Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous group of immature myeloid cells that expand in tumor bearing hosts in response to soluble factors produced by tumor and stromal cells. MDSC expansion has been linked to loss of immune effector cell function and reduced efficacy of immune-based cancer therapies, highlighting the MDSC population as an attractive therapeutic target. Ibrutinib, an irreversible inhibitor of Bruton’s tyrosine kinase (BTK) and IL2-inducible T-cell kinase (ITK), is in clinical use for the treatment of B cell malignancies. Here, we report that BTK is expressed by murine and human MDSCs, and that ibrutinib is able to inhibit BTK phosphorylation in these cells. Treatment of MDSCs with ibrutinib significantly impaired nitric oxide production and cell migration. In addition, ibrutinib inhibited in vitro generation of human MDSCs and reduced mRNA expression of indolamine 2,3-dioxygenase, an immunosuppressive factor. Treatment of mice bearing EMT6 mammary tumors with ibrutinib resulted in reduced frequency of MDSCs in both the spleen and tumor. Ibrutinib treatment also resulted in a significant reduction of MDSCs in wildtype mice bearing B16F10 melanoma tumors, but not in X-linked immunodeficiency mice (XID) harboring a BTK mutation, suggesting that BTK inhibition plays an important role in the observed reduction of MDSCs in vivo. Finally, ibrutinib significantly enhanced the efficacy of anti-PD-L1 (CD274) therapy in a murine breast cancer model. Together, these results demonstrate that ibrutinib modulates MDSC function and generation, revealing a potential strategy for enhancing immune-based therapies in solid malignancies.
Keywords: Myeloid derived suppressor cells, ibrutinib, BTK, monocytes
Introduction
Myeloid derived suppressor cells (MDSC) are immature myeloid cells with immunosuppressive properties that expand in tumor bearing hosts in response to tumor and stroma derived factors (1–4). In mice, MDSC are recognized by the expression of GR-1 and CD11b, whereas in humans these cells are considered to be CD33+/CD11b+/HLA-DRlow/neg (3,5).
MDSC are critical contributors to tumor evasion of immune responses (3,6,7). MDSC promote immune suppression by the production of arginase-1, indolamine 2, 3-dioxygenase (IDO), nitric oxide (NO), reactive oxygen species (ROS), suppressive cytokines (e.g. IL-10 and TGF-β), and the induction of regulatory T-lymphocytes (3,8). Our group has shown that MDSC-derived NO results in the nitration of proteins involved in interferon receptor signal transduction and reduced responsiveness of immune cells to cytokine stimulation (9).
Given the ability of MDSC to suppress anti-tumor immune responses, they have received significant interest as biomarkers and therapeutic targets. The frequency of circulating MDSC correlates with tumor burden and has prognostic value in a variety of solid tumors (10–13). Studies in murine tumor models have shown that reduction of MDSC number or function results in reduced tumor growth and improved anti-tumor immune responses (14,15). Importantly, targeting MDSC in murine tumor models enhances the efficacy of immune based therapies such as PD1/PD-L1 checkpoint blockade (16,17). To date, a variety of agents have been studied for their ability to eliminate or inhibit MDSC function with limited success in translation to the clinical setting (18). As a result, the identification of safe, easily administered and effective agents targeting MDSC could lead to new therapeutic approaches for a number of malignancies.
Ibrutinib is an orally administered irreversible inhibitor of Bruton’s Tyrosine Kinase (BTK) and IL-2 inducible T cell kinase (ITK) that is in clinical use for B cell malignancies. Ibrutinib covalently binds to cysteine residues immediately outside of the ATP binding pocket of BTK and ITK. Targeting BTK in malignant B cells with ibrutinib has been shown to inhibit B cell receptor signaling via reduced activation of ERK and PLCγ2 as well as NF-κB signal transduction (19–21). Inhibition of ITK by ibrutinib limits the development of Th2 cells resulting in a Th1 polarization, which is considered favorable for anti-tumor immune responses (22,23).
BTK also plays a role in the maturation, trafficking, and function of myeloid cells (24–26). Ibrutinib impairs TNF-α and IL-1β production by monocytes in the setting of autoimmune arthritis (27). Reports have also shown a role for BTK in toll-like receptor (TLR) signaling in myeloid cells, which is of interest since TLR signaling has been implicated in MDSC generation and function (28–30).
As a result, it was hypothesized that MDSC would express BTK and treatment with ibrutinib would result in altered MDSC function and/or generation in the setting of cancer. In this report it is demonstrated that MDSC isolated from tumor bearing hosts express BTK and that ibrutinib inhibited BTK phosphorylation in MDSC. Ibrutinib also altered important functional properties of MDSC including NO production and migration. In multiple murine tumor models, ibrutinib significantly reduced the frequency of MDSC in vivo and improved the efficacy of anti-PD-L1 checkpoint blockade. To our knowledge this is the first report to demonstrate expression of BTK in MDSC, and the ability of ibrutinib to impair the generation and function of MDSC. These findings suggest the exciting potential for ibrutinib to improve immune system function in the setting of solid tumors and provide rationale for combining ibrutinib with other immune based therapeutics.
Materials and Methods
Cell lines
The murine MDSC cell line MSC2 (gift from Gregoire Mignot) was cultured in RPMI 1640 media supplemented with 25 mM HEPES, 10% heat-inactivated fetal bovine serum (FBS), 1% antibiotic-antimycotic, and 1 mM sodium pyruvate(31). The EMT6, 4T1, C26, and B16F10 cell lines were purchased from ATCC. EMT6 was maintained in complete IMDM media. 4T1, C26, and B16F10 were maintained in complete DMEM media. All cell lines used were acquired after 2010 and were validated by karyotyping/cytogenetic analysis.
Murine tumor models
Female 4–6 week old BALB/c mice (Jackson Laboratories, Bar Harbor, ME) were injected with 106 EMT6 or 105 4T1 cells in the mammary fat pad to produce tumors. 4–6 week old C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) or BTK mutant X-linked Immunodeficiency (XID) mice (from the laboratory of Dr. John Byrd) were injected with 105 B16F10 cells subcutaneously(32). XID mice have a single basepair substitution that alters the conserved Arg28 residue in the N-terminal unique region of unknown function(33). Ibrutinib or vehicle was administered by drinking water at 25 mg/kg daily. Anti-PD-L1 (Bio X cell, West Lebanon, NJ) was administered intraperitoneal at 100 μg per mouse M/W/F. These studies were conducted under a protocol approved by Ohio State University’s Institutional Animal Care and Use Committee.
Isolation of MDSC from tumor bearing mice
Spleens were harvested aseptically from mice and CD11b+/GR-1+ MDSC were isolated using anti-GR-1 beads (Miltenyi Biotec, Auburn, CA) with purity > 95% by flow cytometry.
Isolation of MDSC from peripheral blood of metastatic melanoma patients
Myeloid cells were enriched from peripheral blood using the RosetteSep HLA-myeloid cell enrichment kit (Stemcell Technologies, Vancouver, BC). MDSC were isolated by subsequent negative selection of HLA-DRneg cells using anti-HLA-DR MicroBeads (Miltenyi Biotec). Samples were acquired following patient signing of informed consent under an IRB approved protocol for human subject research (IRB protocol 1999C0348).
