Abstract
Background
Constraint-based therapy and peripheral nerve stimulation can significantly enhance movement function after stroke. No studies have investigated combining these interventions for cases of chronic, mild-to-moderate hemiparesis following stroke.
Objective
Determine the effects of peripheral nerve stimulation paired with a modified form of constraint-induced therapy on upper extremity movement function after stroke.
Design
Nineteen adult stroke survivors with mild-to-moderate hemiparesis more than 12 months after stroke received 2 hours of either active (n=10) or sham (n=9) peripheral nerve stimulation preceding 4 hours of modified constraint-induced therapy (10 sessions).
Results
Active peripheral nerve stimulation enhanced modified constraint-induced therapy more than sham peripheral nerve stimulation (significance at P<0.05), both immediately after intervention (Wolf Motor Function Test: P=0.006 (timed score); P=0.001 (lift score); Fugl-Meyer Assessment: P=0.022; Action Research Arm Test: P=0.007); and at 1-month follow-up (Wolf Motor Function Test: P=0.025 (timed score); P=0.007 (lift score); Fugl-Meyer Assessment: P=0.056; Action Research Arm Test: P=0.028).
Conclusion
Pairing peripheral nerve stimulation with modified constraint-induced therapy can lead to significantly more improvement in upper extremity movement function than modified constraint-induced therapy alone. Future research is recommended to help establish longitudinal effects of this paired intervention, particularly as it affects movement function and daily life participation.
Keywords: upper extremity, neuroplasticity, occupational therapy, humans
Introduction
Efforts to minimize neurologic damage in acute stroke have met with only limited success1. In turn, there is a crucial need for therapeutic interventions to enhance long-term functional recovery after stroke2. Neuroplastic change (reorganization of neuronal properties) has been associated with functional recovery for neurological populations, including stroke3, 4. Thus, interventions that harness neuroplasticity could be used to enhance recovery of function after stroke. Furthermore, sensory input has been associated with neuroplastic change and recovery of movement function following cortical lesions5, 6. While decrease in afferent input can reduce cortical maps of the deafferented area7, 8, increase in afferent input can increase motor cortical excitability9, 10. This evidence highlights how sensory-based therapeutic interventions may enhance the potential for recovery of movement function after stroke.
A sensory-based intervention called peripheral nerve stimulation (PNS) has been shown to directly affect sensory and motor networks by increasing motor cortical excitability (an indicator of neuroplasticity) and improving post-stroke movement function, especially when delivered as a paired intervention with motor training. For example, in a sham-controlled study, Sawaki and colleagues compared the effects of PNS on voluntary movement of paretic thumb in 7 subjects enrolled at least 6 months after stroke. Results showed significantly more neuroplastic change associated with PNS than with sham conditions11. A study by Ikuno and colleagues enrolled 22 subjects less than 6 months post-ictus, each of whom received a 1-week block of PNS combined with intensive, task-oriented training as well as a 1-week block of training alone. Subjects were randomized to receive either the PNS-training intervention first or the training-alone intervention first. From baseline to 1 week, the PNS-training group showed more improvement in upper extremity (UE) movement function than the training-alone group as measured by the Wolf Motor Function Test (WMFT). Both groups showed significant improvements on the WMFT after their respective periods of PNS combined with task-oriented training. Authors concluded that PNS may enhance outcomes of intensive, task-oriented training for individuals less than 6 months from stroke onset12. A 2011 systematic review by Laufer and colleagues concluded that PNS may enhance aspects of motor recovery after stroke, especially in concert with active motor training. However, for subjects more than 12 months from stroke onset, the review indicated that no evidence exists about the effects of PNS paired with a modified form of constraint-induced therapy (CIT) in cases of mild-to-moderate hemiparesis.
CIT is a form of motor training that has recently emerged to prominence in stroke rehabilitation research12. This approach compels intensive, task-oriented use of the affected limb while the non-affected limb is constrained. CIT developed from groundbreaking studies in which unilaterally deafferented monkeys regained use of their affected limbs after restraint of the non-affected limbs13. Translated to humans, the original CIT protocol (ie, 6 hours daily; 10-12 consecutive weekdays) can significantly improve UE movement function in cases of neurological impairment, even in long-term stages of recovery following stroke14, 15. Moreover, CIT can have superior, more lasting benefit for UE movement function than standard stroke rehabilitation12, 16. Modified forms of CIT (less training time, duration, or intensity than original CIT) have also been shown to lead to significant improvement in UE movement function after stroke17.
