Summary
Loss of pigment epithelium-derived factor (PEDF, SERPINF1) in cancer cells is associated with poor prognosis and metastasis, but the contribution of stromal PEDF to cancer evolution is poorly understood. Therefore, we investigated the role of fibroblast-derived PEDF in melanoma progression. We demonstrate that normal dermal fibroblasts expressing high PEDF levels attenuated melanoma growth and angiogenesis in vivo, whereas PEDF-depleted fibroblasts exerted tumor-promoting effects. Accordingly, mice with global PEDF knockout were more susceptible to melanoma metastasis. We also demonstrate that normal fibroblasts in close contact with PEDF-null melanoma cells lost PEDF expression and tumor suppressive properties. Further mechanistic investigations underlying the crosstalk between tumor and stromal cells revealed that melanoma cells produced PDGF-BB and TGF-β, which blocked PEDF production in fibroblasts. Notably, cancer-associated fibroblasts (CAF) isolated from patient-derived tumors expressed markedly low levels of PEDF. Treatment of patient CAF and TGF-β-treated normal fibroblasts with exogenous PEDF decreased the expression of CAF markers and restored PEDF expression. Finally, expression profiling of PEDF-depleted fibroblasts revealed induction of interleukin-8, SERPINB2, hyaluronan synthase-2, and other genes associated with tumor promotion and metastasis. Collectively, our results demonstrate that PEDF maintains tumor-suppressive functions in fibroblasts to prevent CAF conversion, and illustrate the mechanisms by which melanoma cells silence stromal PEDF to promote malignancy.
Keywords: Cancer-associated fibroblasts, melanoma, pigment epithelium-derived factor
Introduction
Past studies demonstrate that non-neoplastic cells in the tumor microenvironment (TME) are critical in cancer progression (1). These non-malignant cells include fibroblasts, endothelial cells, immune cells and bone marrow-derived myeloid and endothelial progenitors (2). Over time, cancer cells engage in crosstalk with the TME and cause non-neoplastic cells to assume cancer-associated phenotype supportive of tumor growth and dissemination (3).
Cancer-associated-fibroblasts (CAFs) are the most abundant constituents of the TME (2) and atypical fibrotic response in cancers is associated with increased aggressiveness and poor prognosis (4). CAFs largely consist of resident fibroblasts; however they also originate from bone marrow-derived mesenchymal cells, by epithelial-to-mesenchymal transition (EMT) of the resident epithelial and tumor cells (5) and through endothelial-to-mesenchymal transition (EndMT) (6). CAFs support tumor progression by supplying growth factors (e.g. basic fibroblast growth factor (bFGF), epithelial growth factor and hepatocyte growth factor (HGF) and matrix remodeling enzymes, such as matrix metalloproteinases (MMPs) (1). CAFs also supply angiogenic growth factors vascular endothelial growth factor (VEGF), interleukin (IL)-8 and stromal derived factor (SDF)-1, and facilitate VEGF release from the extracellular matrix (ECM) by proteolysis (2, 4). Importantly, CAFs support tumor progression by producing ECM components collagens, fibronectin (FN), hyaluronan, versican and osteopontin (2). Together, CAFs generate ECM, the growth factors and MMPs, which enable and accelerate EMT of the cancer cells and support invasion and metastasis (7).
The conversion of fibroblasts to the tumor-associated phenotype is driven by environmental stress (hypoxia, reactive oxygen species) (8) and cell-cell communications via secreted factors and extracellular vesicles (9). The main cytokines drivers of CAF conversion are transforming growth factor beta (TGF-β) (10) and platelet-derived growth factor-BB (PDGF-BB) (11); together, they are essential for the formation of reactive tumor stroma. Increased PDGF-BB expression is observed in most cancers (colon, brain, breast, prostate and lung cancers) (12), and high stromal expression of PDGF receptor-beta (PDGFR-β) predicts rapid cancer progression and poor prognosis (13). PDGF-BB is critical for fibroblast proliferation and recruitment. In contrast, TGF-β mainly drives the conversion of fibroblasts, endothelial and mesenchymal stem cells to CAFs (14). A less EndMT is caused by TGF-β and HGF (15).
In melanoma, fibroblasts have been identified as a potential cause of therapy resistance; they produce growth factors (e.g. nerve growth factor 1), which reactivate Erk1/2 in tumor cells (16) or provide ECM, which supports focal adhesions and survival signaling (17). We discovered the loss of pigment epithelial-derived factor (PEDF) as a critical step in melanoma transition to the aggressive, metastatic phenotype (18). PEDF is a secreted glycoprotein with anti-angiogenic and direct tumor suppressive properties, whose down-regulation or loss in cancer enables hyperproliferation, migration and invasion (19). PEDF also impedes tumor angiogenesis by restricting migration (20) and causing apoptosis induction in proliferating endothelial cells (21), via peroxisome proliferation-activated receptor-γ (PPAR-γ) and p53 pathways (22, 23) and through extrinsic death signaling by CD95L and TRAIL (21, 24, 25). Recent data suggest that PEDF mitigates tumor-promoting inflammation through conversion of macrophages towards tumor-reactive M1 phenotype (26, 27). Importantly, we have demonstrated that PEDF is produced at high levels by normal melanocytes but is lost in aggressive, metastatic melanoma (18) and this loss alters Rho/Rac signaling causing amoeboid invasion, extravasation and metastasis (28).
To date, most studies were focused on the mechanisms and consequences of PEDF loss in the neoplastic cells. One study describes the tumor-promoting effect of global PEDF loss (29), however, the mechanisms that hinder PEDF expression in the tumor stroma and the specific consequences of this loss are unknown.
Here, we investigate the role of fibroblast-derived PEDF in melanoma progression using an in vivo model that employs PEDF-null primary dermal fibroblasts generated by shRNA knockdown. To elucidate the effects of fibroblast-derived PEDF on melanoma progression, we compared tumor growth in mice inoculated with PEDF-negative, highly aggressive melanoma cells C8161, alone or with normal PEDF-positive or PEDF-null fibroblasts. We discovered that fibroblasts-derived PEDF attenuates the growth and angiogenesis of highly aggressive, metastatic melanoma, and that PEDF-null fibroblasts promote melanoma growth, proliferation and angiogenesis. In agreement, mice with global knockout of SERPINF1 (PEDF) gene had higher incidence of spontaneous melanoma metastasis and enhanced lung colonization in syngeneic melanoma models.
On the other hand, we demonstrate that close proximity of the normal fibroblasts to melanoma cells diminished their tumor suppressive properties in vivo. We also show that PEDF expression was attenuated in fibroblasts subjected to hypoxic stress or exposed to the secretory milieu in the media conditioned by melanoma cells. Among growth factors secreted by the melanoma cells we identified PDGF-BB and TGF-β as critical for the suppression of PEDF production in fibroblasts. Importantly, TGF-β had no direct effect of PEDF expression but augmented PEDF repression by PDGF-BB by up-regulating PDGFR receptor β. We have also found that JNK1/2 and p38 were critical for PEDF repression by PEDFG-BB, while PDGFR-β regulation by TGF-β occurred through PI3K, JNK1/2, p38 and Erk1/2.