In vitro generation of human MDSC
Peripheral blood mononuclear cells (PBMC) were isolated from healthy donor leukopacks (American Red Cross, Columbus, OH) by Ficoll Hypaque (GE Healthcare) density gradient centrifugation. Monocytes were isolated using CD14 MicroBeads (Miltenyi Biotec), and cultured in RPMI 1640 media supplemented with 10% heat-inactivated pooled human AB serum (HAB; C-Six Diagnostics, Germantown, WI), 1% antibiotic-antimycotic, and 10 ng/ml IL-6 and GM-CSF (Peprotech, Rocky Hill, NJ). Where indicated, monocytes were treated with 0.01% DMSO or 1 μM ibrutinib (0.01% DMSO) for 1 hour every day.
Immunoblot analysis
MSC2 cells, murine MDSC, or human MDSC were lysed in RIPA buffer (Sigma, St. Louis, MO). Lysates were probed for phosphorylated BTK (p-BTK) (Cell Signaling, Danvers, MA), total BTK (Cell Signaling), total ITK (Cell Signaling), total Bmx (Epitomics, Eugene, OR) or GAPDH (Santa Cruz Biotechnologies, Dallas, TX) as previously described(23).
Nitric oxide estimation
MSC2 cells or MDSC isolated from metastatic melanoma patients were treated with DMSO or ibrutinib for 1 hour; media was then aspirated and replaced. MSC2 cells were then stimulated with 100 ng/ml LPS for 24 hours (Sigma Aldrich, cat. num. L6529) as previously described (30). Melanoma patient MDSC were cultured with 10 ng/ml IL-6 and 10 ng/ml GM-CSF for 48 hours. Griess reagent (Sigma Aldrich) was used to measure nitrite in supernatants as previously described (34).
Migration assays
MSC2 cells or MDSC from metastatic melanoma patients were treated with DMSO or ibrutinib for one hour. 1 × 105 MSC2 or 2 × 105 patient MDSC were plated in the top chamber of an 8 μm transwell assay. Media conditioned by the EMT6 cell line was used to stimulate MSC2 migration and media supplemented with 200 ng/ml GM-CSF was used stimulate patient MDSC migration. Inserts were collected after 24 hours and stained using the Dip Quick Stain Kit (Jorgensen Laboratories, Inc., Loveland, CO). Photographs were taken using a digital camera and cell numbers enumerated using image J software (2048 × 1536 pixels, Advanced Microscopy Group, Bothell, WA). 2×105 MDSC, isolated from the spleen of EMT6 tumor bearing mice, were treated as above, and migration assayed as previously described (35). Recombinant murine CXCL12, MIP-1α, and VCAM-1 were purchased from R&D systems (Minneapolis, MN).
Cytokine measurement
MSC2 cells were treated with DMSO or ibrutinib for one hour followed by washout and stimulation with LPS. Supernatants were collected after 24 hours and the levels of cytokines measured using a flow cytometric murine cytokine bead assay (BD Bioscience, San Jose, CA).
Real Time PCR
Total RNA was extracted using the Trizol reagent (Life Technologies, Grand Island, NY, USA). Reverse transcription reactions were performed using 500 ng RNA in a 20 μl reaction with the high capacity reverse transcription kit (Life Technologies). cDNA was used as a template to measure the expression of murine and human Arg1, Ido1, Nos2, Ncf1 (p47), and Blk genes by quantitative-Real Time PCR using pre-designed primers (Integrated DNA Technologies, Coralville, IA). Murine and human β-Actin served as an internal control for each reaction (Life Technologies). Real Time PCR reactions were performed using the ABI PRISM 7900HT fast Real Time PCR system with SYBR Green chemistry (Applied Biosystems).
Flow cytometric analysis of murine and human MDSC
Spleens and tumors from mice were processed into single cell suspensions and stained with Alexa 488 anti-GR-1 and APC anti-CD11b antibodies (BD Bioscience). Single cell suspensions from mouse spleens were also stained with fluorochrome-labeled antibodies against CD19 and B220 (BD Bioscience). In vitro generated human MDSC were harvested using non-enzymatic cell dissociation solution (Sigma Aldrich) and stained with APC anti-CD33, PE anti-CD11b, and PECy7 anti-HLA-DR antibodies (Beckman Coulter). Data was acquired using an LSRII flow cytometer (BD Biosciences).
Murine CFSE assay
MDSC were isolated from the spleen of EMT6 tumor bearing mice and T cells were isolated from a non-tumor bearing mouse using the murine T cell isolation kit (Stemcell Technologies). MDSC were treated with DMSO or 1 μM ibrutinib for 1 hour and then incubated overnight with 10 ng/ml IL-6 and GM-CSF. T cells were labeled with CFSE (Life Technologies) and incubated overnight with 10 ng/ml IL-2 (Peprotech). T cells were non-specifically activated with anti-CD3/CD28 beads (Life Technologies) and co-cultured at 1:1, 2:1, and 4:1 ratios of T cells to MDSC. After three days T cell proliferation was assessed by flow cytometry. APC anti-CD4 and anti-CD8 antibodies were used to identify T cell subsets (Biolegend, San Diego, CA).
Statistics
Statistical differences between treatment groups were determined using an ANOVA model and Student’s t-test. For mice tumor studies, linear mixed model was employed to model longitudinal tumor volume for mice under each treatment. Comparisons were done at each time point and averaged across all time points using t-statistics. The Holm-Bonferroni method was used for adjusting raw p-values for multiple comparisons across treatment groups.
Results
Murine and human myeloid derived suppressor cells express Bruton’s Tyrosine Kinase
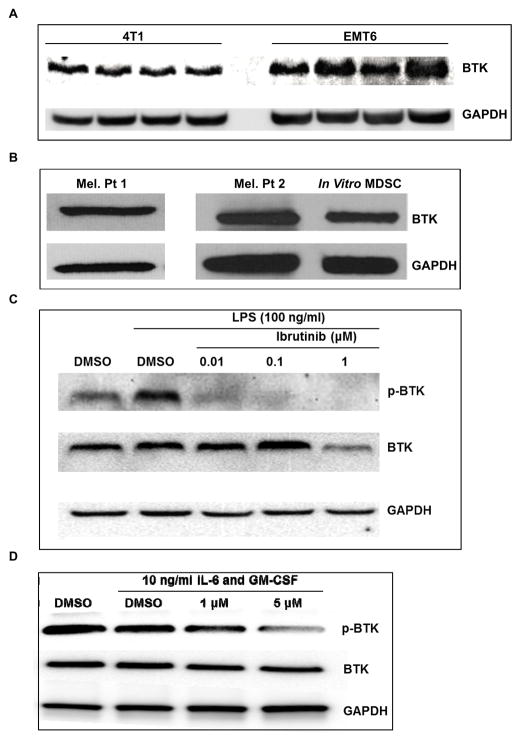
Given the role of BTK in the differentiation and function of myeloid cells it was hypothesized that MDSC would express BTK. To test this, BTK expression was measured in MDSC isolated from Balb/c mice bearing EMT6 and 4T1 mammary carcinoma tumors. As seen in Figure 1A, BTK was expressed by MDSC from both tumor models, but MDSC from the EMT6 model showed higher BTK expression. To investigate this difference, the expression of BTK at the mRNA level was measured by qRT-PCR in FACS sorted granulocytic (G-MDSC) and monocytic (M-MDSC) MDSC subsets. M-MDSC showed more BTK expression than G-MDSC in both tumor models. In addition, there was higher BTK expression in both MDSC subsets from the EMT6 model compared to the 4T1 model (Fig. S1A). In addition, analysis of the MDSC subsets by flow cytometry showed a higher frequency of the M-MDSC subset in the EMT6 model (Fig. S1B). The combination of higher BTK expression and frequency of M-MDSC in the EMT6 model likely explains the difference in BTK expression between these models. The murine MDSC cell line MSC2 and MDSC isolated from Balb/c mice with C26 colon carcinoma tumors also expressed high levels of BTK (Fig. S1C). In addition, MDSC isolated from metastatic melanoma patients as well as human MDSC generated in vitro from monocytes expressed BTK (Fig. 1B).