CIT-based protocols (e.g., original CIT; modified CIT) rely on the premise that motor learning involves entrainment of cortical motor neurons via intensive, repetitive practice of functional tasks3, 18. CIT can lead to significant neuroplastic change after stroke, including enlarged cortical motor maps as measured by transcranial magnetic stimulation16, 19. Additionally, in a pre-post comparison of the effects of CIT, Laible and colleagues found that increases in affected UE motor capacity (as measured by WMFT) were closely related to changes in ipsilesional S1 activation peaks (as measured by functional magnetic resonance imaging (fMRI)) in subjects with moderate UE hemiparesis more than 12 months after stroke20. Furthermore, Hamzei and colleagues found that CIT-related activation changes in sensorimotor cortex (as measured by fMRI) are highly similar to changes resulting from a modified form of CIT21 in subjects with moderate UE hemiparesis more than 24 months after stroke. In sum, research has shown that changes in sensory and motor networks are associated with motor gains induced by CIT or modified CIT in subjects with moderate hemiparesis in long-term stages of recovery following stroke.
The present study adds novel findings by reporting on an investigation of the following central hypothesis: subjects who receive active PNS paired with a modified form of CIT will have significantly more improved UE movement function than subjects who receive sham PNS paired with the same training protocol (ie, a modified form of CIT). The present article reports on the aim to assess effects of intervention on activity-based measures of UE movement function for subjects with mild-to-moderate UE hemiparesis more than 12 months after stroke.
Methods
In accordance with the Declaration of the World Medical Association (www.wma.net), this study was approved by the authorized institutional human research review boards at the institutions governing the research (ie, the University of Kentucky and Cardinal Hill Hospital in Lexington, KY). The research setting was a neurorehabilitation research lab located on the premises of Cardinal Hill Hospital in Lexington, KY. The date range defining the periods of data collection was 11/21/06-02/23/10. All procedures followed in this study were in accordance with institutional guidelines. Subjects were recruited from local and regional communities, including local hospitals and clinics. Inclusion Criteria: Recruitment targeted subjects with mild-to-moderate UE motor deficit after a single ischemic stroke. “Mild-to-moderate” was defined according to standard eligibility criteria for CIT12 (ie, able to extend the affected metacarpophalangeal and interphalangeal joints at least 10°; and the wrist, 20°). Targeted subjects were adults (ie, 18 years of age and older) at least 12 months from stroke onset. Targeting this phase helped mitigate the potential confound of spontaneous motor recovery, which usually occurs within the first 12 months after stroke onset.
Exclusion criteria were established to minimize potential confounding variables. These criteria included a) history of carpal tunnel syndrome and documented peripheral neuropathy; b) within 3 months of recruitment, addition or change in the dosage of drugs known to exert detrimental effects on motor recovery22; and c) aphasia or cognitive deficit severe enough to preclude informed consent.
Figure 1 details the workflow of the study. As required by the authorized institutional human research review boards at the institutions where the research was conducted, all subjects provided written informed consent after receiving a verbal and written explanation of the purposes, procedures, and potential hazards of this study. This study used a parallel-group block design within the conceptual framework of a superiority trial. After enrollment, subjects were evaluated with regard to UE movement function at baseline, after completion of the intervention period, and at 1-month follow-up. Following baseline evaluation, a computer-generated randomizer program was used to generate the simple random allocation sequence (1:1) of subjects into 2 groups (ie, either active PNS paired with a modified CIT protocol, or sham (control) PNS paired with a modified CIT protocol). The PI used an experimental design generator and randomizer program for simple random allocation of subjects into equal-sized groups. The PI generated the random allocation sequence, enrolled subjects, and assigned subjects to interventions. Each intervention session consisted of either active or sham PNS (2 hours) immediately preceding modified CIT (4 hours). PNS was the only independent variable. Subjects, care providers, and assessors of movement function were blinded to group assignment in that they were not made aware of which PNS condition any subject received. Additionally, personnel administering PNS did not administer modified CIT. Subjects were ordered by the randomizer in strict accordance with the order of enrollment.
Figure 1.
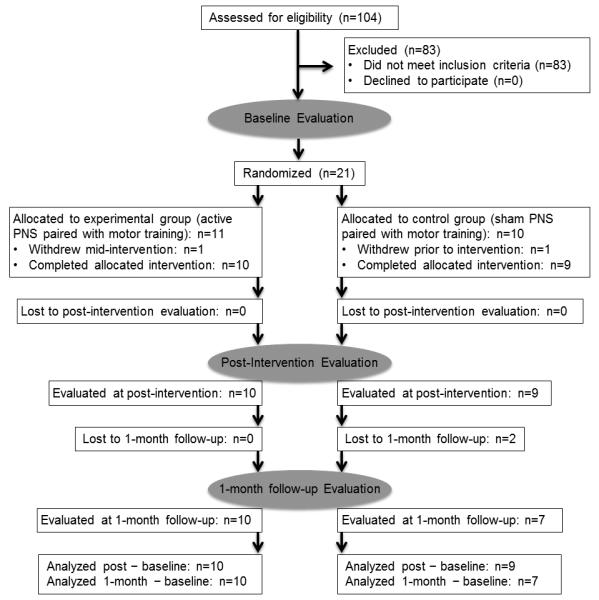
Diagram of Study.
