Abstract
Background
Improving symptoms is a major goal of cancer medicine; however, symptom response is often based on group differences and not individualized. We examined the personalized symptom goal (PSG) for 10 common symptoms in patients with advanced cancer, and identified the factors associated with PSG response.
Methods
In this prospective longitudinal multicenter study, patients from 5 tertiary care hospitals rated the intensity of 10 symptoms using a 0-10 numeric rating scale at first clinic visit and then a second visit 14-34 days later. PSG was determined for each symptom by asking patients: “At what level would you feel comfortable with this symptom?” using the same 0-10 scale for symptom intensity. PSG response was defined as symptom intensity at second visit less than or equal to PSG.
Results
Among 728 patients, the median PSG was 1 for nausea, 2 for depression, anxiety, drowsiness, well being, dyspnea and sleep, and 3 for pain, fatigue, and appetite. A greater proportion of patients achieved a PSG response in second visit compared to first visit (P<0.05 except for drowsiness). Symptom response was associated with lower baseline symptom intensity based on PSG criterion but higher baseline symptom intensity based on the traditional minimal clinically important difference definition (P<0.001 for all symptoms). In multivariable analysis, higher PSG and nationality were associated with greater PSG response.
Conclusion
PSG was ≤3 for a majority of patients. PSG response allows clinicians to tailor treatment goals, while adjusting for individual differences in scale interpretation and factors associated with symptom response.
Keywords: neoplasms, personalized medicine, palliative care, symptom assessment, treatment outcome
Introduction
Cancer patients experience significant symptom burden throughout the disease trajectory, including pain, fatigue, dyspnea, anorexia, anxiety and depression [1, 2]. Currently, patient reported outcomes represent the gold standard for symptom assessment and are used in clinical trials to determine symptom response and in clinical practice to guide decision making [3]. Because of its simplicity in administration and interpretation, the unidimensional numeric rating scale that ranges from 0 (no symptom) to 10 (worse possible) is used ubiquitously for symptom distress screening and assessment by multiple disciplines, such as oncology and palliative care worldwide [4-7].
Despite its widespread use, the 0-10 scale has some important limitations. First, because of its intrinsic subjectivity, significant variations exist in how individual patients interpret the scale and express their symptom intensity [8]. Second, response is difficult to determine because the minimal clinically important difference (MCID) is not always established. Even when available, the cutoffs for response only apply to group averages instead of individual patients, making it difficult to judge if a patient has achieved a response [9]. Furthermore, the use of absolute decrease as a criterion for response is potentially biased because patients with higher baseline symptom intensity were more likely to report a greater improvement [10].
In the era of personalized cancer care, clinicians need to tailor treatment recommendations to the individual patient. This applies not only to anti-neoplastic therapeutics, but also supportive care [11]. Personalized symptom goals are novel measures that may help to individualize supportive care therapies while overcoming some of the traditional challenges with patient reported outcomes. Personalized symptom goals are determined by asking the patient “At what level of symptom intensity would you feel comfortable?” Thus, personalized symptom goals provide a simple yet individualized therapeutic “target” for each symptom, and allows for intra-patient determination of symptom response that is both practical and meaningful. In a retrospective study, Dalal et al. first reported the personalized pain goal in 465 cancer patients seen at a Supportive Care Clinic at a Comprehensive Cancer Center [12]. The median personalized pain goal was 3/10 (interquartile range 2-3), and it did not change significantly (P=0.57) at followup visit that occurred a median of 2 weeks later [12]. To date, personalized symptom goal has been examined for pain only. Furthermore, the factors associated with baseline intensity of personalized symptom goals and achievement of these goals response have not been examined. A better characterization of personalized symptom goals, and factors associated with personalized symptom goal level and response would help clinicians to personalize symptom management. In this study, we characterized the personalized symptom goal for 10 common symptoms in patients with advanced cancer seen at supportive care clinics, and identified the patient factors associated with personalized symptom response. We hypothesize that a higher personalized symptom goal, because of the lower symptom threshold, is associated with a greater likelihood of achieving a personalized symptom response.
Methods
Participants
This is an international longitudinal observational study that examined symptom response in cancer patients [13]. We enrolled patients with advanced cancer, age 18 years or greater, who were seen at an outpatient supportive care clinic at one of the 5 participating centers, and have a return clinic visit scheduled 14 to 34 days later. Patients with delirium (Memorial Delirium Assessment Scale [MDAS] of 13 or greater) were excluded. The institution review boards at all participating centers approved the study. All participants provided written informed consent.