Importantly, we show that PEDF repealed CAF conversion by TGF-β, where it inhibited SMAD signaling and attenuated CAF markers, smooth muscle actin (SMA) or vimentin in patient-derived CAFs. Finally, PEDF knockdown in fibroblasts caused increased expression of multiple genes associated with tumor promotion and metastasis including IL-8, SERPINB2 and hylauronan synthase. Together, our results demonstrate the importance of fibroblast-derived PEDF in controlling tumor progression and identify crosstalk between tumor cells and fibroblasts, which overcomes the tumor-suppressive effects of stromal PEDF.
Materials and Methods
Experimental Animals
Mice were maintained at Northwestern University Center for Comparative Medicine according to the National Institutes of Health guidelines and following protocols approved by Northwestern University Animal Use & Care Committee. HsdNu/Nu nude mice ere from Harlan (Indianapolis, IN); C57BL6 mice were from Jackson Laboratories; C57Bl6 SERPINF1−/− mice (Regeneron, Tarrytown, NY) were bred at Northwestern University. PEDF silencing was ascertained by RT-PCR/ LacZ expression (Supplementary Fig. 1).
Cells
Human melanoma cell line C8161-HA from Dr. Hendrix (Children's Memorial Hospital, Chicago, IL) was cultured in RPMI1640 (GIBCO, Grand Island, NY) with 10% FBS (HyClone, Waltham, MA), penicillin and streptomycin (P/S, GIBCO). The human (A375) and murine (B16F10) melanoma cell lines (American Type Culture Collection, Manassas, VA) were maintained in DMEM (GIBCO) with 10% FBS and P/S. Human neonatal dermal fibroblasts (HDFs, Northwestern University Skin Disease Research Center, SDRC) were cultured in DMEM with 10%FBS and P/S and used between passages 3–10. Pooled fibroblasts from multiple donors were used to reduce sample variations. Cells were grown at 37°C, 5% CO2. Primary CAFs (see below) were cultured in DMEM with 10% FBS and P/S.
Authentication
Human melanoma cell lines were were authenticated by Biosynthesis, Inc. (Lewisville, TX) using STR DNA profiling methods. The initial vials were expanded and cryopreserved the lab and used no longer than five consecutive passages. Authentication certificate is provided (see Supplementary Materials).
Gene silencing
We have used pGIPz lentiviral vectors (Open Biosystems, Huntsville, AL) encoding shRNA-mir VL2HS_221662 (sh-PEDF) or scrambled, non-silencing control (sh-Scr). Lentiviruses were produced at Northwestern University SDRC Viral Core. Silencing was verified by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and Western blot.
Subcutaneous tumor inoculation
(a) Co-injection
C8161-HA cells (106) mixed with 4.0×106 Sh-Scr Sh-PEDF HDFs in PBS or 100μl vehicle were injected subcutaneously (s.c.) in the flanks of nude mice.
(b) Inoculation at remote sites
C8161-HA cells were injected s.c. into the flanks of nude mice, (106/ 100μl/ mouse). When palpable tumors formed, the mice were randomized into groups (n=5) and injected in the dorsal midline with Sh-PEDF or Sh-Scr fibroblasts (106/ 100μl/ mouse) or 100μl PBS. Tumors were measured twice weekly.
Angiogenesis Assessment
Five-μm sections of paraffin-embedded tissue (Northwestern University Mouse Phenotyping Core) were incubated overnight at 4°C with anti-CD31 antibody (BD Pharmingen, Billerica, MA); 2 h at RT with Rhodamine-conjugated 2° antibodies (Jackson ImmunoResearch) and counterstained with DAPI. Digital images of 10 random fields/ group were taken with 20X objective and CD31-positive structures quantified (Nikon Elements software, Nikon, Melville, NY).
Proliferation analysis, macrophage infiltration and apoptosis were assessed on paraffin-embedded sections stained at Northwestern University Mouse Phenotypic Core for Ki67, F4/80 o subjected to TUNEL assay. Digital images were taken with 20X objective and 10 random fields/ group assessed as above.
Metastasis Assays
(a) Spontaneous metastasis
B16F10 cells harvested at 80–90% confluence were injected s.c in hindquarters of wild type (WT) SEPINF1KO mice (104/ 100μl/ mouse, n=8). After 4 weeks, the mice were euthanized, metastases measured with micro-calipers and counted.
(b) Lung colonization
B16F10 cells were injected via 27-gauge needle in the tail veins of WT and SEPINF1 KO mice (105/ 100 μl/ mouse, n=5). The animals were sacrificed after 14 days and the lungs photographed.
(c) Extravasation Assay
Lung extravasation was assessed as described (28). B16F10 cells were labeled with fluorescent dye CSFE (Life technologies, Grand Island, NY), re-suspended in PBS (106/ 100μl) and injected into the tail veins of mice (100μl/ mouse, n=3). The mice were sacrificed 2 and 24 h post-injection, and fluorescent colonies visualized using Nikon AZ-100 microscope (Nikon, Melville, NY, 5X objective) in > 5 random fields.
Conditioned media (CM) preparation
Cells were grown to 70–80% confluence, rinsed twice in PBS and placed in serum-free media for 48 h. CM was cleared by centrifugation (1500g × 5 min, 4°C), the supernatants concentrated by spin-filtration (3-kDa MWCO, Millipore, Billerica, MA) and stored at −80°C. Protein concentration was measured using Bradford Assay (Thermo Scientific, Waltham, MA).
Treatments
HDFs plated in 6-well plates were serum-starved overnight and treated 24h with 150μg/ml CM, growth factors (b-FGF, IL-8, PDGF-β, TGF-β and VEGF) or serum-free DMEM (control). Small molecule inhibitors (Supplementary Table 1) were applied 2 h before treatments.
CAFs isolation
Patient tumor samples were de-identified with 6-digit numbers. The protocol was approved by Northwestern University Institutional Review Board, and all patients provided informed consent. Fragments of tumor samples or patient-derived xenografts were cut into 1–2 mm pieces, and plated in GlutaMax DMEM or RPMI1640 with 10% of FBS. After 2–16 weeks the cells were harvested with 0.25% Trypsin/EDTA (Life Technologies) and cryopreserved at passage 3.
Immunoblotting
Cells were lysed with M-PER buffer with protease/phosphatase inhibitors (Thermo Scientific, Waltham, MA), lysates cleared by centrifugation (8000g, 10 min, 4°C) and total protein measured. The lysares were resolved by SDS-PAGE (4–20% Tris-HCl polyacrylamide gels, Biorad, Hercules, CA) and transferred to PVDF membranes (GE Healthcare Life Sciences, Pittsburg, PA). The membranes were rinsed in Tris-buffered saline with 0.1% Tween 20 (TBS-T) and blocked 30 min in TBS-T with 5% non-fat milk. After incubation with 1° antibodies (Supplementary Table 2), the membranes were washed with TBS-T and incubated with secondary antibodies (Supplementary Table 3; 1 h, 5% non-fat milk, TBS-T). Blots were developed with Enhanced Chemoluminescence kit (Thermo Scientific, Waltham, MA) and quantified by densitometry (ImageJ32 software, National Institutes of Health, Bethesda, MD). All values were corrected for the integrated optical density (iOD) and normalized to loading controls.