Sample Size
Prior to the present study, a proof-of-concept study was conducted to compare outcomes of PNS paired with modified CIT (n=3) versus modified CIT only (n=2). Thus, the present study required a sample size of 10 evaluable subjects per group to detect the same effect size as that observed in the proof-of-concept study with 80% power, assuming a similar standard deviation in the change. This estimate was felt to be conservative since the variance was estimated from the change in a group that received an intervention and appeared to have an average effect. In the present study, 21 subjects were enrolled. Nineteen subjects completed baseline and post evaluations; 17 of the 19 went on to complete 1-month follow-up evaluations.
Evaluation and Outcome Measures
The WMFT served as the primary outcome measure. The WMFT is a time- and function-based assessment encompassing a battery of 17 tasks that simulate functional tasks and that are ordered according to complexity 12. The non-timed subcomponents of the WMFT comprise 1) a lift portion, which measures the maximum amount of weight the tested individual can lift to a height requiring 90° shoulder flexion; and 2) grip force dynamometer measurements. The WMFT has established reliability and validity and has been extensively applied in several CIT studies to evaluate UE motor capacity12. Secondary outcome measures included the Fugl-Meyer Assessment Scale (FMA; UE motor score only) and the Action Research Arm Test (ARAT). The FMA is a quantitative measure of motor recovery, balance, sensation, coordination and speed and is based on the principle that motor recovery occurs in a predictable progression23. The inter-rater reliability (=0.886~0.984 according to the subset for lower or UE) and test-retest reliability (=0.99) of FMA are also high24. FMA is extensively applied in cases of stroke; it is feasible for use with this condition23. The highest possible FMA UE motor score for a tested UE is 66. The ARAT was developed specifically to measure rehabilitation-related changes in UE motor capacity25 and has measures for grasp, grip, pinch, and gross UE motor capacity. The highest possible ARAT score for a tested UE is 57.
Intervention Component 1: PNS
PNS was delivered in 120-minute sessions each day during a period of 10 consecutive weekdays. Optimal positions to stimulate the posterior interosseous, median, and ulnar nerves were determined by applying a surface bar electrode with the cathode placed distally on the affected UE. To stimulate each nerve trunk, gold-plated stimulating electrodes were placed with the cathode positioned proximally over each of the optimal positions identified by the bar electrode26. Disposable surface EMG electrodes were placed over the belly of the extensor digitorum communis, abductor pollicis brevis, and abductor digiti minimi muscles. EMG activity was amplified and filtered (bandpass, 10-3000Hz) and recorded using a data collection program written in LabVIEW (National Instruments, Austin, TX). For active PNS, the stimulus intensity was adjusted to elicit small compound muscle action potentials of approximately 50 to 100μV without the absence of visible muscle movements 26. This low stimulus intensity and the stimulus duration of 1ms has been shown to preferentially activate large cutaneous and proprioceptive sensory fibers27. For sham PNS, an identical protocol was implemented except that the amplitude was set to 0V. Since attention appears to play an important role in neuroplastic change9, subjects were required to stay awake during PNS.
Intervention Component 2: Modified CIT
Each subject participated in 4 hours of modified CIT immediately following PNS. An occupational therapist blinded to PNS condition administered the training, which included rest breaks and grading of activities according to subject fatigue. Per the original CIT regimen, subjects were requested to wear a padded constraining mitt for at least 90% of waking hours on the non-affected extremity as well as fill out a diary to monitor the time spent wearing the mitt. Also in keeping with original CIT, the protocol focused on constraining the non-affected extremity while compelling highly repetitive use of the affected extremity in task-oriented motor activities 12. Tasks with progressive difficulty were applied where the extended motor ability was kept just beyond the performance already achieved (shaping). Tasks were repeatable and targeted functional goals of importance to each subject (such as activities of daily living) or prerequisites to function (eg, releasing; grasping; reaching; supination). For example, an individual who had difficulty with thumb movement performed activities that required use of the thumb and second digit in order to strengthen thumb movements in activities identified as meaningful by the individual (eg, fastening jewelry; handwriting; manipulating coins). Verbal or visual (graph) positive feedback was provided after small improvements beyond the already achieved skill level. Rest breaks were provided, lasting no longer than the practice segment. The number of repetitions for each designated task were documented to evaluate the effort of each patient. The target range for repetitions of any given task was 10 to 50 according to the demands of the task as well as reported levels of fatigue and engagement of each subject with a given task. Transfer package was not provided in this study. Therapy took place in a 1:1 therapist-to-subject ratio.