Participating centers included MD Anderson Cancer Center in Houston, United States; King Hussein Cancer Center in Amman, Jordan; Barretos Cancer Hospital in Barretos, Brazil; and Pontificia Universidad Catolica de Chile in Santigo, Chile, and Tata Memorial Center in Mumbai, India. All 5 institutions were tertiary care hospitals with access to comprehensive cancer treatments and concurrent supportive care. All patients underwent symptom evaluation and treatment by a specialist palliative care team. With the exception of Chile, all centers were part of the Sister Institution Research Network, a multi-national cancer research cooperative.
Data collection
Data collection took place between December 8, 2011 and April 30, 2014. We collected baseline patient characteristics, including age, sex, race, education level, cancer diagnosis, Karnofsky Performance status, CAGE questionnaire [14] and MDAS [15] during the first study visit. The CAGE questionnaire consists of 4 questions (Cut down, Annoyed, Guilt, Eye opener), and has been widely used for screening of alcoholism (indicated by 2 or more affirmative responses) [14]. MDAS is a validated 10-item instrument for assessment of delirium. The total score ranges from 0 to 30 points, with a score ≥13 suggesting the presence of delirium [15].
We assessed the intensity of 10 common symptoms (i.e. pain, fatigue, nausea, depression, anxiety, drowsiness, shortness of breath, appetite, feelings of well-being and sleep) at both the first and second study visits using the Edmonton Symptom Assessment Scale (ESAS). ESAS is a validated symptom battery that uses a 0 (no symptom) to 10 (worst intensity) point numeric rating scale to examine the average intensity of each symptom over the past 24 hours [16, 17]. It has been psychometrically and linguistically validated and is available in the languages in respective countries (i.e. English, Arabic, Portugese, Spanish and Hindi) [18-23].
For each of the 10 symptoms, we assessed the personalized symptom goal at the second study visit by asking the patient “At what level would you feel comfortable with this symptom?” using the same 0-10 numeric rating scale. A previous study has shown that personalized symptom goal was stable over time, with minimal change between consultation and followup visits [12].
The study questions were translated to the local languages to facilitate data collection and back-translated to ensure accuracy of translation. The site investigators visited Houston to understand the data collection process, and provided training to the local research staff. Teleconferences were held between the principal investigator and each site investigator twice a month to provide data monitoring.
Statistical analysis
This study was powered to examine the MCID for each of the 10 ESAS symptoms as the primary analysis [13]. The original study also included a 6th site (medical oncology clinic) but this was excluded to ensure homogeneity of our study population.
We summarized our data with descriptive statistics. We examined two response criteria in this study: the MCID response, defined as symptom intensity improvement from visit 1 to visit 2 of 1/10 point or greater [13]; and personalized symptom response (or achievement of personalized symptom goal) defined as symptom intensity at visit 2 ≤ personalized symptom goal. For example, if a patient stated her personalized symptom goal for dyspnea was 4, then any dyspnea intensity less than or equal to 4/10 was considered a personalized symptom response. We compared the proportion of patients who achieved their personalized symptom goal at visit 1 and visit 2 using the McNemar test. We also compared the response rates between the MCID response criterion and personalized symptom response criterion at each baseline symptom intensity category using the same test.
We examined the factors associated with personalized symptom response using multivariate logistic regression analysis. To minimize over-fitting, we limited the number of covariates in the model, which included age, sex, study site, cancer diagnosis, CAGE positivity and personalized symptom goal intensity.
The Statistical Analysis Software 9.3 (SAS Institute Inc., Cary, NC, USA) software was used for statistical analysis. Statistically significance was declared when the P-value is <0.05.
Results
Patient characteristics
The baseline demographics are shown in Table 1. The average age was 57 (range 19-85). 361 (50%) were female; 229 (31%) were Caucasian; and 157 (22%) had gastrointestinal cancers. A majority of patients (n=632, 87%) had metastatic disease. The median duration between the two study visits was 21 days (interquartile range 18-28 days).