Figure 1. Murine and human MDSC express BTK and its phosphorylation is inhibited by ibrutinib.
(A) Immunoblot showing BTK and GAPDH expression in MDSC isolated from the spleen of mice bearing 4T1 and EMT6 mammary carcinoma tumors. (B) Immunoblot of BTK and GAPDH expression in human MDSC isolated from patients with metastatic melanoma and in vitro generated MDSC. Results displayed are from two separate immunoblots. (C) Immunoblot showing ibrutinib reduces the level of phosphorylated BTK (p-BTK) in LPS stimulated MSC2 cells. (D) In vitro generated MDSC were treated with DMSO or ibrutinib for 1 hour followed by stimulation with IL-6 and GM-CSF. Lysates were collected 15 minutes after stimulation and probed for expression of p-BTK, BTK, and GAPDH.
The ability of ibrutinib to inhibit the phosphorylation of BTK (p-BTK) in MDSC was next determined. The murine MDSC cell line MSC2, which has been shown to phenotypically and functionally resemble the monocytic MDSC subset, was first employed (31). MSC2 cells were treated with DMSO or ibrutinib at doses ranging from 0.01 μM to 1 μM for one hour followed by stimulation with 100 ng/ml of LPS (TLR 4 agonist) for four hours, and the level of p-BTK was assessed by immunoblot. These experiments revealed that BTK was constitutively phosphorylated in MSC2 cells, but treatment with ibrutinib inhibited p-BTK even at the 0.01 μM dose in the presence or absence of LPS stimulation (Fig. 1C and S1D). In a similar set of experiments, human MDSC generated in vitro from healthy donor monocytes were treated with DMSO or ibrutinib followed by treatment with 10 ng/ml IL-6 and GM-CSF. In vitro generated human MDSC were found to express high levels of p-BTK that was not further enhanced by IL-6 and GM-CSF. However, similar to MSC2 cells, ibrutinib treatment resulted in a decrease in the level of p-BTK (Fig. 1D).
Ibrutinib does not induce apoptosis of murine or human MDSC
MSC2 cells, melanoma patient MDSC, and in vitro generated MDSC were treated with various concentrations of ibrutinib and cell viability was determined 24 hours later by annexin/PI staining. Treatment with ibrutinib resulted in a slight increase in the frequency of apoptotic MSC2 cells, but this effect was not statistically significant. Likewise, ibrutinib was not cytotoxic to human MDSC. Trypan blue exclusion also showed no effect of ibrutinib on MDSC viability. (Fig. S2 and data not shown).
Gene expression and cytokine production in MSC2 cells following ibrutinib treatment
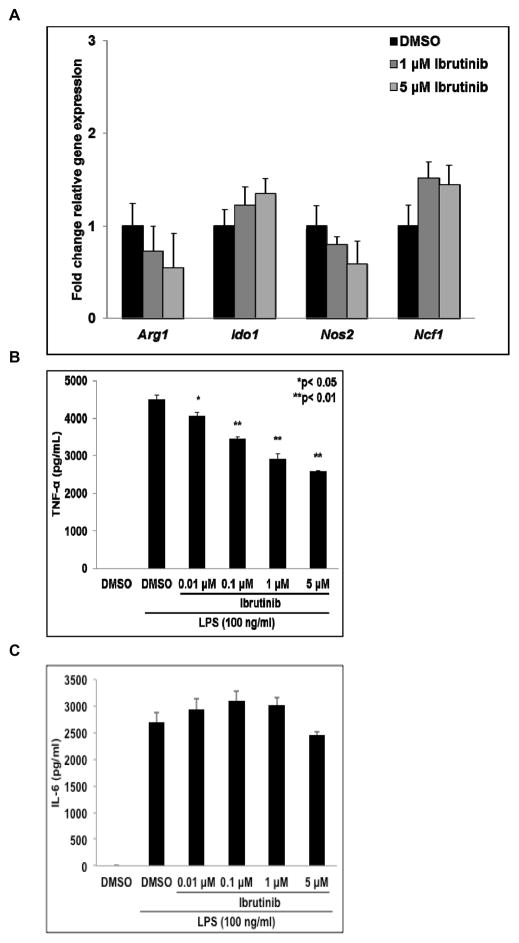
The effect of ibrutinib on the expression of genes known to mediate immune suppression by MDSC was examined. MSC2 cells were treated with DMSO or ibrutinib for 1 hour followed by activation with LPS. Cells were harvested 24 hours later and the relative expression of arginase-1 (Arg1), indolamine 2, 3-dioxygenase-1 (Ido1), inducible nitric oxide synthase-2 (Nos2), and neutrophil cytosolic factor-1 (Ncf1 or p47) mRNA was determined by qRT-PCR. Treatment with ibrutinib caused Arg1 and Nos2 expression to trend lower while Ido1 and Ncf1 trended towards increased expression, but none of these differences were statistically significant (Fig. 2A). In addition, the expression of these genes was measured in MDSC isolated from the spleen of EMT6 tumor bearing mice treated with ibrutinib or vehicle. This analysis showed similar trends in expression of these genes following treatment with ibrutinib as the MSC2 cells (Fig. S3).
Figure 2. Effect of ibrutinib on gene expression and cytokine production by activated MSC2 cells.
(A) Effect of ibrutinib on expression of Arg1, Ido1, Nos2, and Ncf1 by LPS activated MSC2 cells. Total RNA was collected 24 hours later and expression of Arg1, Ido1, Nos2, and Ncf1 determined by qRT-PCR. Values displayed are means ± SE from three independent experiments. (B and C) After 24 hours the supernatants of cells treated as in Fig. 2A were collected and the levels of TNF-α and IL-6 were measured by flow cytometry using a cytokine bead assay. Values displayed are means ± SE from three independent experiments.