Statistics
For each outcome of interest, a longitudinal repeated measures model that accounts for time, trial arm, and their interaction was fit. Each model incorporates an unstructured working covariance matrix, and the Kenward and Roger28 degrees of freedom method was used for inference. These analyses correspond to the use of repeated measures MANOVA, but with the allowance of missing data. Primary interest was in the comparison of mean changes in outcomes from baseline to immediately post-intervention and to 1-month follow-up for the 2 trial arms. Corresponding results are presented in Table 2. For more detail from the models, the separate impacts of each trial arm on the mean change of each outcome are presented. All available data were utilized for analyses. All tests were 2-sided, with statistical significance pre-specified as P<0.05. Analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC).
Table 2.
Estimated means, 95% confidence intervals, and p-values corresponding to mean change.
| Outcome Measure |
Test Portion |
Post-intervention – Baseline | 1-month follow-up – Baseline | ||||
|---|---|---|---|---|---|---|---|
| Active | Sham | Active - Sham | Active | Sham | Active - Sham | ||
| Wolf Motor Function Test (WMFT; primary outcome measure) |
Timed (log): more affected upper extremity (UE) |
−0.36 (−0.45, −0.28) p<0.001 |
−0.18 (−0.27, −0.09) p=0.001 |
−0.19 (−0.31, −0.06) p=0.006 |
−0.37 (−0.49, −0.24) p<0.001 |
−0.14 (−0.29, 0.004) p=0.056 |
−0.23 (−0.42, −0.03) p=0.025 |
| Lift: more affected UE |
4.60 (3.72, 5.48) p<0.001 |
2.11 (1.19, 3.03) p<0.001 |
2.49 (1.22, 3.76) p=0.001 |
4.30 (2.50, 6.10) p<0.001 |
0.30 (−1.78, 2.39) p=0.763 |
4.00 (1.24, 6.75) p=0.007 |
|
| Grip: more affected UE |
5.89 (1.56, 10.22) p=0.011 |
1.86 (−2.70, 6.42) p=0.402 |
4.03 (−2.26, 10.32) p=0.194 |
6.19 (1.36, 11.03) p=0.015 |
1.61 (−3.56, 6.78) p=0.521 |
4.59 (−2.49, 11.66) p=0.189 |
|
| Fugl- Meyer Assessment (FMA; secondary outcome measure) |
Motor: more affected UE |
10.20 (7.52, 12.88), p<0.001 |
5.56 (2.73, 8.38) p=0.001 |
4.64 (0.75, 8.54) p=0.022 |
12.50 (8.61, 16.39) p<0.001 |
6.58 (1.89, 11.26) p=0.009 |
5.92 (−0.16, 12.01) p=0.056 |
| Action Research Arm Test (ARAT; secondary outcome measure) |
More affected UE |
9.60 (6.79, 12.41) p<0.001 |
3.67 (0.70, 6.63) p=0.018 |
5.93 (1.84, 10.02) p=0.007 |
11.10 (7.10, 15.10) p<0.001 |
4.29 (−0.13, 8.72) p=0.057 |
6.81 (0.83, 12.78) p=0.028 |
Results
Analysis was by original assigned groups. Table 1 summarizes demographics of the sample. No significant difference existed between groups on any outcome measured at baseline, as determined by simple unpaired t-testing. No treatment complications or serious adverse events occurred during the study. Two subjects were withdrawn from the study after baseline testing and prior to post-intervention evaluation. One of these 2 subjects was assigned to the active PNS group and was subsequently withdrawn due to non-compliance with the study protocol (inconsistent attendance during the intervention period). The other subject was assigned to the sham PNS group and subsequently requested to withdraw secondary to sequelae of a fall sustained at home. This fall was determined by the study doctor to be a non-serious adverse event unrelated to the study procedures. Two other subjects were lost to 1-month follow-up testing as a result of transportation issues. Both of these subjects had been assigned to the sham PNS group. The trial ended because funding was completed.
Table 1.