Table 1. Patient Characteristics (N=728).
| Variables | N (%)* |
|---|---|
| Age, average (range) | 57 (19-85) |
| Female | 361 (50) |
| Race | |
| Caucasian | 229 (31) |
| Black | 37 (5) |
| Hispanic | 224 (31) |
| Asian | 55 (8) |
| Other | 183 (25) |
| Marital status | |
| Single | 98 (13) |
| Married | 502 (69) |
| Divorced | 126 (17) |
| Education | |
| Illiterate | 6 (1) |
| High school or less | 355 (49) |
| Some college up to Bachelor's | 299 (41) |
| Advanced degree | 68 (9) |
| Cancer | |
| Breast | 131 (18) |
| Gastrointestinal | 157 (22) |
| Genitourinary | 77 (11) |
| Gynecological | 64 (9) |
| Head and neck | 70 (10) |
| Hematological | 31 (4) |
| Other | 84 (12) |
| Respiratory | 114 (16) |
| Stage | |
| Advanced, non-metastatic | 96 (13) |
| Metastatic | 632 (87) |
| CAGE positive† | 100 (14) |
| Memorial Delirium Assessment Scale, median (Q1-Q3) | 2 (1,3) |
| Karnofsky Performance Status, average (SD)‡ | 69 (13) |
| Duration between visits, median (Q1-Q3) | 21 (18,28) |
| Edmonton Symptom Assessment Scale, median (Q1-Q3) | |
| Pain | 3 (1, 4) |
| Fatigue | 3 (1, 4) |
| Nausea | 1 (0, 3) |
| Depression | 2 (0, 3) |
| Anxiety | 2 (0, 3) |
| Drowsiness | 2 (1, 4) |
| Appetite | 3 (1, 4) |
| Well Being | 2 (1, 3.5) |
| Dyspnea | 2 (0, 3) |
| Sleep | 2 (1, 4) |
Abbreviations: Q1-Q3, interquartile range
unless otherwise specified
CAGE was considered to be positive if patients scored 2 or more point out of 4
During second visit
Personalized Symptom Goal and Response
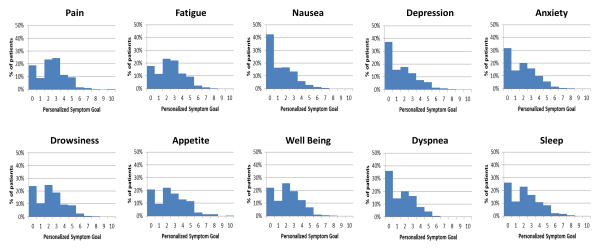
The median level of personalized symptom goal was 1 for nausea, 2 for depression, anxiety, drowsiness, well being, dyspnea and sleep and 3 for pain, fatigue, and appetite (Table 2). A majority of patients had personalized symptom goal of 3 or less (Figure 1).
Table 2. Achievement of Personalized Symptom Goal.
| Symptom | N | PSG intensity, median (Q1-Q3) | Proportion of patients with PSG ≤3 N (%) | Proportion of patients who achieved PSG at first visit N (%)* | Proportion of patients who achieved PSG at second visitN (%)* | Difference, % | P-value† |
|---|---|---|---|---|---|---|---|
| Pain | 722 | 3 (1, 4) | 548 (76) | 215 (30) | 301 (42) | 12 | <0.0001 |
| Fatigue | 722 | 3 (1, 4) | 541 (75) | 182 (25) | 235 (33) | 8 | <0.0001 |
| Nausea | 721 | 1 (0, 3) | 645 (89) | 485 (67) | 526 (73) | 6 | 0.007 |
| Depression | 722 | 2 (0, 3) | 603 (84) | 395 (55) | 425 (59) | 4 | 0.03 |
| Anxiety | 723 | 2 (0, 3) | 593 (82) | 333 (46) | 392 (54) | 8 | <0.0001 |
| Drowsiness | 722 | 2 (1, 4) | 564 (78) | 342 (47) | 354 (49) | 2 | 0.41 |
| Appetite | 722 | 3 (1, 4) | 505 (70) | 279 (39) | 334 (46) | 7 | 0.0002 |
| Well Being | 721 | 2 (1, 3.5) | 569 (79) | 211 (29) | 246 (34) | 5 | 0.009 |
| Dyspnea | 722 | 2 (0, 3) | 626 (87) | 403 (56) | 460 (64) | 8 | <0.0001 |
| Sleep | 723 | 2 (1, 4) | 552 (76) | 255 (35) | 333 (46) | 11 | <0.0001 |
Abbreviation: PSG, personalized symptom goal; Q1-Q3, interquartile range
Achievement of personalized symptom goal was defined as symptom intensity less than or equal to personalized symptom goal for that symptom.