Image acquisition
Photomicrographs were taken with Nikon Diaphot 2000 microscope with bright-light and fluorescent digital cameras, Nikon Elements and Spot 5 software. Images were analyzed using Elements or ImageJ32 software. Western Blots were digitalized using Epson Scan software and plot profiles generated. The values for each band were calculated as (area under the curve) × iOD. Macroscopicl images were taken with Nikon CoolPix camera and quantified using Object Count function (ImageJ32).
Gene array analysis
Dermal fibroblasts were transduced with Sh-PEDF or Sh-Scr. Replicates of 3 were used for statistical evaluation. mRNA was extracted with Qiagen RNeasy kit (Qiagen, Valencia, CA) and analyzed at the University of Illinois at Chicago Genomic Core (Chicago, IL). Statistical significance was calculated by Student's t-test and P <0.05 considered significant. The expression differences > 1.5 were further validated by qRT-PCR.
RNA amplification
Total mRNA was reverse-transcribed using iScript cDNA kit (Bio-Rad, Hercules, CA) and amplified with SsoAdvance SYBR Green Supermix, (Bio-Rad, Hercules, CA) in Bio-Rad CSF96 Real-Time Detection System. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Tubulin or L19 were used as reference.
Statistical Analysis
All experiments were performed > 3 times and the samples tested in triplicates. Statistical significance was assessed by One-Way Anova with Bonferroni Multiple Comparison posttest for normally distributed datasets (established by Kolmogorov-Smirnov test, GraphPad Prizm). For smaller/ non-normal datasets, non-parametric Kruskal-Wallis or Mann-Whitney tests were applied and P < 0.05 set as significant.
Results
PEDF-null fibroblasts enhanced tumor growth
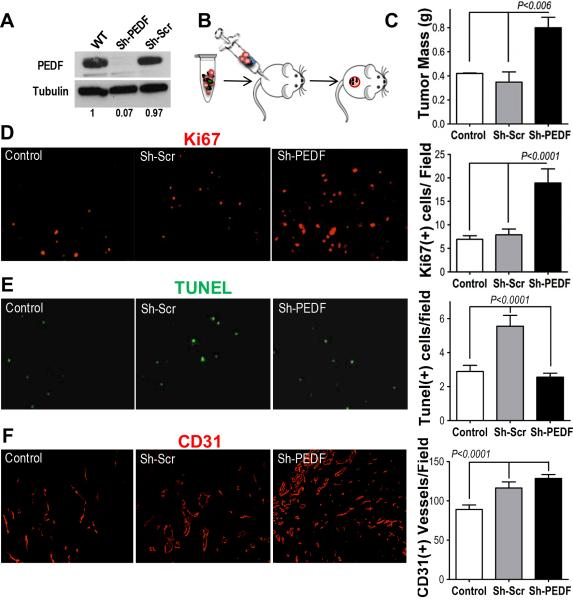
Fibroblasts are the second richest source of PEDF in skin (18) and PEDF from dermal fibroblasts could compensate for its lack in aggressive melanoma cells. To investigate this possibility, we generated PEDF-null human dermal fibroblasts (HDFs) using lentivirally expressed shRNA (sh-PEDF). Scrambled sequence was used as control (Sh-Scr). Western blot and qRT-PCR showed ~ 85% and ~ 60% reduction in PEDF protein and mRNA, respectively (Fig 1A, Supplementary Fig. 2). Sh-PEDF or Sh-Scr fibroblasts mixed 4:1 with C8161-HA melanoma cells, which express no detectable PEDF (18) were injected into the flanks of nude mice (Fig 1B). C8161-HA cells alone served as control. After 14 days tumor growth was significantly enhanced by Sh-PEDF but not Sh-Scr fibroblasts as was evidenced by tumor weights and volumes (Fig 1C, Supplementary Fig. 3A). Accelerated growth in Sh-PEDF was accompanied by increased proliferation (Ki67 staining, Fig 2D). The observed increase in proliferation occurred mainly in the tumor cells as was evidenced by minimal Ki67-positivity in GFP-expressing fibroblasts (Supplementary Fig. 4C).
Figure 1. PEDF Loss in Resident Fibroblasts Enhanced Tumor Growth.
(A) PEDF-null fibroblasts were generated by knockdown with lentiviral shRNA specific to PEDF (sh-PEDF). Scrambled shRNA (sh-Scr) was used as a negative control. The knockdown was verified by Western blot. WT, wild type, uninfected fibroblasts. (B) Experimental design. Nude mice were inoculated s.c. with C8161-HA melanoma cells (106) alone (control) or admixed with 1:4 with sh-Src or sh-PEDF fibroblasts; two weeks post-injection the tumors were harvested and the following parameters assessed: (C) tumor mass; (D) Proliferation (Ki67 immunofluorescence); (E) Apoptosis (in situ TUNEL); (F) MVD (CD31 immunofluorescence). Digital images were analyzed using “Object Count” function of the Elements software. The SEM and P values were calculated using Graph Pad Prism software.
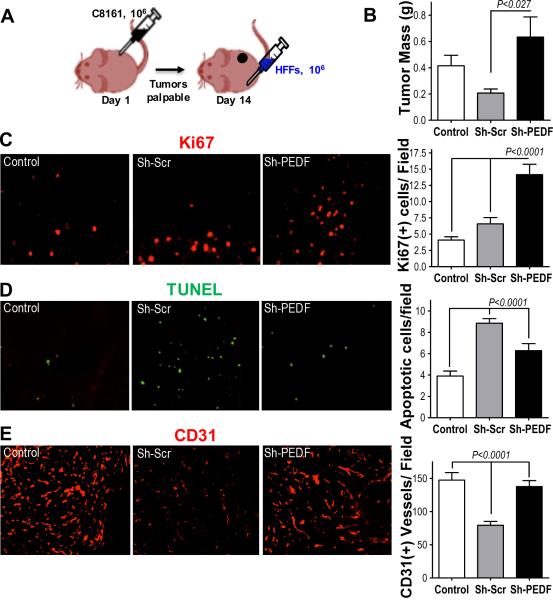
Figure 2. PEDF-expressing fibroblasts injected at a distant site inhibited tumor growth and angiogenesis.
(A) C8161-HA cells (106) were injected subcutaneously in the right flanks of nude mice. Fourteen days post injection palpable tumors formed and the mice were injected with 106 HDFs (PEDF status indicated) or 100 μl saline (control) at the dorsal midline. Two weeks after HDF injection tumors were harvested and evaluated. (B) Tumor mass; (C) Proliferation (Ki67 immunofluorescence); (D) Apoptosis (TUNEL); (E) Microvascular density (CD31 immunofluorescence, red). Digital images were taken at 20× magnification and no less than 5 random field per section evaluated. The SEM values are shown. P values were determined using Graph Pad Prism software.
Forced PEDF expression in cancer cells attenuates tumor growth by simultaneously inducing apoptosis and blocking angiogenesis; however, the contribution of fibroblast-derived PEDF has not been explored. We observed significantly higher apoptosis in the presence of Sh-Scr fibroblasts compared to the control and Sh-PEDF groups (Fig 2E). In contrast, the difference in the microvascular density (MVD) was not significant and the tumors containing Sh-Scr or Sh-PEDF fibroblasts presented with significantly higher MVD (Fig 1F).