Supernatants from parallel treatments of MSC2 cells were collected and cytokine production measured using a flow cytometry cytokine bead assay. LPS treatment induced the release of IL-6 and TNF-α whereas production of IL-10 was not significantly affected. Ibrutinib significantly reduced TNF-α production at all doses tested, but had no impact on IL-6 secretion (p< 0.05, Fig. 2B and 2C). These findings are consistent with the reported role of BTK in promoting stabilization of TNF-α mRNA but not IL-6 mRNA following TLR4 stimulation (36). As IL-6 is known to drive the expression of MDSC immune suppressive genes such as Arg1 and Ido1, the lack of a change in IL-6 secretion following ibrutinib treatment is consistent with the data showing no significant change in the expression of these genes.
Ibrutinib reduces murine and human MDSC NO production and migration
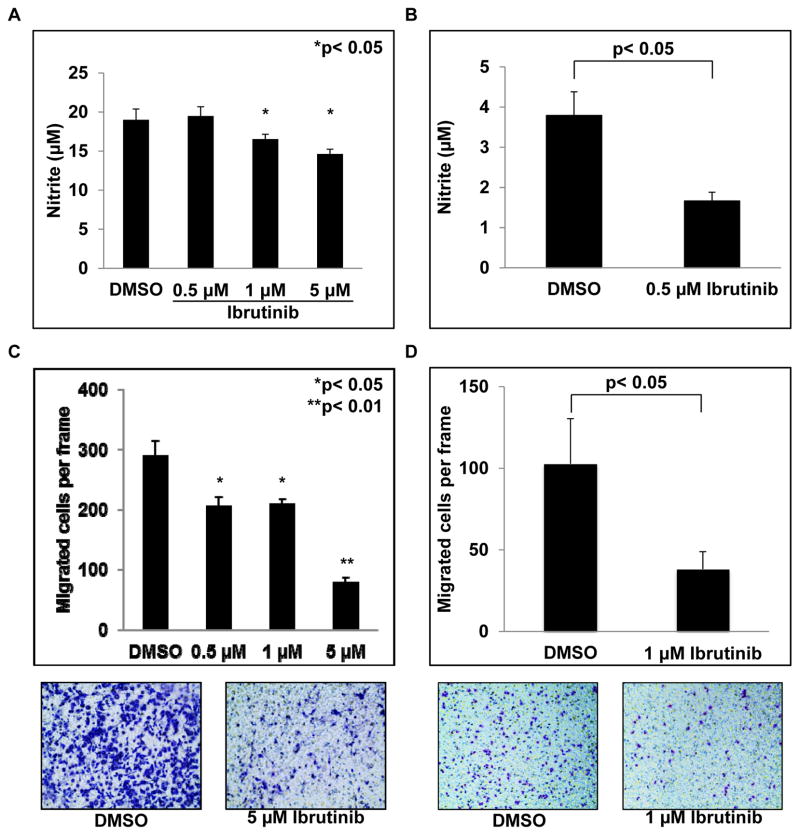
NO is a key molecule produced by MDSC that is involved in mediating immune suppression (9). As a result, the ability of ibrutinib to reduce NO production by murine and human MDSC was tested. MSC2 cells were treated with ibrutinib followed by stimulation with LPS. Supernatants were harvested 24 hours later and nitrite levels quantified using the Griess reagent as described (34). Ibrutinib treatment led to decreased NO production that reached statistical significance at the 1 μM dose (p< 0.05, Fig. 3A). Similarly, treatment of MDSC isolated from metastatic melanoma patients with ibrutinib resulted in a significant decrease in NO production compared to DMSO treated controls (Fig. 3B).
Figure 3. Ibrutinib reduces MDSC NO production and migraton.
(A) MSC2 cells were treated with DMSO or ibrutinib followed by stimulation with LPS. Supernatants were collected after 24 hrs and nitrite levels were measured using Griess reagent. Values displayed are means ± SE from three independent experiments, *p< 0.05. (B) MDSC isolated from metastatic melanoma patients were treated with DMSO or ibrutinib for 1 hour. After treatment, MDSC were cultured with 10 ng/ml IL-6 and 10 ng/ml GM-CSF for 48 hours and nitrite measured as above. Values represent mean ± SE from three patients, p< 0.05. (C) MSC2 cells were treated with DMSO or ibrutinib for one hour. Media conditioned by the EMT6 cell line served to stimulate migration. Values represent mean ± SE from three independent experiments. (D) MDSC isolated from patients with melanoma were treated with DMSO or ibrutinib. 200 ng/ml GM-CSF was used to stimulate chemotaxis and PBS supplemented media served as a negative control. Migration proceeded for 12 hrs. Values displayed are means ± SE from 3 patients. Representative images are given below migration results. Migrated cells are stained purple against the light blue transwell insert.
To promote local immune suppression MDSC must migrate into the tumor microenvironment, and disruption of this process may affect anti-tumor immune responses (4,17). Notably, ibrutinib has been shown to impair the chemotaxis of malignant B cells (35). To investigate the ability of ibrutinib to impair MDSC migration MSC2 cells were treated with various doses of ibrutinib and plated in a transwell assay with EMT6 cancer cell conditioned media as a stimulus for migration. These experiments showed that ibrutinib significantly reduced MSC2 migration at the 0.5 μM dose (p< 0.05, Fig. 3C). Similarly, MDSC isolated from patients with metastatic melanoma were treated with DMSO or 1 μM ibrutinib and migration stimulated using media supplemented with 200 ng/ml GM-CSF. Results from these experiments revealed that ibrutinib also significantly inhibited the migration of human MDSC (p< 0.05, Fig. 3D). To validate these findings the ability of ibrutinib to impair MDSC migration in response to the chemokines CXCL12 (SDF-1) and CCL3 (MIP-1α) was tested as previously described (35). Ibrutinib was able to inhibit MDSC migration in response to SDF-1, but not MIP-1α (Fig. S4 and data not shown). Importantly SDF-1 has previously been shown to regulate MDSC accumulation within tumors (37). In addition, ibrutinib significantly reduce the expression of CD49D and CD11a, adhesion molecules known to play a role in myeloid cell migration, whereas expression of CD11b and CD62L was unaffected (Fig. S5A–S5D). This reduction in adhesion molecule expression could provide an explanation for the observed reduction in MDSC migration.