We compared the proportion of patients who achieved PSG between visit 1 and visit 2 using the McNemar test.
Figure 1. Distribution of Personalized Symptom Goal for 10 Symptoms.
A majority of patients reported a personalized symptom goal of 3 or less.
The personalized symptom response ranged between 33% and 73% at the second visit (Table 2). Compared to the initial visit, a greater proportion of patients achieved their personalized symptom goal for a majority of ESAS symptoms.
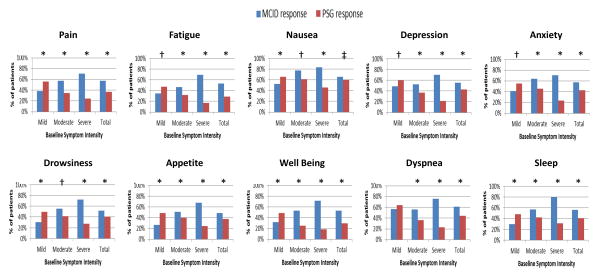
We compared the performance of personalized symptom response against the tradition MCID response criterion in Figure 2. Patients with higher baseline symptom intensity were more likely to achieve a response based on the MCID criteria. However, use of the personalized symptom goal yielded the opposite finding, with patients who had a lower baseline symptom intensity being significantly more likely to achieve a symptom response (P<0.001 for all symptoms).
Figure 2. Response Rates Differences by Baseline Symptom Intensity and Response Criteria.
We plotted the response rates by two criteria (minimal clinically important difference [MCID] and personalized symptom goal [PSG]) according to baseline symptom intensity (i.e. mild 1-3, moderate 4-6, and severe 7-10). Using the MCID criteria, patients with higher baseline symptom intensity were more likely to achieve a response and vice versa; in contrast, the personalized symptom response criteria resulted in the opposite conclusion. P-values were computed based on the McNemer test (* P<0.0001, † P<0.001, ‡ P<0.05)
Factors Associated with Personalized Symptom Response at Visit 2
In multivariable analysis, higher personalized symptom goal and nationality were associated with personalized symptom response for almost all the symptoms (Table 3). Older age was associated with lower response for anorexia, male sex was associated with greater response for anxiety, while CAGE positivity was associated with lower response for anxiety. Cancer diagnosis was also associated with altered response for several symptoms.
Table 3. Multivariate Logistic Regression Analysis of Factors Associated with Achievement of Personalized Symptom Goal at Followup Visit.
| Odds ratio (95% confidence interval) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pain | Fatigue | Nausea | Depression | Anxiety | Drowsiness | Appetite | Well Being | Dyspnea | Sleep | |
| Age (per year increase) | 1.01 (0.99-1.02) | 0.99 (0.98-1) | 1 (0.98-1.01) | 0.99 (0.98-1.01) | 1 (0.99-1.02) | 0.99 (0.98-1.01) | 0.99 (0.97-1) | 1 (0.98-1.01) | 0.99 (0.98-1) | 1 (0.99-1.01) |
| P-value | 0.42 | 0.14 | 0.67 | 0.23 | 0.59 | 0.40 | 0.04 | 0.60 | 0.10 | 0.97 |
|
| ||||||||||
| Sex | ||||||||||
| Female | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Male | 1.34 (0.93-1.95) | 1.19 (0.79-1.79) | 1.36 (0.91-2.04) | 1.32 (0.91-1.9) | 1.58 (1.1-2.27) | 1.21 (0.83-1.77) | 1.14 (0.79-1.66) | 0.86 (0.57-1.28) | 0.81 (0.56-1.18) | 0.81 (0.55-1.19) |
| P-value | 0.12 | 0.41 | 0.13 | 0.14 | 0.01 | 0.32 | 0.48 | 0.45 | 0.28 | 0.29 |
|
| ||||||||||
| Cancer Diagnosis | ||||||||||
| Breast | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| GI | 0.98 (0.56-1.71) | 1.49 (0.8-2.77) | 0.78 (0.44-1.39) | 1.22 (0.71-2.1) | 1.04 (0.61-1.77) | 0.83 (0.48-1.46) | 0.6 (0.35-1.04) | 1.76 (0.96-3.25) | 1.37 (0.79-2.39) | 0.54 (0.3-0.95) |