To exclude potential fibroblasts' proliferation and apoptosis from analyses, C8161HA cells were injected in the right flanks of mice and the tumors allowed to establish 14 days, at which point mice were randomized into groups and injected in the dorsal midline with Sh-Scr or Sh-PEDF fibroblasts or saline (Fig. 2A). In this setting, Sh-Scr fibroblasts significantly reduced tumor growth (Fig 2B, Supplementary Fig. 3C). The proliferative index (Ki67) remained higher in Sh-PEDF compared to Sh-Scr and control groups (Fig. 2C) and Sh-Scr tumors displayed increased apoptosis (Fig. 2D). There was no significant recruitment of GFP-tagged fibroblasts to the tumors (Supplementary Fig. 3D). Importantly, in contrast to the admixing experiment, Sh-Scr fibroblasts caused a significant reduction in MVD compared to Sh-PEDF and control groups (Fig. 2E).
This indicates that fibroblast-derived PEDF attenuates tumor growth and angiogenesis and suggest that anti-cancer effects of Sh-Scr fibroblasts are reduced when they are in close contact with tumor cells.
The lack of host PEDF increased metastasis
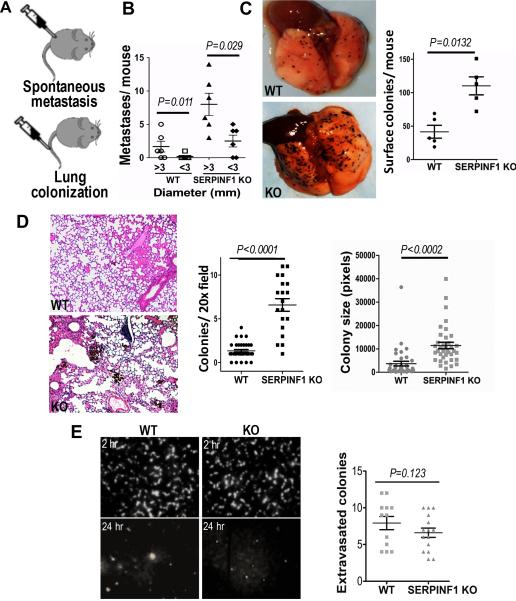
Ectopic PEDF reduces metastatic potential of melanoma cells by concomitant disruption of the amoeboid migration and MT1-MMP proteolysis (28). A recent study showed increased uveal melanoma metastasis in mice with global PEDF knockdown (29) but did not uncover the underlying mechanism. In agreement, subcutaneously injected of B16F10 melanoma cells formed larger metastasis with higher incidence in SERPINF1 KO mice compared to the wild-type controls (Fig. 3A, supplementary Table 3). Similarly, in SERPINF1 KO mice tail-vein inoculation of B16F10 cells yielded more surface lung colonies, with increased the number and size of parenchymal micrometastases (Fig. 3B, C). This difference was not due increased extravasation, since the number of single-cell colonies formed by B16F10 cells 24h post inoculation were similar in SERPINF1 KO and control mice (Fig. 3D).
Figure 3. Lack of PEDF in the tumor microenvironment promotes metastasis.
(A) B16F10 melanoma cells (105) were injected into the flanks of wild type (WT) and PEDF-null (SERPINF1 KO) mice (n=8) to evaluate spontaneous metastasis. Alternatively, 105 cells were injected into the tail vein of WT and SERPINF1 KO mice (n=5) to assess lung colonization. (B) Three weeks after primary tumor implantation, autopsy was performed and spontaneous abdominal and pleural metastasis counted and measured. (C) Two weeks after tail vein injection, mice were sacrificed, lungs photographed (representative images are shown) and surface colonies counted using ImageJ software. (D) Lungs from were also formalin-fixed and paraffin-embedded, 5 μm thick sections stained with Hematoxylin/ Eosine and micrometastases number and area per animal determined using ImageJ software. (E) Extravasation of CDFA-SE labeled B16F10 cells from the lung vasculature was assessed in WT and SERPINF1 KO mice 2 h and 24 h after tail vein inoculation. Shown are the representative fluorescence images of the lung surface at 2 h and 24 h, obtained with Nikon AZ-100 microscope (5× objective) and quantitative analysis at a 24 h time point (Nikon Elements software).
Therefore, the invasive capacity of melanoma cells was unaffected by PEDF in the host tissue, and the observed differences were likely caused by decreased overall survival, due to reduced proliferation and elevated apoptosis.
Tumor cells suppress PEDF production in the fibroblasts
PEDF-positive fibroblasts attenuated tumor growth and angiogenesis if injected independently of C8161-HA melanoma cells but lost their tumor suppressive properties when co-injected with cancer cells. We reasoned that fibroblasts in admixed tumors undergo partial CAF conversion, possibly due to PEDF decrease, and examined factors that could reduce PEDF expression.
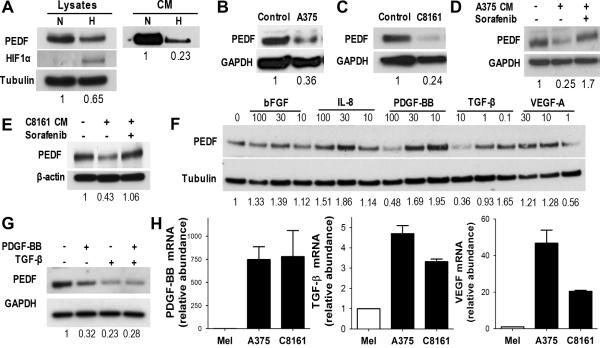
In a growing tumor CAFs are exposed to hypoxic stress, which attenuates PEDF expression in melanoma cells (30). Similarly, exposure to hypoxia significantly reduced PEDF in the HDFs as was determined by Western blot (Fig. 4A).
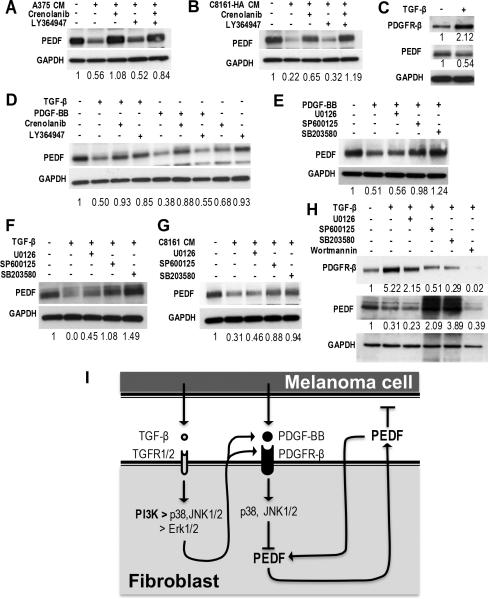
Figure 4. Tumor-associated Factors Down-regulate PEDF Expression in Fibroblasts.
PEDF levels were measured in primary HDFs, exposed to hypoxic (H) or normoxic (N) conditions or treated with CM from melanoma cells. (A) HDFs were serum starved and exposed to hypoxa for 24 h; PEDF levels in cell lysates and in CM were assessed by Western blot. (B, C) HDFs were treated 24 h with CM from A375 or C8161 cells (150 μg/ml protein final concentration). (D, E) HDFs were treated 24 h with A375 or C8161-HA CM in the presence of 10 μM Sorafenib. (F) HDFs were exposed for 24 h to increasing concentrations of growth factors and PEDF levels evaluated by Western blot. (G) HDFs were treated with PDGF-BB and TGF-β alone and in combination and PEDF levels assessed by Western blot. (H) QRT-PCR measurement of mRNA levels of PDGF-β and TGF-β in A375 and C8161-HA melanoma cell lines and primary melanocytes (normalized to GAPDH).