Ibrutinib reduces MDSC suppression of T cell proliferation
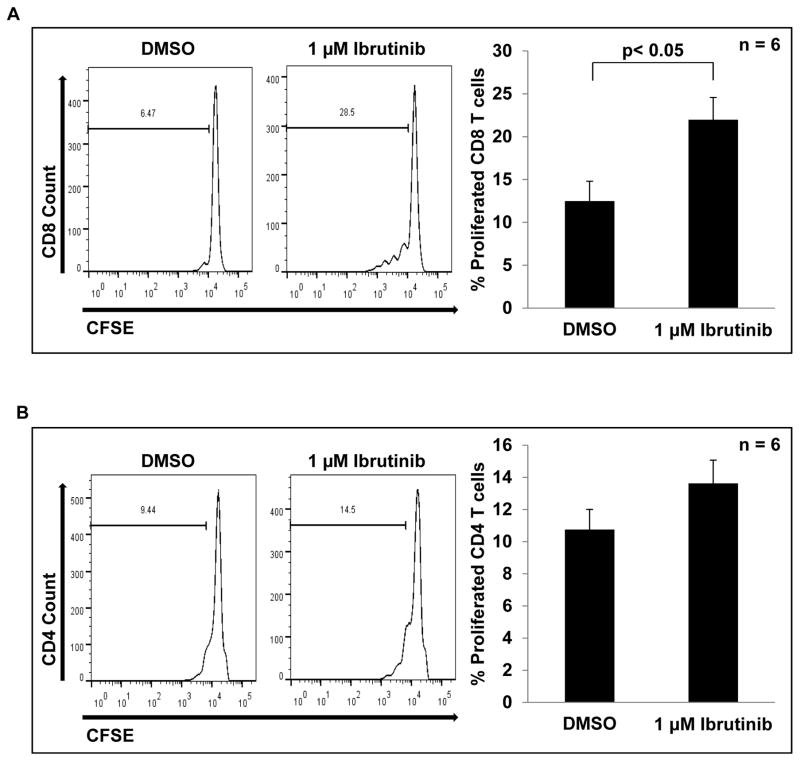
Given the ability of ibrutinib to attenuate MDSC NO production and migration it was hypothesized that treatment of MDSC with ibrutinib would reduce their ability to suppress T cell proliferation. To test this, MDSC isolated from the spleens of EMT6 tumor bearing mice were treated with DMSO or ibrutinib and co-cultured with CFSE-labeled T cells isolated from healthy mice that were activated with anti-CD3/CD28 beads. After 3 days, T cell proliferation was assessed by flow cytometry, and it was found that CD8+ T cells cultured with ibrutinib-treated MDSC showed significantly more proliferation than those cultured with DMSO treated MDSC (12.49% vs. 21.98%, p< 0.05, Fig 4A). There was also a trend towards increased CD4+ T cell proliferation in the presence of ibrutinib treated MDSC as compared to control-treated MDSC, but this result did not reach statistical significance (Fig. 4B).
Figure 4. Ibrutinib reduces MDSC suppression of T cell proliferation.
DMSO or ibrutinib treated MDSC were co-cultured with anti-CD3/CD28 bead activated T cells labelled with CFSE at a 2:1 ratio. After three days cells were collected and stained with anti-CD8 (A) and anti-CD4 (B) antibodies and proliferation assessed by flow cytometry. Histograms show results from one representative experiment. Bar graphs represent means ± SE from six independent experiments, p< 0.05 for CD8 T cells.
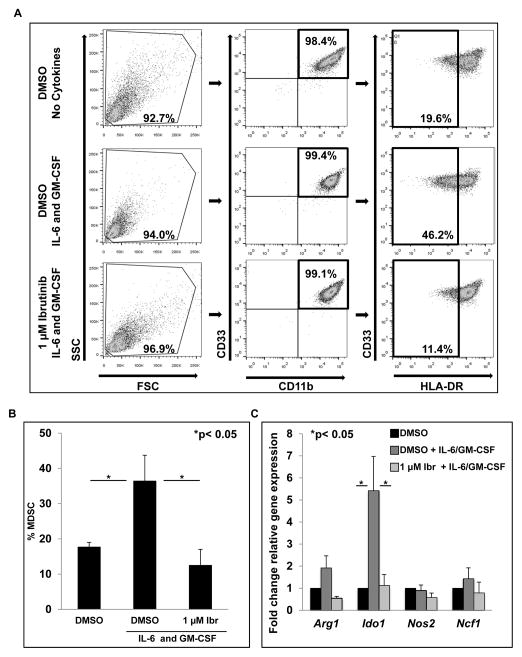
Ibrutinib inhibits in vitro generation of human MDSC from healthy donor monocytes
BTK signaling has been shown to play a role during the differentiation of myeloid cell subsets (24,38). In particular, BTK plays a negative role in the maturation of dendritic cells; BTK-deficient dendritic cells have high HLA-DR expression compared to wild type counterparts (39). As a result, it was hypothesized that BTK inhibition with ibrutinib could limit the generation of human MDSC by promoting HLA-DR expression. To test this hypothesis, three experimental conditions were employed: 1.) monocytes cultured without cytokines and treated daily with DMSO (control), 2.) monocytes cultured with IL-6 and GM-CSF treated daily with DMSO, 3.) monocytes cultured with IL-6 and GM-CSF treated daily with 1 μM ibrutinib. Six-day culture of human monocytes in IL-6 and GM-CSF produced a significant increase in CD33+/CD11b+/HLA-DRlow MDSC (46.2%, p< 0.05, Fig. 5A and 5B). Importantly, these cells were fully able to suppress T cell proliferation (Fig. S6). Daily treatment with ibrutinib reduced MDSC to levels comparable to those in the no cytokine control culture (12.49%, p< 0.05, Fig. 5A and 5B). Expression of Arg1, Ido1, Nos2, and Ncf1 (p47) in these treatment groups was measured by qRT-PCR on day 6 of culture. Ibrutinib treatment resulted in a significant reduction in Ido1 transcript (p< 0.05, Fig. 5C), while expression of Arg1, Nos2, and Ncf1 (p47) was lower compared to cells cultured with IL-6 and GM-CSF treated with DMSO, but not in a statistically significant manner. These results are in contrast to those reported in Fig. 2, where ibrutinib did not significantly alter the expression of these genes in cells that already possessed a MDSC phenotype. This may indicate that ibrutinib has a more potent ability to block the development of MDSC than impair the expression of immune suppressive genes by MDSC.
Figure 5. Ibrutinib inhibits in vitro generation of human MDSC.
Monocytes were cultured for 6 days in the three different conditions. (A) Gating strategy and flow panels from a representative experiment. (B) Percent MDSC in different culture conditions after 6 days. Values represented are means ± SE from three independent experiments. MDSC were identified by a CD33+/CD11b+/HLA-DRlow phenotype, *p<0.05. (C) Total RNA was collected on day 6 of monocyte cultures and expression of Arg1, Ido1, Nos2, and Ncf1 was determined by qRT-PCR. Values represent mean ± SE from three independent experiments, *p< 0.05 for Ido1.