| GU | 1.07 (0.53-2.16) | 1.44 (0.66-3.12) | 1.12 (0.52-2.41) | 1.17 (0.58-2.33) | 1.62 (0.81-3.25) | 0.74 (0.37-1.49) | 0.68 (0.34-1.37) | 3.4 (1.62-7.15) | 3.5 (1.6-7.62) | 1.22 (0.6-2.47) |
| Others | 1.04 (0.64-1.7) | 1.13 (0.65-1.97) | 1.23 (0.73-2.07) | 1.35 (0.84-2.18) | 1.76 (1.1-2.81) | 0.81 (0.49-1.33) | 0.76 (0.47-1.23) | 1.48 (0.86-2.55) | 1.31 (0.8-2.13) | 0.55 (0.33-0.91) |
| Respiratory | 0.97 (0.53-1.77) | 1.38 (0.71-2.69) | 0.86 (0.46-1.6) | 1.71 (0.95-3.07) | 1.02 (0.57-1.81) | 0.85 (0.47-1.55) | 0.98 (0.55-1.76) | 2.1 (1.09-4.05) | 0.84 (0.47-1.51) | 0.8 (0.44-1.46) |
| P-value | >0.99 | 0.71 | 0.37 | 0.43 | 0.03 | 0.92 | 0.27 | 0.022 | 0.002 | 0.009 |
|
| ||||||||||
| Nationality | ||||||||||
| US | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Brazil | 1.57 (1.01-2.44) | 3.08 (1.93-4.93) | 1.13 (0.67-1.9) | 1.91 (1.17-3.13) | 1.49 (0.93-2.39) | 0.93 (0.59-1.47) | 2.05 (1.29-3.27) | 0.98 (0.43-2.22) | 1.05 (0.5-2.21) | 0.55 (0.24-1.27) |
| Chile | 0.83 (0.47-1.47) | 1.16 (0.6-2.24) | 1.44 (0.75-2.74) | 0.55 (0.32-0.95) | 0.73 (0.42-1.25) | 0.45 (0.25-0.81) | 1.2 (0.68-2.1) | 1.77 (1.16-2.7) | 1.6 (1.05-2.43) | 1.72 (1.15-2.59) |
| India | 1.3 (0.67-2.5) | 1.62 (0.82-3.2) | 1.27 (0.56-2.88) | 0.38 (0.19-0.77) | 0.77 (0.39-1.53) | 4.84 (1.81-12.89) | 1.77 (0.9-3.48) | 1.39 (0.72-2.67) | 1.49 (0.76-2.93) | 4.87 (2.25-10.56) |
| Jordan | 0.43 (0.28-0.65) | 0.47 (0.29-0.76) | 0.64 (0.42-0.96) | 0.64 (0.43-0.93) | 0.57 (0.39-0.83) | 0.35 (0.24-0.53) | 0.73 (0.49-1.09) | 0.48 (0.29-0.78) | 0.63 (0.42-0.95) | 0.88 (0.58-1.35) |
| P-value | <0.001 | <0.001 | 0.06 | <0.001 | 0.003 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 |
|
| ||||||||||
| CAGE | ||||||||||
| Negative | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Positive | 0.68 (0.42-1.09) | 0.63 (0.37-1.06) | 0.74 (0.45-1.24) | 0.93 (0.57-1.5) | 0.61 (0.38-0.97) | 1.54 (0.95-2.52) | 1.34 (0.83-2.18) | 0.92 (0.56-1.51) | 1.39 (0.83-2.32) | 1.09 (0.66-1.78) |
| P-value | 0.11 | 0.08 | 0.25 | 0.75 | 0.04 | 0.08 | 0.23 | 0.74 | 0.21 | 0.74 |
|
| ||||||||||
| Personalized symptom goal (per 1 point increase) | 1.21 (1.1-1.34) | 1.44 (1.29-1.61) | 1.01 (0.9-1.14) | 1.03 (0.93-1.14) | 1.06 (0.96-1.17) | 1.22 (1.11-1.35) | 1.23 (1.12-1.34) | 1.53 (1.37-1.7) | 0.99 (0.9-1.1) | 1.41 (1.28-1.55) |
| P-value | <0.001 | <0.001 | 0.89 | 0.55 | 0.26 | <0.001 | <0.001 | <0.001 | 0.91 | <0.001 |
Abbreviations: GI, gastrointestinal; GU, genitourinary
Discussion
We examined the personalized symptom goal for 10 symptoms in an international cohort of cancer patients. The personalized symptom goals were 3 or less for a majority of patients, although wide variations exist. We found that the traditional response criteria using MCID was biased toward patients with high baseline symptom intensity compared to the personalized symptom response. Moreover, personalized symptom response was associated with the level of personalized symptom goal and nationality. This novel concept has the potential to revolutionize how patients, clinicians and researchers interpret symptom assessment scales, shifting the paradigm toward a more personalized approach in symptom management.
