, Sheng M, Zhu J. Rap2-JNK removes synaptic AMPA receptors during depotentiation. Neuron. 2005;46:905–916. doi: 10.1016/j.neuron.2005.04.037. [DOI] [PubMed] [Google Scholar]