In addition, tumor cells secrete growth factors and cytokines, which affect non-neoplastic cells in the TME (1). To investigate whether these factors attenuate PEDF levels in CAFs, HDFs were treated with CM from A375 and C8161-HA melanoma cells. Both CM considerably decreased PEDF in fibroblast lysates (Fig. 4B–C). This decrease was reversed by pan-RTK inhibitor Sorafenib (Selleckchem, Houston, TX, Fig. 4D–E), which has limited selectivity for VEGF and PDGF receptors. We then tested a series of growth factors expressed by aggressive melanoma, VEGF, bFGF, PDGF-BB, TGF-β and IL-8. Of these, PDGF-BB and TGF-β (used at concentrations in the range typically produced by melanoma cells (31)) caused a dose-dependent reduction of PEDF levels in fibroblasts (Fig. 4F); however, combined use of PDGF-BB and TGF-β had no additive effect (Fig. 4G). Q-PCR and ELISA confirmed significantly higher expression of PDGF-BB and TGF-β in A375 and C8161-HA cells (Fig. 4H and data not shown). Our findings indicate that melanoma cells attenuate PEDF expression by CAFs, which can be reduced by hypoxia and by tumor-derived PDGF-BB and TGF-β. Interestingly, recombinant PDGF-BB dramatically reduced PEDF mRNA in cultured HDFs (Supplementary Fig. 5).
TGF-β enhances PEDF suppression by paracrine and autocrine PDGF-BB via JNK1/2, p38, PI3K and Erk1/2
PDGF-BB and TGF-β were sufficient to attenuate PEDF expression in CAFs. To establish whether these factor(s) are also necessary, we treated HDFs with A375 or C8161-HA CM in the presence of the inhibitors of PDGF-BB (Crenolanib) and TGF-β (LY364947). PDGF-BB inhibitor consistently reversed PEDF down-regulation by melanoma CM (Fig. 5A, B). In contrast, TGF-β inhibitor was ineffective at restoring PEDF expression but slightly augmented the effect of Crenolanib (Fig. 5A, B). We hypothesized that PDGF-BB, whose levels in melanoma cells exceed normal by 400–500 fold, directly inhibits PEDF expression, while the TGF-β effect is indirect. In agreement, PDGF-BB knockdown alleviated PEDF suppression by C8161-HA CM; this reversal was more pronounced in HDFs with TGFRB1 knockdown (Supplementary Fig. 6).
Figure 5. TGF-β and PDGF-BB down-regulation of PEDF expression in Fibroblasts via Paracrine and Autocrine molecular events.
(A, B) Normal HDFs were incubated 24 h with CM from A375 and C8161 cells with and without PDGF-BB (Crenolanib) or TGF-β (LY364947) inhibitors; PEDF in cell lysates was measured by western blot. (C) PEDF expression in HDFs treated with PDGF-β (100 ng/ml) or TGF-β (10 ng/ml) with or without their respective inhibitors Crenolanib or Ly364947. (D) To assess the effects of autocrine PDGF-BB, fibroblasts were Crenolanib; TGF-β was added were indicated. (E & F) HDFs were treated 24h with PDGF-BB or TGF-β in the presence or in the absence of inhibitors of ERK ½ (U0126), JNK1/2 (SP600125) or p38 (SB203580). For concentrations see Supplementary Table 1. PEDF in cell lysates was detected by western blot. (G) PEDF expression in primary HDFs treated with C8161 CM (150 μg/ml) in the presence Erk1/2, JNK1/2 and p38 inhibitors as above. (H) PDGFR expression in primary HDFs treated with TGF-β in the presence of inhibitors of Erk1/2 JNK1/2, p38 and PI3K (Wortmannin), respectively. (I) Schematic presentation of the interplay between melanoma cells and fibroblasts, including secreted factors and signaling intermediates.
TGF-β is known to up-regulate PDGF receptors in hepatic cells (32). Similarly, PDGFR-β was significantly higher in primary fibroblasts treated with TGF-β (Fig. 5C). Intriguingly, PEDF suppression by TGF-β was reversed by Crenolanib (Fig. 5D). We reasoned that in melanoma, PDGF-BB is the main factor, which blocks PEDF expression in GCAFs and that TGF-β exacerbates this effect by up-regulating PDGFR. The PDGFR-α and β mRNAs were unaltered by TGF-β (Supplementary Fig. 7). In contrast, TGF-β increased PDGF-BB mRNA in HDFs (Supplementary Fig. 8), suggesting an autocrtine feedback loop that further attenuates PEDF expression.
Seeking protein kinases involved in PEDF regulation, we found that JNK1/2 and p38 inhibitors but not ERK1/2 inhibitor reversed PEDF down-regulation by PDGF-BB, TGF-β or C8161-HA CM (Fig. 5E–G). Further, the PDGFR upregulation by TGF-β was abolished by the PI3K inhibitor Wortmannin, diminished by 50–30% with JNK1/2, p38 and Erk1/2 inhibitors (Fig. 5H, Supplementary Fig. 9).
Our results define a crosstalk between melanoma cells and skin fibroblasts in which fibroblast-derived PEDF inhibits melanoma growth and angiogenesis. Aggressive melanomas secrete PDGF-BB, which reduces PEDF expression in adjacent fibroblasts, a process that requires JNK1/2 and p38, and TGF-β, which further sensitizes fibroblasts to PDGF-BB by upregulating PDGFR-β, mainly via p38 and PI3K and increased PDGF-BB expression by fibroblasts (Fig. 5I).
PEDF repeals CAF Conversion
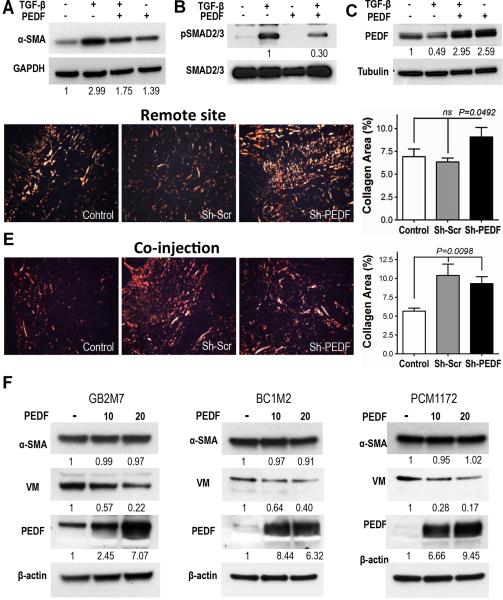
Our findings suggest that PEDF loss enables CAF conversion. In TGF-β-activated HDFs. PEDF significantly dampened the increase of CAF marker α-SMA (6) (Fig. 6A) and inhibited TGF-β signaling as was evidenced by ~ 3-fold decrease of SMAD2/3 phosphorylation (Fig. 6B). PEDF also non-canonical TGF-β signaling ias was evidenced by decreased Erk1/2 activation (Supplementary Fig. 10). Interestingly, PEDF treatment strongly elevated cytoplasmic PEDF levels in the activated fibroblasts (2.5 - 6 fold, Fig. 6C and F), suggesting a positive feedback loop. In agreement, PicoSirius staining of the tumors from the in vivo experiments showed increased collagen deposition in C8161 tumors when Sh-PEDF but not Sh-Scr fibroblasts were injected in a remote site (Fig. 6D). Consistent attenuated PEDF expression on contact with melanoma cells, both Sh-PEDF and Sh-Scr fibroblasts augmented collagen deposition when comixed with melanoma cells (Fig. 6E). Moreover, expression profiling of Sh-PEDF and Sh-Scr fibroblasts showed significant upregulation of Hyaluronan Synthase 2 (HAS2), IL-8, Serpin B2, Stanninocalcin (STC)-1 and NADPH Oxidase (NOX)4 in response to PEDF knockdown, (Supplementary Fig. 11).