Personalized symptom goal provides important insights into how patients interpret the 0-10 scale. Although 0, 1-3, 4-6 and 7-10 points in a 0-10 scale generally correspond to none, mild, moderate and severe symptom burden, not all patients interpret the scale similarly [24]. In clinical practice, it is not uncommon to see some patients report a 9/10 symptom intensity appear comfortable and functional. Because it is not feasible to assess symptom perception, clinicians often rely on symptom expression as documented by patient reported outcomes using 0-10 scales. Previous studies showed that symptom expression varies with many patient-related factors. For example, older patients generally have a lower symptom expression, while patients with a history of alcoholism often report higher symptom burden [25, 26]. This raises the question if the altered symptom expression is due to differences in symptom perception and/or scale interpretation. The variation in personalized symptom goal observed in this study suggests that each patient interprets the scale differently and sets her own internal barometer for when she feels comfortable.
We found that not all patients set their personalized symptom goal as 0, which is consistent with a previous study demonstrating that the median personalized pain goal was 3 [12]. This study revealed that a majority of patients would feel comfortable for almost all their symptoms if it could be lowered to mild intensity (<=3/10). The exception was nausea (median personalized symptom goal was 0) in which the baseline intensity was low (median level 1). Further studies are needed to examine patient factors associated with variations in personalized symptom goal, particularly why some patients have high thresholds. To validate this concept further, future research should also examine if achievement of personalized symptom goal is associated with improvement in other outcomes such as medication dose adjustment and patient experience.
By asking the patient to specify his/her desired level of symptom control, personalized symptom goals set individualized therapeutic targets for each symptom, and in the process, establishes a novel outcome for both symptom management and research. Instead of aiming to reduce the symptom intensity by a pre-defined magnitude as defined by the MCID, it may be more meaningful to improve the symptom to a level in which the patient would like to achieve. For example, reduction of dyspnea from 7 to 6 out of 10 may be considered a clinical response based on MCID of 1 point [13]. However, a patient may not perceive this difference, and may remain quite uncomfortable when the personalized goal was 3/10. In another example, it may seem paradoxical for a patient to state that he was pleased with the current pain regimen when his pain was still 6/10; however, this would be logical if the personalized pain goal for this patient was actually 6.
In the research setting, personalized symptom goal allows clinicians to determine a reasonable target for each patient and the proportion of patients who achieve this goal, rather than an average change. This is similar to the concept of tumor response in which we need to document the intra-individual improvement rather than average change in tumor size. The use of personalized symptom goal also helps investigators to address a concern with the MCID criterion—that patients with higher symptom intensity were more likely to have a response, when in reality many of those who “responded” continue to have suboptimal symptom control (e.g. pain decreased from 9 to 7) [10]. As observed in this study and in a previous report on pain [12], MCID response criteria often resulted in over-estimation of response when pain intensity is high, and under-estimation of response when pain intensity is low. In contrast, personalized symptom response is based on a clear, individualized and meaningful target, and patients with higher baseline symptom scores were less likely to achieve their personalized response. Future clinical trials may consider incorporating personalized symptom response as a novel outcome in addition to the traditional MCID response criterion.