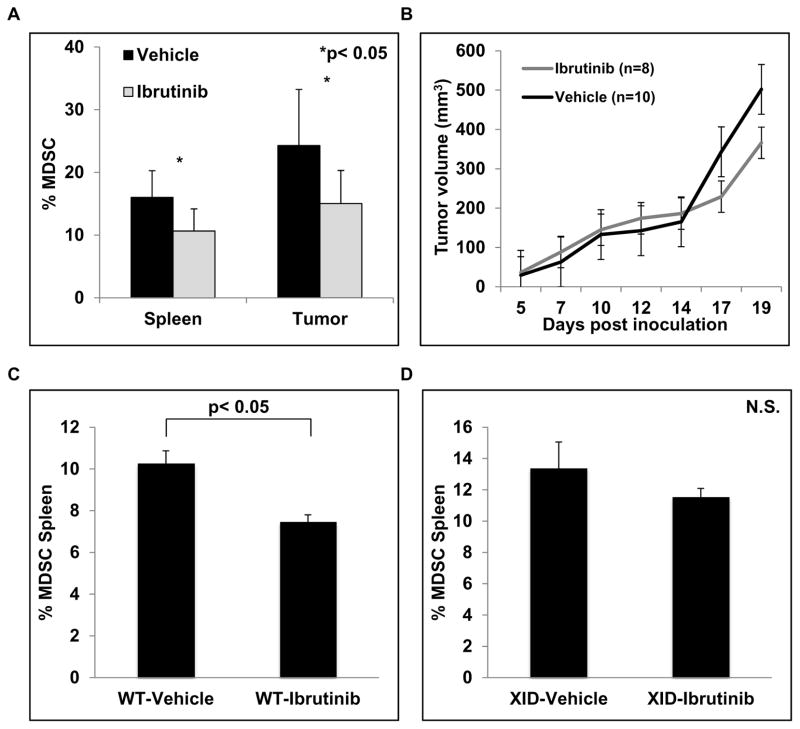
Ibrutinib reduces the frequency of MDSC in tumor-bearing mice
The effect of ibrutinib treatment on MDSC in vivo was evaluated next. Immune competent Balb/c mice bearing EMT6 murine mammary carcinoma tumors were treated with ibrutinib (25 mg/kg daily) or vehicle via drinking water as previously reported (23). At the end of the study, spleens and tumors were analyzed for the presence of MDSC. Ibrutinib treatment resulted in a significant reduction of CD11b+/GR-1+ MDSC in both the spleen and tumor (p< 0.05, Fig. 6A). This reduction in MDSC was accompanied by a significant reduction in spleen weight, suggesting reduced extra-medullary myelopoiesis(40) (Fig. S7A). Importantly, no difference in B220+/CD19+ B cell frequency in the spleen was observed after ibrutinib treatment, indicating ibrutinib was able to specifically deplete MDSC (Fig. S7B). Ibrutinib treated mice also showed a small reduction in tumor volume, but this difference was not statistically significant (Fig. 6B). MDSC levels are known to correlate with tumor burden. As a result, the lack of a difference in tumor volume between vehicle and ibrutinib treated mice suggests that the reduction of MDSC frequency is a direct result of ibrutinib, and not simply a reflection of differences in tumor burden. In addition, EMT6 cancer cells do not express BTK, thus likely excluding a direct anti-tumor effect of ibrutinib in this model (Fig. S7C).
Figure 6. Ibrutinib reduces MDSC frequency in vivo.
Eight mice were included in the ibrutinib treatment group and ten mice in the vehicle control group. (A) Splenocytes and single cell suspensions of the tumors were stained with GR-1 and CD11b antibodies. Values are the mean ± SE of GR-1+/CD11b+ MDSC in the spleen and tumor, *p< 0.05. (B) Tumor volumes were measured three times weekly. Values are the mean ± SE of tumor volumes at each time point. (C) Wild type C57BL/6 mice were inoculated with 1×105 B16F10 melanoma cells subcutaneously. After two weeks of treatment mice were sacrificed and the frequency of GR-1+/CD11b+ MDSC in spleen was measured by flow cytometry. Values represented are means ± SE of MDSC from 5 mice for each treatment group, p< 0.05. (D) BTK mutant C57BL/6 XID mice were inoculated with 1×105 B16F10 melanoma cells subcutaneously. After two weeks of treatment mice were sacrificed and the frequency of GR-1+/CD11b+ MDSC in spleen was measured by flow cytometry. Values represented are means ± SE of MDSC from 5 mice for each treatment group, p = 0.73.
To further confirm the ability of ibrutinib to reduce the frequency of MDSC in vivo, C57BL/6 mice inoculated with B16F10 melanoma cells were treated with vehicle or ibrutinib as above once tumors had successfully engrafted (5 mm diameter). Similar to the results obtained with the EMT6 mammary carcinoma model, ibrutinib treatment did not significantly affect B16F10 tumor growth (Fig. S8), but it did result in a significant reduction in the frequency of GR-1+/CD11b+ MDSC in the spleen of B16F10 tumor bearing hosts (Fig. 6C).
While ibrutinib is a potent inhibitor of BTK it is also known to effectively inhibit several other kinases including ITK, Bmx, and Blk. To investigate the specific role of BTK inhibition in the observed reduction of MDSC in tumor bearing hosts, X-linked immunodeficiency (XID) mice that have a point mutation in BTK were utilized. It was hypothesized that if additional kinases, besides BTK, played an important role in the observed reduction of MDSC in tumor bearing hosts, then BTK mutant XID mice treated with ibrutinib would also show a significant reduction of MDSC. To test this hypothesis XID mice were engrafted with B16F10 melanoma tumors and treated with vehicle or ibrutinib as described above. After two weeks the mice were sacrificed and the frequency of splenic MDSC was determined by flow cytometry. This analysis revealed that treatment of XID mice with ibrutinib did not significantly reduce MDSC frequency in the spleen compared to vehicle treated XID mice (Fig. 6D). This suggests that BTK inhibition by ibrutinib plays an important role in the observed reduction of MDSC in EMT6 and B16F10 tumor bearing hosts. To further clarify the potential off target effects of ibrutinib, the expression of Bmx and ITK was measured by immunoblot analysis. No significant expression was observed in mouse and human MDSC (Fig. S9A and S9B). In addition, Blk expression by mouse and human MDSC was measured by qRT-PCR and only low levels of expression were detected (Fig. S9C and S9D). However, these results do not completely rule out the possibility that inhibition of alternative kinases besides BTK also play some role in the effect of ibrutinib on MDSC, given that the frequency of MDSC in ibrutinib treated XID mice was lower compared to vehicle treated XID mice.
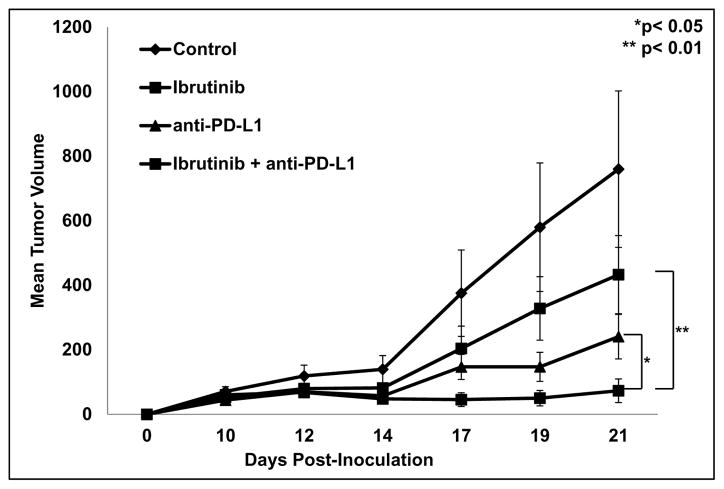
Ibrutinib improves the efficacy of immune based cancer therapies
Given previous reports of strategies that target MDSC leading to enhanced efficacy of immune checkpoint blockade, the ability of ibrutinib to improve anti-PD-L1 therapy was tested(17). For this study Balb/c mice were inoculated with EMT6 mammary carcinoma cells as above. Once tumors were palpable (5 mm diameter) mice were treated with vehicle/IgG as control, ibrutinib (25 mg/kg daily), anti-PD-L1 antibody (100 μg/mouse), or the combination of ibrutinib and anti-PD-L1. As shown in Fig. 7 the combination of ibrutinib and anti-PD-L1 produced a significant reduction in tumor growth compared to either agent alone (p< 0.05 and p< 0.01, Fig. 7). In addition, 50% of mice in the combination treatment group showed a complete response compared to 11.1% with anti-PD-L1 alone. Importantly, all mice that had a complete response are still tumor free 6 weeks after the last treatment.