Figure 6. PEDF attenuates fibroblast conversion to CAFs.
(A) Primary HDFs were treated 72 h with TGF-β with and without exogenous PEDF (20 nM). Myofibroblast marker α-SMA was assessed in cell lysates by Western blot. Densitometry results normalized to GAPDH are shown below each panel. (B) HDFs were treated for 24 h as in (A). Phospho-SMAD2/3, and total SMAD were assessed in cell lysates and the results normalized to the total SMAD2/3 (representative experiment of 5 is shown). (C) Cells were treated as above and PEDF levels assessed (normalized to GAPDH). (D, E) C8161-HA tumors from experiments in Fig. 1 and 2 were stained with PicoSirius Red to assess matrix deposition and quantified with ImageJ software. (F) CAFs from patient tumors (glioblastoma, GB2M7, breast, BC1M2, and prostate carcinoma, PCM1172) were evaluated for PEDF expression and treated with 10 and 20 nM exogenous PEDF. Note decreased vimentin expression in PEDF-treated fibroblasts and low pre-treatment PEDF levels in the cytoplasm, which increase after PEDF exposure.
Fibroblasts isolated from the patient tumors of varying origin all showed strikingly low levels of PEDF (Fig. 6F). As in TGF-β treated fibroblasts, exogenous PEDF significantly increased its own expression. While it had no significant effect on α-SMA, there was a significant decrease in mesenchymal marker vimentin, which could be indicative of normalization process due to disrupted TGF-β signaling.
Together, our results support the notion that PEDF is an endogenous factor that maintains tumor suppressive qualities of the fibroblasts (see discussion). This is important, because the microenvironment factors that maintain fibroblasts in their normal, tumor-suppressive state are not known and PEDF may be the first of such factors described.
Discussion
To date, most studies focused on the tumor-promoting role of CAFs, however, some clearly demonstrate the heterogeneity of CAFs and their potent tumor-suppressive effects (reviewed in (33, 34)). Fibroblast depletion of in a mouse model of pancreatic carcinoma is causes accelerated tumor progression, immunosuppression, and drug resistance (35). Another study shows potent tumor-suppressive effects of CAFs from breast cancer specimens expressing Slit2 (36). Similarly, PTEN expression by stromal fibroblasts conveys tumor suppressive effects, by stimulating miR-320, an inhibitor of Ets2 dependent transcription (37–39). A more recent study shows tumor suppressive properties of podoplanin-expressing fibroblasts in lung cancer (40). In light of these observations, a new model has been proposed, where CAFs, like tumor-associated macrophages, can be polarized towards F1 (tumor-suppressive) and F2 (tumor-promoting) states (33). While the factors driving F1-F2 conversion are well known (TGF-β, PDGF-BB, SDF-1), little is known about extracellular molecules that can cause or maintain F1 state and PEDF may be the first such protein identified.
The consequences and underlying mechanisms of PEDF loss by the tumor cells have been extensively studied; however, PEDF role and regulation in the tumor microenvironment is poorly understood. We have focused on the role of fibroblast-derived PEDF. Recent studies in PEDF null mice established its role as an anti-fibrotic factor in the liver and pancreas (41, 42) suggesting that PEDF down-regulation in fibroblasts, where it is normally high, may be essential for their conversion to tumor-promoting, F2 CAFs.
Using in vivo models where PEDF-positive or PEDF-null fibroblasts generated by shRNA knockdown are combined with melanoma cells, we were able to corroborate and in part explain earlier findings where host-derived PEDF interferes with tumor dissemination (29). In mice with pre-established subcutaneous melanoma, we showed that fibroblasts lacking PEDF accelerated tumor growth by decreasing apoptosis, enhancing proliferation and promoting angiogenesis. In contrast, PEDF-positive, normal fibroblasts reduced tumor angiogenesis as was evidenced by a significant decrease in MVD compared to control tumors in the absence of exogenous fibroblasts. Importantly, the effect of exogenously added PEDF-positive fibroblasts was significantly dampened by contact with the tumor cells. Co-injection with C8161-HA melanoma cells attenuated HDF's ability to halt tumor growth and angiogenesis, in contrast with their effect when inoculated remotely. This could be explained if close proximity to melanoma cells attenuated PEDF expression in fibroblasts.
Indeed, we discovered that even 24-h exposure of primary dermal fibroblasts to the media conditioned the PEDF null melanoma cells significantly reduced PEDF expression. Using pharmacologic inhibitors and direct screening, we identified PDGF-BB and TGF-β among factors secreted by the melanoma cells, as sufficient to attenuate PEDF expression. Both PDGF-BB and TGF-β play prominent roles in the formation of reactive stroma in solid tumors. PDGF-BB is thought to be critical for fibroblast recruitment and proliferation, while TGF-β is considered one of the main factors that cause F1 - F2 conversion (normal fibroblasts to CAFs), with up-regulated expression of ECM components, pro-fibrotic and pro-invasive factors (43). We found that JNK and p38, but not Erk1/2 were required for this PEDF reduction. Intriguingly, using specific inhibitors of PDGFR and TGF-β signaling, we found that only PDGF-BB directly inhibited PEDF protein expression, while TGF-β1 modulated PEDF expression indirectly, by up-regulating PDGF receptor. This was suggested by the fact that PDGF inhibitor, Crenolanib, reversed PEDF reduction caused by TGF-β. Our findings are consistent with prior results, were TGF-β1 was found to enhance PDGFR-β expression in hepatic stellate cells (32). Further analysis showed that PDGFR-β induction by TGF-β1 required PI3K, JNK1/2, p38 and to a lesser degree Erk1/2. Moreover, we found that TGF-β also up-regulated PDGF-BB ligand. Together our findings suggest that PDGF-BB is a direct regulator of PEDF expression, whereas TGF-β controls PEDF levels by enhancement of PDGF-BB expression and signaling (summarized in Fig. 5I).