We found that personalized symptom response was associated with higher personalized symptom goal and nationality in multivariate analysis. Logically, patients who set a higher personalized symptom goal have a lower threshold for response. For instance, it is much more likely for patients to achieve a pain score at or below a personalized pain goal of 6/10 than 1/10. Variations among countries in personalized symptom response rates may be explained by differences in culture, patient expectations, clinic population, baseline symptom intensity and/or treatment effectiveness. Males were more likely to meet their anxiety goals, which may be related to psychological factors and/or pharmacogenetic factors. More research is needed to further examine factors that influence personalized symptom response.
The strengths of this study include the examination of a novel concept, the systematic examination of 10 symptoms which allows us to understand the pattern of symptom expression, and the multi-center nature making the findings more generalizable. Our study shows that a simple question can help clinicians calibrate the 0-10 symptom scale and personalize treatment goals. This concept explains the under-reporting and elevated expression of symptoms, and helps to define a meaningful treatment target tailored to the individual. Because personalize symptom goal is relatively stable over 3 weeks [12], it does not need to be asked every visit.
This study has several limitations. This study included only patients with advanced cancer seen by palliative care teams in large academic centers. The personalized symptom goals may differ in other clinical settings and patient populations. We also did not assess the test-test reliability of this scale because it has been examined previously [12]. Finally, we only followed patients for up to 34 days in this prospective study. Longer term follow up is needed to determine the stability of PSG over time.
The goal of medicine is to help patients achieve their unique care goals and to maximize comfort. The use of personalized symptom goals may help patients to communicate their goals for symptom management, clinicians to understand the internal barometer for each unique patient, and investigators to assess symptom response in a more individualized and meaningful manner. The use of personalized symptom goals may allow us to enhance delivery of personalized care for our patients.
Acknowledgments
We thank all the patients who participated in this study. We also thank Swati Bansal, Dr. Odai Khamash, Dr. Abdelrahman Alhawamdeh, Natalia Campacci, Camila Souza Crovador, Won Ji Yuen, Dr. Sarika Sane, and Dr. Mrunal Marathe for their assistance with data collection.
Funding: this research is supported by the sister institution network fund from the University of Texas MD Anderson Cancer Center, King Hussein Cancer Center, Barretos Cancer Hospital and Tata Memorial Center. EB was supported in part by National Institutes of Health Grants R01NR010162-01A1, R01CA122292-01, AND R01CA124481-01. DH is supported in part by an American Cancer Society Mentored Research Scholar Grant in Applied and Clinical Research (MRSG-14-1418-01-CCE) and a National Institutes of Health grant (R21CA186000-01A1).
Footnotes
Conflict of interest disclosures: None reported
Author Contributions: David Hui: Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing – original draft, writing – review and editing, visualization, supervision, project administration, and funding acquisition. Minjeong Park: Methodology, software, formal analysis, writing – review and editing, and visualization. Omar Shamieh: Methodology, investigation, resources, writing – review and editing, project administration, and funding acquisition. Carlos Eduardo Paiva: Methodology, investigation, resources, writing – review and editing, project administration, and funding acquisition. Pedro Emilio Perez-Cruz: Methodology, investigation, resources, writing – review and editing, project administration, and funding acquisition. Mary Ann Muckaden: Methodology, investigation, resources, writing – review and editing, project administration, and funding acquisition. Eduardo Bruera: Conceptualization, methodology, writing – review and editing, supervision, project administration, and funding acquisition.