Demographics of sample.
| Age (y) |
Group | Sex | Time Since Stroke (months) |
Stroke Type | Stroke Site | Handedness Before Stroke |
More Affected Upper Extremity |
|---|---|---|---|---|---|---|---|
| 50 | Sham | F | 26 | Ischemic | Middle cerebral artery (MCA) territory |
Right | Right |
| 35 | Sham | F | 29 | Ischemic | MCA territory |
Right | Right |
| 65 | Sham | M | 15 | Ischemic | MCA territory |
Right | Left |
| 63 | Sham | M | 20 | Ischemic | Basal ganglia |
Right | Right |
| 62 | Sham | M | 22 | Ischemic | MCA territory |
Right | Right |
| 58 | Sham | F | 84 | Ischemic | Basal ganglia |
Right | Left |
| 54 | Sham | M | 21 | Ischemic | MCA territory |
Right | Right |
| 61 | Sham | M | 41 | Ischemic | Corona radiata |
Right | Right |
| 43 | Sham | M | 64 | Hemorrhagic | MCA territory |
Left | Right |
| 66 | Active | M | 12 | Ischemic | Basal ganglia |
Right | Right |
| 58 | Active | F | 60 | Ischemic | MCA territory |
Right | Left |
| 48 | Active | M | 12 | Ischemic | Corona radiata |
Right | Right |
| 61 | Active | M | 12 | Ischemic | Basal ganglia |
Right | Right |
| 61 | Active | F | 60 | Ischemic | MCA territory |
Right | Left |
| 52 | Active | F | 52 | Hemorrhagic | MCA territory |
Right | Left |
| 56 | Active | F | 24 | Ischemic | MCA territory |
Right | Right |
| 65 | Active | F | 24 | Ischemic | MCA territory |
Right | Left |
| 62 | Active | F | 21 | Ischemic | Basal ganglia |
Right | Left |
| 38 | Active | F | 18 | Ischemic | MCA territory |
Right | Left |
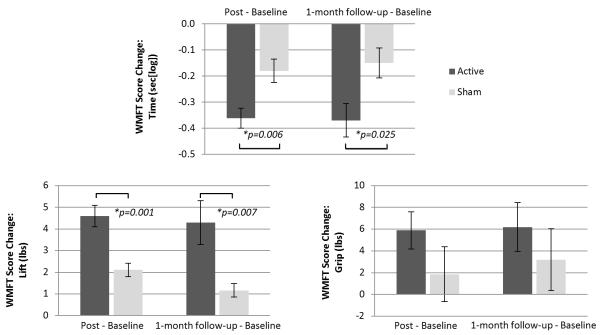
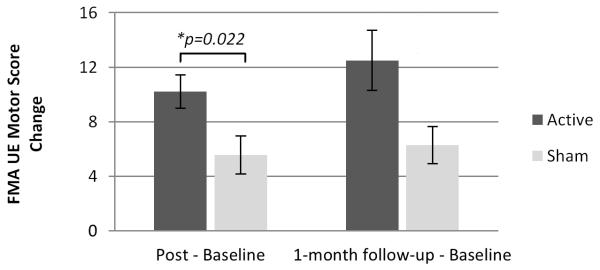
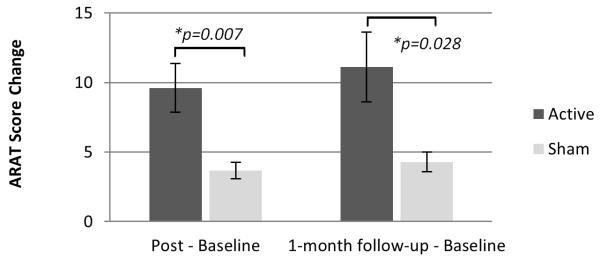
For the active PNS group, score changes for the affected UE were significantly greater than score changes for the sham PNS group on the WMFT timed and lift portions (Figure 2); the UE motor portion of the FMA (Figure 3); and the ARAT (Figure 4). These significant differences between groups were evident on all outcome measures immediately post-intervention. Significant differences between groups were also evident at 1-month follow-up except for FMA (Table 2). No significant difference between groups was evident at any timepoint after intervention with regard to the grip portion of the WMFT (Figure 2; Table 2). However, mean changes were significant in the active PNS group, and estimated mean changes were more favorable than for the sham PNS group. For both groups, the WMFT showed non-significant changes in the less affected UE; the ARAT showed no change in this regard. No evidence of unintended effects in each group was found.
Figure 2.
Comparison of Groups’ Score Changes on Wolf Motor Function Test (WMFT). Results of the WMFT (primary outcome measure) show that active peripheral nerve stimulation (PNS) can enhance outcomes of a modified form of constraint-induced therapy (CIT) significantly more than sham PNS. Score decrease on the timed portion of the WMFT, as well as score increase on the lift and the grip portions, indicate improvement in affected upper extremity (UE) motor capacity. Immediately post-intervention, as well as at 1-month follow-up, there was a significant difference between groups on the timed portion (upper image) and the lift portion (lower left image). No significant difference emerged between groups on the grip portion (lower right image).
Figure 3.
Comparison of Groups’ Score Changes on Upper Extremity (UE) Motor Score of the Fugl-Meyer Assessment (FMA). Increase in FMA score indicates improvement in affected UE motor function. Results at immediately post-intervention show that active peripheral nerve stimulation (PNS) can enhance outcomes of a modified form of constraint-induced therapy (CIT) significantly more than sham PNS.