Figure 7. Ibrutinib improves anti-PD-L1 immune checkpoint blockade.
Once tumors were palpable (5mm diameter) mice were divided into 4 treatment groups: vehicle/IgG control, ibrutinib (25 mg/kg daily), anti-PD-L1 (100 μg per mouse MWF), or the combination of ibrutinib and anti-PD-L1 therapy. 9–10 mice were included in each treatment group. Tumor volumes were measured three times weekly with digital calipers. Treatment with the combination of ibrutinib and anti-PD-L1 resulted in a significant reduction in tumor growth compared to treatment with ibrutinib (**p< 0.01) or anti-PD-L1 (*p<0.05) alone.
Discussion
BTK is a key component of B cell receptor signaling, but it is now being recognized that BTK also plays a role in the function of other immune cells (24). This work evaluated the expression of BTK in MDSC and the effect of a BTK inhibitor, ibrutinib, on MDSC function and generation. BTK was found to be highly expressed by MDSC isolated from multiple tumor models and human MDSC. Ibrutinib was able to inhibit the phosphorylation of BTK in both murine and human MDSC. Furthermore, ibrutinib reduced NO production and migration of MDSC. Ibrutinib also inhibited the in vitro generation of human MDSC, which was associated with reduced expression of Ido1 mRNA in these cultures. Using mouse models of breast cancer and melanoma, ibrutinib treatment resulted in a significant reduction of MDSC in vivo. However, ibrutinib treatment of XID mice expressing a non-functional mutant form of BTK engrafted with B16F10 melanoma tumors showed no significant reduction of MDSC compared to vehicle treated XID mice. Finally, ibrutinib was able to improve the efficacy of anti-PD-L1 immune checkpoint blockade, a T cell directed therapy.
Ibrutinib, an irreversible inhibitor of BTK, is clinically used for the treatment of B cell malignancies. Inhibition of BTK via ibrutinib impairs the activation of signaling pathways important to malignant B cells including the B cell receptor and NF-κB pathways (19,20). Several reports have demonstrated the ability of ibrutinib to alter the activation and function of immune cell subsets including basophils, monocytes, and T helper cells, indicating ibrutinib has broader immune modulatory effects (41). The current work builds on these studies by showing that MDSC express BTK and that their generation/function can be inhibited by ibrutinib.
To date four strategies have been employed to target MDSC: depletion, deactivation, differentiation, or the blockage of development (18). Depletion of MDSC has been studied using MDSC specific depleting peptides, pleiotropic agents such as epigenetic modifiers, and non-specific chemotherapeutic agents (42–44). Inhibitors of NO, arginase, and ROS have been used to deactivate MDSC function (18). In addition, agents such as CSF-1R inhibitors and inhibitors of chemokine signaling have been used to block MDSC migration into tumors (17,18). Finally, several groups have studied the ability of kinase inhibitors such as sunitinib or inhibitors of transcriptions factors (e.g. STAT3) to block MDSC generation (18,45–47). Results from the present study show that ibrutinib has the ability to reduce NO production, migration, and inhibit the generation of MDSC. These characteristics make ibrutinib a potentially promising therapeutic agent to target MDSC.
These findings are of particular interest given a recent report by Sagiv-Barfi et al. that showed ibrutinib enhanced the anti-tumor effect of PD-L1 immune checkpoint blockade (48). However, using the 4T1 mammary carcinoma model ibrutinib did not significantly alter the frequency of MDSC. The authors concluded that the action of the combination therapy was not due an effect on MDSC, but the result of direct activation of T cells (23,48). The results of this study are consistent with those of Sagiv-Barfi et al. in that the combination of ibrutinib and anti-PD-L1 therapy shows improved efficacy, the discrepancy between our findings of reduced MDSC frequency in tumor bearing mice treated with ibrutinib and the lack of this effect observed by Sagiv-Barfi et al. could be explained by differences in the dose, duration and route of ibrutinib administration and/or the tumor models.
Notably, in the study by Sagiv-Barfi et al. ibrutinib was delivered by intraperitoneal injection at a dose of 6 mg/kg daily for 8 days, whereas in the current study ibrutinib was delivered orally at a dose of 25 mg/kg daily for two weeks. It is possible that the lower dose and shorter treatment duration was insufficient to have an effect on MDSC number. Significant differences between the 4T1 and EMT6 models have also been reported (49). The 4T1 model is associated with significantly higher IL-6 levels and recruits more MDSC to primary tumor and metastatic sites compared to the EMT6 model (49). In addition, Sagiv-Barfi et al. did not examine the effect of ibrutinib on the function of MDSC. The results of the current study shows that ibrutinib can modulate MDSC function and development demonstrating several potential mechanisms by which ibrutinib could improve T cell function. Importantly, the improved CD8+ T cell proliferation in the presence of ibrutinib treated MDSC suggests that ibrutinib results in improved immune function through impairment of MDSC function.
In conclusion, this report demonstrates murine and human MDSC express BTK, and that ibrutinib is able to inhibit the phosphorylation of BTK in these cells. Treatment of MDSC with ibrutinib results in impaired NO production, migration, and generation of MDSC in vitro. In addition, ibrutinib treatment of tumor bearing mice resulted in a significant reduction of MDSC and improved the anti-tumor effect of anti-PD-L1 checkpoint blockade. Thus, ibrutinib-induced suppression of MDSC function and generation could potentially enhance the efficacy of immune based therapies such as cancer vaccines, cytokine therapy, and checkpoint blockade potentially leading to novel combination therapies for testing in clinical trials.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by National Institutes of Health Grants P01CA95426, K24 CA93670 (W.E. Carson.), T32CA90338-27 (W.E. Carson), P30 CA016058 (W.E. Carson, S. Tridandapani, J.C. Byrd, M.A. Caliguiri), Translational grant K12 CA133250 in experimental therapeutics National Cancer Institute (R.C. Wesolowski), and Ohio State Translational Therapeutics Award TT142 (W.E. Carson).
Abbreviations
- MDSC
myeloid derived suppressor cell
- NO
nitric oxide
- IDO
indolamine 2,3-dioxygenase
- BTK
Bruton’s Tyrosine Kinase
- ITK
IL-2 inducible T cell kinase
- TLR4
Toll-like receptor 4
Footnotes
The authors have no conflicts of interest to disclose.