References
- 1.Seow H, Barbera L, Sutradhar R, et al. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol. 2011;29:1151–8. doi: 10.1200/JCO.2010.30.7173. [DOI] [PubMed] [Google Scholar]
- 2.Mercadante S, Casuccio A, Fulfaro F. The course of symptom frequency and intensity in advanced cancer patients followed at home. J Pain Symptom Manage. 2000;20:104–12. doi: 10.1016/s0885-3924(00)00160-3. [DOI] [PubMed] [Google Scholar]
- 3.Fashoyin-Aje LA, Martinez KA, Dy SM. New patient-centered care standards from the commission on cancer: opportunities and challenges. J Support Oncol. 2012;10:107–11. doi: 10.1016/j.suponc.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Dudgeon D, King S, Howell D, et al. Cancer Care Ontario's experience with implementation of routine physical and psychological symptom distress screening. Psychooncology. 2012;21:357–64. doi: 10.1002/pon.1918. [DOI] [PubMed] [Google Scholar]
- 5.Bultz BD, Waller A, Cullum J, et al. Implementing routine screening for distress, the sixth vital sign, for patients with head and neck and neurologic cancers. J Natl Compr Canc Netw. 2013;11:1249–61. doi: 10.6004/jnccn.2013.0147. [DOI] [PubMed] [Google Scholar]
- 6.Farrar JT, Pritchett YL, Robinson M, et al. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. J Pain. 2010;11:109–18. doi: 10.1016/j.jpain.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Gift AG, Narsavage G. Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care. 1998;7:200–04. [PubMed] [Google Scholar]
- 8.Hui D, Bruera E. Supportive and Palliative Oncology: A New Paradigm for Comprehensive Cancer Care. Hematology & Oncology Review. 2013;9:68–74. [Google Scholar]
- 9.Sloan JA. Assessing the minimally clinically significant difference: scientific considerations, challenges and solutions. COPD. 2005;2:57–62. doi: 10.1081/copd-200053374. [DOI] [PubMed] [Google Scholar]
- 10.Kang JH, Kwon JH, Hui D, et al. Changes in Symptom Intensity Among Cancer Patients Receiving Outpatient Palliative Care. J Pain Symptom Manage. 2013;46:652–60. doi: 10.1016/j.jpainsymman.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Hui D, Bruera E. A personalized approach to assessing and managing pain in patients with cancer. J Clin Oncol. 2014;32:1640–6. doi: 10.1200/JCO.2013.52.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalal S, Hui D, Nguyen L, et al. Achievement of personalized pain goal in cancer patients referred to a supportive care clinic at a comprehensive cancer center. Cancer. 2012;118:3869–77. doi: 10.1002/cncr.26694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui D, Shamieh O, Paiva C, et al. Minimal Clinically Important Differences in the Edmonton Symptom Assessment Scale in Cancer Patients: A Prospective Study. Cancer. 2015;121:3027–35. doi: 10.1002/cncr.29437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–07. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 15.Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Assessment Scale. J Pain Symptom Manage. 1997;13:128–37. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 16.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 17.Hannon B, Dyck M, Pope A, et al. Modified Edmonton Symptom Assessment System including constipation and sleep: validation in outpatients with cancer. J Pain Symptom Manage. 2015;49:945–52. doi: 10.1016/j.jpainsymman.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–71. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Carvajal A, Centeno C, Watson R, et al. A comprehensive study of psychometric properties of the Edmonton Symptom Assessment System (ESAS) in Spanish advanced cancer patients. Eur J Cancer. 2011;47:1863–72. doi: 10.1016/j.ejca.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Kwon JH, Nam SH, Koh S, et al. Validation of the Edmonton Symptom Assessment System in Korean patients with cancer. J Pain Symptom Manage. 2013;46:947–56. doi: 10.1016/j.jpainsymman.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton Symptom Assessment System: a 15-year retrospective review of validation studies (1991--2006) Palliat Med. 2008;22:111–22. doi: 10.1177/0269216307087659. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe SM, Nekolaichuk CL, Beaumont C. The Edmonton Symptom Assessment System, a proposed tool for distress screening in cancer patients: development and refinement. Psychooncology. 2012;21:977–85. doi: 10.1002/pon.1996. [DOI] [PubMed] [Google Scholar]
- 23.Paiva CE, Manfredini LL, Paiva BS, et al. The Brazilian Version of the Edmonton Symptom Assessment System (ESAS) Is a Feasible, Valid and Reliable Instrument for the Measurement of Symptoms in Advanced Cancer Patients. PLoS ONE. 2015;10:e0132073. doi: 10.1371/journal.pone.0132073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oldenmenger WH, de Raaf PJ, de Klerk C, et al. Cut points on 0-10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: a systematic review. J Pain Symptom Manage. 2013;45:1083–93. doi: 10.1016/j.jpainsymman.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Parsons HA, Delgado-Guay MO, El Osta B, et al. Alcoholism screening in patients with advanced cancer: impact on symptom burden and opioid use. J Palliat Med. 2008;11:964–8. doi: 10.1089/jpm.2008.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dev R, Parsons HA, Palla S, et al. Undocumented alcoholism and its correlation with tobacco and illegal drug use in advanced cancer patients. Cancer. 2011;117:4551–6. doi: 10.1002/cncr.26082. [DOI] [PMC free article] [PubMed] [Google Scholar]