Figure 4.
Comparison of Groups’ Score Changes on Action Research Arm Test (ARAT). Increase in ARAT score indicates improvement in affected upper extremity (UE) motor capacity. Results at immediately post-intervention, as well as at 1-month follow-up, show that active peripheral nerve stimulation (PNS) can enhance outcomes of a modified form of constraint-induced therapy (CIT) significantly more than sham PNS.
Discussion
Because results indicated that active PNS can enhance a modified form of CIT significantly more than sham PNS can, it appears that PNS has enormous promise as a clinical intervention to enhance outcomes of motor training for stroke survivors with mild to moderate hemiparesis. Additionally, that the active PNS group showed more significant improvement than the sham PNS group 1 month after intervention had ended (ie, on the WMFT timed portion) highlights the potential translational value of this study. More specifically, this evidence suggests that compared with the sham PNS group, the active PNS group may have made more extensive use of the more affected upper extremity in settings outside the lab, such as in activities of daily living. Future studies are recommended to provide conclusive evidence in this regard.
The lack of more significant improvement for the active PNS group compared with the sham PNS group on WMFT grip was somewhat surprising, particularly in consideration of a 2002 study by Conforto and colleagues that showed improvement in pinch force associated with 2 hours of PNS to median nerve (no motor training provided) for subjects more than 12 months post-stroke (n=8) 29. Likewise, a separate study by Klaiput and colleagues reported gains in pinch force associated with 2 hours of dual PNS to median and ulnar nerve (without motor training) for 20 subjects less than 6 months following stroke30. On the other hand, in 2010, Conforto and colleagues showed a lack of effects on pinch force associated with different intensities of single nerve (median) PNS paired with motor training in 22 subjects at 2 months or less since stroke31. Additionally, Sawaki and colleagues showed that while there was a trend towards improved WMFT grip associated with CIT, no significant between-groups difference was evident in comparing early with late phase of recovery (ie, less than 9 months post-stroke versus greater than 12 months post-stroke)32. Taken together, these inconsistent findings with regard to WMFT measurement of isometric force may reflect the lack of homogeneity in PNS protocols and/or phases of recovery across these cited studies.
In order to maximize adjuvancy of PNS with CIT or its modified forms, further research is recommended to optimize parameters of multiple nerve stimulation sites (ie, proximal vs distal; multiple versus single). The literature reports only 1 study that, similar to the present study, applied simultaneous stimulation of median, radial, and ulnar nerves33. (However, in delivering PNS at the elbow, this protocol differed from the present study, which delivered multiple nerve stimulation more distally. Additionally, the present study delivered individual PNS trains with an offset of 35ms between each stimulation channel to prevent stimulation of distal nerves from being blocked by stimulation of more proximal nerves, a phenomenon known as “collision” in nerve conduction studies 34, 35.) Fleming and colleagues administered 2 hours of PNS immediately prior to 30 minutes of task-specific training for 33 subjects with chronic stroke33. At post-intervention, more significant improvement on the ARAT was evident for the active PNS group compared with the sham PNS group; but no significant between-groups difference was evident on secondary measures (ie, FMA; Motor Activity Log; Goal Attainment Scale) or at long-term follow-up.
Other future investigations are recommended to help establish strategies for translating intervention used in the present study, as well as other PNS-based interventions, to settings beyond research. Examples would include PNS paired with standard occupational therapy or PNS paired with daily living tasks in in the home and community. Studies are also recommended to determine the effect of PNS paired with different CIT-based protocols (ie, various frequencies or durations). Such studies would build on the systematic review and meta-analysis by Peurala and colleagues17 regarding optimal parameters of different CIT-based protocols targeting various outcomes (eg, functional independence; reduction of motor impairment).
Because the reduced training time, duration, or intensity of modified forms of CIT may have greater insurance-related compatibility than CIT36, studying how PNS enhances different CIT protocols could help optimize the intervention for settings that may not support protracted therapy. To this end, the modified CIT in the present study required comparatively less daily time than CIT. However, full clinical translation would require establishing the minimum time required for efficacy of this paired intervention.
The main possible limitations to the present study included small sample size and need for longer-term follow-up. Other possible limitations included lack of multiple baselines. Additionally, the customization of motor training to each subject could be considered a lack of standardization, even though this tailored approach was associated with improvement for both groups. Finally, the present study, as well as the other PNS/motor training studies we have cited here, did not focus on stroke survivors with severe motor deficit (ie, almost no finger or hand movement) more than 12 months post-stroke. Future studies in this regard would establish the generalizability of findings from the present study to other stroke sub-populations in great need of effective interventions.