Bibliography
- 1.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. Journal of immunology (Baltimore, Md : 1950) 2010;185(4):2273–84. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102(6):2138–45. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 3.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews Immunology. 2009;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature reviews Immunology. 2012;12(4):253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trikha P, Carson WE., 3rd Signaling pathways involved in MDSC regulation. Biochimica et biophysica acta. 2014;1846(1):55–65. doi: 10.1016/j.bbcan.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer immunology, immunotherapy : CII. 2010;59(10):1593–600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. Journal of immunology (Baltimore, Md : 1950) 2009;182(1):240–9. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 8.Jitschin R, Braun M, Buttner M, Dettmer-Wilde K, Bricks J, Berger J, et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood. 2014;124(5):750–60. doi: 10.1182/blood-2013-12-546416. [DOI] [PubMed] [Google Scholar]
- 9.Mundy-Bosse BL, Lesinski GB, Jaime-Ramirez AC, Benninger K, Khan M, Kuppusamy P, et al. Myeloid-derived suppressor cell inhibition of the IFN response in tumor-bearing mice. Cancer research. 2011;71(15):5101–10. doi: 10.1158/0008-5472.CAN-10-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markowitz J, Brooks TR, Duggan MC, Paul BK, Pan X, Wei L, et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer immunology, immunotherapy : CII. 2015;64(2):149–59. doi: 10.1007/s00262-014-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. Journal of immunology (Baltimore, Md : 1950) 2013;190(2):794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- 12.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer immunology, immunotherapy : CII. 2011;60(10):1419–30. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer immunology, immunotherapy : CII. 2009;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. The Journal of experimental medicine. 2006;203(12):2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer research. 2007;67(23):11438–46. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridle BW, Chen L, Lemay CG, Diallo JS, Pol J, Nguyen A, et al. HDAC inhibition suppresses primary immune responses, enhances secondary immune responses, and abrogates autoimmunity during tumor immunotherapy. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21(4):887–94. doi: 10.1038/mt.2012.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Science translational medicine. 2014;6(237):237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesolowski R, Markowitz J, Carson WE., 3rd Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer. Journal for immunotherapy of cancer. 2013;1:10. doi: 10.1186/2051-1426-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman SE, Mustafa RZ, Gyamfi JA, Pittaluga S, Chang S, Chang B, et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123(21):3286–95. doi: 10.1182/blood-2014-02-548610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byrd JC, O’Brien S, James DF. Ibrutinib in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2013;369(13):1278–9. doi: 10.1056/NEJMc1309710. [DOI] [PubMed] [Google Scholar]
- 22.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nature reviews Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 23.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539–49. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiedler K, Sindrilaru A, Terszowski G, Kokai E, Feyerabend TB, Bullinger L, et al. Neutrophil development and function critically depend on Bruton tyrosine kinase in a mouse model of X-linked agammaglobulinemia. Blood. 2011;117(4):1329–39. doi: 10.1182/blood-2010-04-281170. [DOI] [PubMed] [Google Scholar]
- 25.Mueller H, Stadtmann A, Van Aken H, Hirsch E, Wang D, Ley K, et al. Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma2 and PI3Kgamma pathways. Blood. 2010;115(15):3118–27. doi: 10.1182/blood-2009-11-254185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honda F, Kano H, Kanegane H, Nonoyama S, Kim ES, Lee SK, et al. The kinase Btk negatively regulates the production of reactive oxygen species and stimulation-induced apoptosis in human neutrophils. Nature immunology. 2012;13(4):369–78. doi: 10.1038/ni.2234. [DOI] [PubMed] [Google Scholar]
- 27.Chang BY, Huang MM, Francesco M, Chen J, Sokolove J, Magadala P, et al. The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis research & therapy. 2011;13(4):R115. doi: 10.1186/ar3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KG, Xu S, Kang ZH, Huo J, Huang M, Liu D, et al. Bruton’s tyrosine kinase phosphorylates Toll-like receptor 3 to initiate antiviral response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(15):5791–6. doi: 10.1073/pnas.1119238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Zhan Z, Li D, Xu L, Ma F, Zhang P, et al. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nature immunology. 2011;12(5):416–24. doi: 10.1038/ni.2015. [DOI] [PubMed] [Google Scholar]
- 30.Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. Journal of leukocyte biology. 2009;85(6):996–1004. doi: 10.1189/jlb.0708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apolloni E, Bronte V, Mazzoni A, Serafini P, Cabrelle A, Segal DM, et al. Immortalized myeloid suppressor cells trigger apoptosis in antigen-activated T lymphocytes. Journal of immunology (Baltimore, Md : 1950) 2000;165(12):6723–30. doi: 10.4049/jimmunol.165.12.6723. [DOI] [PubMed] [Google Scholar]
- 32.Woyach JA, Bojnik E, Ruppert AS, Stefanovski MR, Goettl VM, Smucker KA, et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL) Blood. 2014;123(8):1207–13. doi: 10.1182/blood-2013-07-515361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawlings DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, et al. Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science (New York, NY) 1993;261(5119):358–61. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 34.Sawant A, Deshane J, Jules J, Lee CM, Harris BA, Feng X, et al. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer research. 2013;73(2):672–82. doi: 10.1158/0008-5472.CAN-12-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119(11):2590–4. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 36.Horwood NJ, Page TH, McDaid JP, Palmer CD, Campbell J, Mahon T, et al. Bruton’s tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. Journal of immunology (Baltimore, Md : 1950) 2006;176(6):3635–41. doi: 10.4049/jimmunol.176.6.3635. [DOI] [PubMed] [Google Scholar]
- 37.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71(24):7463–70. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ni Gabhann J, Hams E, Smith S, Wynne C, Byrne JC, Brennan K, et al. Btk regulates macrophage polarization in response to lipopolysaccharide. PloS one. 2014;9(1):e85834. doi: 10.1371/journal.pone.0085834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawakami Y, Inagaki N, Salek-Ardakani S, Kitaura J, Tanaka H, Nagao K, et al. Regulation of dendritic cell maturation and function by Bruton’s tyrosine kinase via IL-10 and Stat3. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(1):153–8. doi: 10.1073/pnas.0509784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strauss L, Sangaletti S, Consonni Francesca M, Szebeni G, Morlacchi S, Totaro Maria G, et al. RORC1 Regulates Tumor-Promoting “Emergency” Granulo-Monocytopoiesis. Cancer Cell. 28(2):253–69. doi: 10.1016/j.ccell.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Ansell SM. Two targets for the price of one. 2013. pp. 2529–31. [DOI] [PubMed] [Google Scholar]
- 42.Qin H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nature medicine. 2014;20(6):676–81. doi: 10.1038/nm.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(32):11774–9. doi: 10.1073/pnas.1410626111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng Y, Dou Y, Duan L, Cong C, Gao A, Lai Q, et al. Using chemo-drugs or irradiation to break immune tolerance and facilitate immunotherapy in solid cancer. Cellular immunology. 2015;294(1):54–59. doi: 10.1016/j.cellimm.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer research. 2013;73(10):3007–18. doi: 10.1158/0008-5472.CAN-12-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(6):2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 47.Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. International immunopharmacology. 2011;11(7):856–61. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(9):E966–72. doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh K, Lee OY, Shon SY, Nam O, Ryu PM, Seo MW, et al. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast cancer research : BCR. 2013;15(5):R79. doi: 10.1186/bcr3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.