Our in vivo results combined with in vitro findings led us to conclude that PEDF may control the activation state of the fibroblasts whereby high PEDF levels are consistent with quiescence and normal (F1) polarization state, while low PEDF levels signify activation and conversion to CAFs and F2 polarization. This hypothesis is in agreement with the previous findings that PEDF is expressed at high levels in non-stimulated early-passage fibroblasts and is down-regulated when they become proliferative (44) or senescencent (45). Loss of fibroblastic PEDF appears permissive to F2 polarization (CAF conversion) since PEDF inhibits TGF-β responses in primary fibroblasts, including SMAD signaling, Erk1/2 signaling and the F2 marker, α-SMA. Moreover, PEDF knockdown fibroblasts caused overall changes in fibroblast expression profile, which favor tumor progression. The up-regulated genes included HAS2, which is responsible for deposition of hyaluronan (HA). HA is a CD44 ligand and predictor of poor prognosis and metastasis in multiple cancers types, due in part to CD44-dependent recruitment of cancer stem cells (46). IL-8 is a known pro-inflammatory, pro-angiogenic chemokine whose with well established role in cancer including melanoma (47). Stanniocalcin-1 expression specifically by CAFs also is associated with poor prognosis and metastasis in cancers including melanoma (48). Most interesting, SERPINB2 (Plasminogen Activator Inhibitor 2) functions as a tumor suppressor, when expressed by cancer cells (49) but becomes a clear-cut oncogene if expressed by fibroblasts. In breast cancer models, it contributes to the formation of autophagic stroma, which fuels mitochondrial metabolism in cancer cells and promotes metastasis (50) or enables cancer cell survival and vascular cooption in the brain (51).
Our results point to PEDF as a critical factor that maintains F1 polarization of fibroblasts and limits their contribution to tumor progression. Tumors expressing high PDGF-BB levels overcome the negative pressure exerted by F1 fibroblasts by decreasing their PEDF production. This effect is further augmented by TGF-β, which up-regulates PDGFR-β. Our findings were corroborated by the fact that PEDF expression was extremely low in CAFs cultured from three types of human tumors and that exogenous PEDF attenuated some of the CAF F2 markers, and restored its own expression. Based on our observations, it is conceivable that pharmacologic inhibitors of PDGF-B and TGF-β can restore PEDF expression in fibroblasts in vivo, and could restrain or reverse fibroblasts activation and restore PEDF in the tumor milieu. In turn, fibroblasts derived-PEDF may elicit paracrine effects in the TME including inhibition of endothelial and tumor cell proliferation, induction of apoptosis and anti-tumor immune response (M1 macrophage polarization). Our findings also support the use of PEDF small peptide mimetics as therapeutic approach to enforce the normal state of tumor stroma and prevent tumor fibrosis and metastasis.
Supplementary Material
Acknowledgements
This study was funded by the following grants: T32 Carcinogenesis Training grant CA60013828 (NN), NCI R01 CA172669 (OV) and SAF2014-53819-R grant from Ministerio de Ciencia y Competitividad of Spain (BJ). AM, AU and OD were supported by the Baskes Family Research Fund. Primary fibroblast cultures and lentiviral stocks were generated at NU Skin Disease Research Core). CAFs were isolated at NU Center for Developmental therapeutics. We also thank Dr. Stanley Wiegand (Regeneron) for the gift of SERPINF1 KO mice.
Footnotes
Conflict of Interest: There is no conflict of interest to declare
CITATIONS
- 1.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell. 2012;21(3):309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature reviews Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 3.Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Current opinion in genetics & development. 2001;11(1):54–9. doi: 10.1016/s0959-437x(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 4.Paulsson J, Micke P. Prognostic relevance of cancer-associated fibroblasts in human cancer. Seminars in cancer biology. 2014;25:61–8. doi: 10.1016/j.semcancer.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Hwang SM, Lee JM, Kang JH, Chung IY, Chung BG. Epithelial-to-mesenchymal transition of human lung alveolar epithelial cells in a microfluidic gradient device. Electrophoresis. 2013;34(3):441–7. doi: 10.1002/elps.201200386. [DOI] [PubMed] [Google Scholar]
- 6.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. British journal of cancer. 2008;99(9):1375–9. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miles FL, Sikes RA. Insidious changes in stromal matrix fuel cancer progression. Molecular cancer research : MCR. 2014;12(3):297–312. doi: 10.1158/1541-7786.MCR-13-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santi A, Caselli A, Paoli P, Corti D, Camici G, Pieraccini G, et al. The effects of CA IX catalysis products within tumor microenvironment. Cell communication and signaling : CCS. 2013;11:81. doi: 10.1186/1478-811X-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman RM. Stromal-cell and cancer-cell exosomes leading the metastatic exodus for the promised niche. Breast cancer research : BCR. 2013;15(3):310. doi: 10.1186/bcr3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calon A, Tauriello DV, Batlle E. TGF-beta in CAF-mediated tumor growth and metastasis. Seminars in cancer biology. 2014;25:15–22. doi: 10.1016/j.semcancer.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Donovan J, Abraham D, Norman J. Platelet-derived growth factor signaling in mesenchymal cells. Front Biosci (Landmark Ed) 2013;18:106–19. doi: 10.2741/4090. [DOI] [PubMed] [Google Scholar]
- 12.Braicu C, Tudoran O, Balacescu L, Catana C, Neagoe E, Berindan-Neagoe I, et al. The significance of PDGF expression in serum of colorectal carcinoma patients--correlation with Duke's classification. Can PDGF become a potential biomarker? Chirurgia (Bucur) 2013;108(6):849–54. [PubMed] [Google Scholar]
- 13.Paulsson J, Sjoblom T, Micke P, Ponten F, Landberg G, Heldin CH, et al. Prognostic significance of stromal platelet-derived growth factor beta-receptor expression in human breast cancer. The American journal of pathology. 2009;175(1):334–41. doi: 10.2353/ajpath.2009.081030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lisanti MP, Martinez-Outschoorn UE, Sotgia F. Oncogenes induce the cancer-associated fibroblast phenotype: metabolic symbiosis and “fibroblast addiction” are new therapeutic targets for drug discovery. Cell Cycle. 2013;12(17):2723–32. doi: 10.4161/cc.25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taddei ML, Giannoni E, Comito G, Chiarugi P. Microenvironment and tumor cell plasticity: an easy way out. Cancer letters. 2013;341(1):80–96. doi: 10.1016/j.canlet.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Cheng H, Terai M, Kageyama K, Ozaki S, McCue PA, Sato T, et al. Paracrine effect of NRG1 and HGF drives resistance to MEK inhibitors in metastatic uveal melanoma. Cancer research. 2015 doi: 10.1158/0008-5472.CAN-15-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, et al. Intravital Imaging Reveals How BRAF Inhibition Generates Drug-Tolerant Microenvironments with High Integrin beta1/FAK Signaling. Cancer cell. 2015;27(4):574–88. doi: 10.1016/j.ccell.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orgaz JL, Ladhani O, Hoek KS, Fernandez-Barral A, Mihic D, Aguilera O, et al. `Loss of pigment epithelium-derived factor enables migration, invasion and metastatic spread of human melanoma'. Oncogene. 2009;28(47):4147–61. doi: 10.1038/onc.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becerra SP, Notario V. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nature reviews Cancer. 