Conclusions
All outcome measures in this study reflected improvement in behavioral measures of UE activity for both groups after intervention. However, significantly more improvement was evident for the active PNS group compared with the sham PNS group. Overall, the results of this study provide a strong rationale for a full-scale investigation of the effects of PNS paired with a modified form of CIT for stroke survivors with chronic, mild-to-moderate UE hemiparesis.
Supplementary Material
Acknowledgements
Heartfelt appreciation is extended to our study participants and to Dr. David Jackson for referrals.
This study was funded by NIH R03 HD049408-01A1 as well as the Cardinal Hill Rehabilitation Hospital Endowed Chair in Stroke and Spinal Cord Injury Rehabilitation (0705129700).
Footnotes
Disclosures: There are no financial benefits to the authors. There are no conflicts of interest related to this research or this manuscript.
Results of this study were first presented in poster form at the 2013 International Stroke Conference in conjunction with the Travel Award for Junior Investigators.
The clinical trial registration number with clinicaltrials.gov is NCT02587234.
Contributor Information
Cheryl Carrico, University of Kentucky, Department of Physical Medicine and Rehabilitation, Lexington, KY..
Kenneth C. Chelette, II, University of Kentucky, Department of Physical Medicine and Rehabilitation, Lexington, KY..
Philip M. Westgate, University of Kentucky, Department of Biostatistics, College of Public Health, Lexington, KY..
Elizabeth Salmon-Powell, University of Kentucky, Department of Physical Medicine and Rehabilitation, Lexington, KY..
Laurie Nichols, University of Kentucky, Department of Physical Medicine and Rehabilitation, Lexington, KY; Cardinal Hill Rehabilitation Hospital, Lexington, KY..
Lumy Sawaki, University of Kentucky, Department of Physical Medicine and Rehabilitation, Lexington, KY; Cardinal Hill Rehabilitation Hospital, Lexington, KY; Wake Forest University, Department of Neurology, Winston-Salem, NC..
References
- 1.Wardlaw JM, Zoppo G, Yamaguchi T, Berge E. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2003:CD000213. doi: 10.1002/14651858.CD000213. [DOI] [PubMed] [Google Scholar]
- 2.Hallett M. Plasticity of the human motor cortex and recovery from stroke. Brain Res Brain Res Rev. 2001;36:169–174. doi: 10.1016/s0165-0173(01)00092-3. [DOI] [PubMed] [Google Scholar]
- 3.Nudo RJ. Adaptive plasticity in motor cortex: Implications for rehabilitation after brain injury. J Rehabil Med. 2003;41(Suppl):7–10. doi: 10.1080/16501960310010070. [DOI] [PubMed] [Google Scholar]
- 4.Barbay S, Plautz EJ, Friel KM, Frost SB, Dancause N, Stowe AM, et al. Behavioral and neurophysiological effects of delayed training following a small ischemic infarct in primary motor cortex of squirrel monkeys. Exp Brain Res. 2006;169:106–116. doi: 10.1007/s00221-005-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merzenich MM, Jenkins WM. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J Hand Ther. 1993;6:89–104. doi: 10.1016/s0894-1130(12)80290-0. [DOI] [PubMed] [Google Scholar]
- 6.Fraser C, Power M, Hamdy S, Rothwell J, Hobday D, Hollander I, et al. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron. 2002;34:831–840. doi: 10.1016/s0896-6273(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 7.Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J. Neurosci. 1998;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen LG, Brasil NJ, Pascual LA, Hallett M. Plasticity of cortical motor output organization following deafferentation, cerebral lesions, and skill acquisition. Adv Neurol. 1993;63:187–200. [PubMed] [Google Scholar]
- 9.Ridding MC, McKay DR, Thompson PD, Miles TS. Changes in corticomotor representations induced by prolonged peripheral nerve stimulation in humans. Clin Neurophysiol. 2001;112:1461–1469. doi: 10.1016/s1388-2457(01)00592-2. [DOI] [PubMed] [Google Scholar]
- 10.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 11.Sawaki L, Wu CW, Kaelin-Lang A, Cohen LG. Effects of somatosensory stimulation on use-dependent plasticity in chronic stroke. Stroke. 2006;37:246–247. doi: 10.1161/01.STR.0000195130.16843.ac. [DOI] [PubMed] [Google Scholar]
- 12.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The excite randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 13.Taub E, Uswatte G. Constraint-induced movement therapy: Bridging from the primate laboratory to the stroke rehabilitation laboratory. J Rehabil Med. 2003;34:40. doi: 10.1080/16501960310010124. [DOI] [PubMed] [Google Scholar]
- 14.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 15.Wittenberg GF, Chen R, Ishii K, Croarkin E, Eckloff S, Gerber L, et al. Effects of constraint-induced therapy on motor function and cortical physiology in chronic stroke. 2nd. World Congress of Neurorehabilitation proceedings.1999. [Google Scholar]