2013;13(4):258–71. doi: 10.1038/nrc3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285(5425):245–8. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 21.Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, et al. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nature medicine. 2002;8(4):349–57. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- 22.Biyashev D, Veliceasa D, Kwiatek A, Sutanto MM, Cohen RN, Volpert OV. Natural angiogenesis inhibitor signals through Erk5 activation of peroxisome proliferator-activated receptor gamma (PPARgamma) The Journal of biological chemistry. 2010;285(18):13517–24. doi: 10.1074/jbc.M110.117374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho TC, Chen SL, Yang YC, Liao CL, Cheng HC, Tsao YP. PEDF induces p53-mediated apoptosis through PPAR gamma signaling in human umbilical vein endothelial cells. Cardiovascular research. 2007;76(2):213–23. doi: 10.1016/j.cardiores.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Yao YC, Fang SH, Ma CQ, Cen Y, Xu ZM, et al. Pigment epithelial-derived factor (PEDF)-triggered lung cancer cell apoptosis relies on p53 protein-driven Fas ligand (Fas-L) up-regulation and Fas protein cell surface translocation. The Journal of biological chemistry. 2014;289(44):30785–99. doi: 10.1074/jbc.M114.590000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho TC, Chen SL, Shih SC, Chang SJ, Yang SL, Hsieh JW, et al. Pigment epithelium-derived factor (PEDF) promotes tumor cell death by inducing macrophage membrane tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) The Journal of biological chemistry. 2011;286(41):35943–54. doi: 10.1074/jbc.M111.266064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelius T, Samathanam C, Martinez-Marin D, Gaines N, Stevens J, Hickson J, et al. Positive correlation between PEDF expression levels and macrophage density in the human prostate. The Prostate. 2013;73(5):549–61. doi: 10.1002/pros.22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halin S, Rudolfsson SH, Doll JA, Crawford SE, Wikstrom P, Bergh A. Pigment epithelium-derived factor stimulates tumor macrophage recruitment and is downregulated by the prostate tumor microenvironment. Neoplasia. 2010;12(4):336–45. doi: 10.1593/neo.92046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladhani O, Sanchez-Martinez C, Orgaz JL, Jimenez B, Volpert OV. Pigment epithelium-derived factor blocks tumor extravasation by suppressing amoeboid morphology and mesenchymal proteolysis. Neoplasia. 2011;13(7):633–42. doi: 10.1593/neo.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lattier JM, Yang H, Crawford S, Grossniklaus HE. Host pigment epithelium-derived factor (PEDF) prevents progression of liver metastasis in a mouse model of uveal melanoma. Clinical & experimental metastasis. 2013;30(8):969–76. doi: 10.1007/s10585-013-9596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Barral A, Orgaz JL, Gomez V, del Peso L, Calzada MJ, Jimenez B. Hypoxia negatively regulates antimetastatic PEDF in melanoma cells by a hypoxia inducible factor-independent, autophagy dependent mechanism. PloS one. 2012;7(3):e32989. doi: 10.1371/journal.pone.0032989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elias EG, Hasskamp JH, Sharma BK. Cytokines and growth factors expressed by human cutaneous melanoma. Cancers. 2010;2(2):794–808. doi: 10.3390/cancers2020794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah R, Reyes-Gordillo K, Arellanes-Robledo J, Lechuga CG, Hernandez-Nazara Z, Cotty A, et al. TGF-beta1 up-regulates the expression of PDGF-beta receptor mRNA and induces a delayed PI3K-, AKT-, and p70(S6K) -dependent proliferative response in activated hepatic stellate cells. Alcoholism, clinical and experimental research. 2013;37(11):1838–48. doi: 10.1111/acer.12167. [DOI] [PubMed] [Google Scholar]
- 33.Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Frontiers in oncology. 2014;4:62. doi: 10.3389/fonc.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth--bystanders turning into key players. Current opinion in genetics & development. 2009;19(1):67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer cell. 2014;25(6):719–34. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang PH, Hwang-Verslues WW, Chang YC, Chen CC, Hsiao M, Jeng YM, et al. Activation of Robo1 signaling of breast cancer cells by Slit2 from stromal fibroblast restrains tumorigenesis via blocking PI3K/Akt/beta-catenin pathway. Cancer research. 2012;72(18):4652–61. doi: 10.1158/0008-5472.CAN-12-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bronisz A, Godlewski J, Wallace JA, Merchant AS, Nowicki MO, Mathsyaraja H, et al. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nature cell biology. 2012;14(2):159–67. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace JA, Li F, Leone G, Ostrowski MC. Pten in the breast tumor microenvironment: modeling tumor-stroma coevolution. Cancer research. 2011;71(4):1203–7. doi: 10.1158/0008-5472.CAN-10-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461(7267):1084–91. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi A, Ishii G, Neri S, Yoshida T, Hashimoto H, Suzuki S, et al. Podoplanin-expressing cancer-associated fibroblasts inhibit small cell lung cancer growth. Oncotarget. 2015;6(11):9531–41. doi: 10.18632/oncotarget.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmitz JC, Protiva P, Gattu AK, Utsumi T, Iwakiri Y, Neto AG, et al. Pigment epithelium-derived factor regulates early pancreatic fibrotic responses and suppresses the profibrotic cytokine thrombospondin-1. The American journal of pathology. 2011;179(6):2990–9. doi: 10.1016/j.ajpath.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai TH, Shih SC, Ho TC, Ma HI, Liu MY, Chen SL, et al. Pigment Epithelium-Derived Factor 34-mer Peptide Prevents Liver Fibrosis and Hepatic Stellate Cell Activation through Down-Regulation of the PDGF Receptor. PloS one. 2014;9(4):e95443. doi: 10.1371/journal.pone.0095443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. American journal of cancer research. 2011;1(4):482–97. [PMC free article] [PubMed] [Google Scholar]
- 44.Coljee VW, Rotenberg MO, Tresini M, Francis MK, Cristofalo VJ, Sell C. Regulation of EPC-1/PEDF in normal human fibroblasts is posttranscriptional. Journal of cellular biochemistry. 2000;79(3):442–52. doi: 10.1002/1097-4644(20001201)79:3<442::aid-jcb90>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 45.Pignolo RJ, Cristofalo VJ, Rotenberg MO. Senescent WI-38 cells fail to express EPC-1, a gene induced in young cells upon entry into the G0 state. The Journal of biological chemistry. 1993;268(12):8949–57. [PubMed] [Google Scholar]
- 46.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 47.Singh S, Singh AP, Sharma B, Owen LB, Singh RK. CXCL8 and its cognate receptors in melanoma progression and metastasis. Future Oncol. 2010;6(1):111–6. doi: 10.2217/fon.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pena C, Cespedes MV, Lindh MB, Kiflemariam S, Mezheyeuski A, Edqvist PH, et al. STC1 expression by cancer-associated fibroblasts drives metastasis of colorectal cancer. Cancer research. 2013;73(4):1287–97. doi: 10.1158/0008-5472.CAN-12-1875. [DOI] [PubMed] [Google Scholar]
- 49.McMahon B, Kwaan HC. The plasminogen activator system and cancer. Pathophysiology of haemostasis and thrombosis. 2008;36(3–4):184–94. doi: 10.1159/000175156. [DOI] [PubMed] [Google Scholar]
- 50.Castello-Cros R, Bonuccelli G, Molchansky A, Capozza F, Witkiewicz AK, Birbe RC, et al. Matrix remodeling stimulates stromal autophagy, “fueling” cancer cell mitochondrial metabolism and metastasis. Cell Cycle. 2011;10(12):2021–34. doi: 10.4161/cc.10.12.16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156(5):1002–16. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.