- 16.Sawaki L, Butler AJ, Leng X, Wassenaar PA, Mohammad YM, Blanton S, et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair. 2008;22:505–513. doi: 10.1177/1545968308317531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peurala SH, Kantanen MP, Sjogren T, Paltamaa J, Karhula M, Heinonen A. Effectiveness of constraint-induced movement therapy on activity and participation after stroke: A systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2012;26:209–223. doi: 10.1177/0269215511420306. [DOI] [PubMed] [Google Scholar]
- 18.Bach y Rita P. Central nervous system lesions: Sprouting and unmasking in rehabilitation. Arch Phys Med Rehabil. 1981;62:413–417. [PubMed] [Google Scholar]
- 19.Liepert J, Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250:5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- 20.Laible M, Grieshammer S, Seidel G, Rijntjes M, Weiller C, Hamzei F. Association of activity changes in the primary sensory cortex with successful motor rehabilitation of the hand following stroke. Neurorehabil Neural Repair. 2012;26:881–888. doi: 10.1177/1545968312437939. [DOI] [PubMed] [Google Scholar]
- 21.Hamzei F, Dettmers C, Rijntjes M, Weiller C. The effect of cortico-spinal tract damage on primary sensorimotor cortex activation after rehabilitation therapy. Exp Brain Res. 2008;190:329–336. doi: 10.1007/s00221-008-1474-x. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein LB, Davis JN. Restorative neurology. Drugs and recovery following stroke. Stroke. 1990;21:1636–1640. doi: 10.1161/01.str.21.11.1636. [DOI] [PubMed] [Google Scholar]
- 23.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 24.Duncan PW, Propst M, Nelson SG. Reliability of the fugl-meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 25.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540:623–633. doi: 10.1113/jphysiol.2001.012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panizza M, Nilsson J, Roth BJ, Basser PJ, Hallett M. Relevance of stimulus duration for activation of motor and sensory fibers: Implications for the study of h-reflexes and magnetic stimulation. Electroencephalography and clinical neurophysiology. 1992;85:22–29. doi: 10.1016/0168-5597(92)90097-u. [DOI] [PubMed] [Google Scholar]
- 28.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 29.Conforto AB, Kaelin-Lang A, Cohen LG. Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol. 2002;51:122–125. doi: 10.1002/ana.10070. [DOI] [PubMed] [Google Scholar]
- 30.Klaiput A, Kitisomprayoonkul W. Increased pinch strength in acute and subacute stroke patients after simultaneous median and ulnar sensory stimulation. Neurorehabil Neural Repair. 2009;23:351–356. doi: 10.1177/1545968308324227. [DOI] [PubMed] [Google Scholar]
- 31.Conforto AB, Ferreiro KN, Tomasi C, dos Santos RL, Moreira VL, Marie SK, et al. Effects of somatosensory stimulation on motor function after subacute stroke. Neurorehabil Neural Repair. 2010;24:263–272. doi: 10.1177/1545968309349946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawaki L, Butler AJ, Leng X, Wassenaar PA, Mohammad YM, Blanton S, et al. Differential patterns of cortical reorganization following constraint-induced movement therapy during early and late period after stroke: A preliminary study. NeuroRehabilitation. 2014;35(3):415–26. doi: 10.3233/NRE-141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleming MK, Sorinola IO, Roberts-Lewis SF, Wolfe CD, Wellwood I, Newham DJ. The effect of combined somatosensory stimulation and task-specific training on upper limb function in chronic stroke: A double-blind randomized controlled trial. Neurorehabil Neural Repair. 2015;29:143–152. doi: 10.1177/1545968314533613. [DOI] [PubMed] [Google Scholar]
- 34.Kimura J. Collision technique. Physiologic block of nerve impulses in studies of motor nerve conduction velocity. Neurology. 1976;26:680–682. doi: 10.1212/wnl.26.7.680. [DOI] [PubMed] [Google Scholar]
- 35.Humm AM, Z'Graggen WJ, von Hornstein NE, Magistris MR, Rosler KM. Assessment of central motor conduction to intrinsic hand muscles using the triple stimulation technique: Normal values and repeatability. Clin Neurophysiol. 2004;115:2558–2566. doi: 10.1016/j.clinph.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Ikuno K, Kawaguchi S, Kitabeppu S, Kitaura M, Tokuhisa K, Morimoto S, et al. Effects of peripheral sensory nerve stimulation plus task-oriented training on upper extremity function in patients with subacute stroke: A pilot randomized crossover trial. Clin Rehabil. 2012;26:999–1009. doi: 10.1177/0269215512441476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.