Abstract
Purpose:
In 2011, an Advanced Notice of Proposed Rulemaking proposed that de-identified human data and specimens be included in biobanks only if patients provide consent. The National Institutes of Health Genomic Data Sharing policy went into effect in 2015, requiring broad consent from almost all research participants.
Genet Med 18 7, 663–671.
Methods:
We conducted a systematic literature review of attitudes toward biobanking, broad consent, and data sharing. Bibliographic databases included MEDLINE, Web of Science, EthxWeb, and GenETHX. Study screening was conducted using DistillerSR.
Genet Med 18 7, 663–671.
Results:
The final 48 studies included surveys (n = 23), focus groups (n = 8), mixed methods (n = 14), interviews (n = 1), and consent form analyses (n = 2). Study quality was characterized as good (n = 19), fair (n = 27), and poor (n = 2). Although many participants objected, broad consent was often preferred over tiered or study-specific consent, particularly when broad consent was the only option, samples were de-identified, logistics of biobanks were communicated, and privacy was addressed. Willingness for data to be shared was high, but it was lower among individuals from under-represented minorities, individuals with privacy and confidentiality concerns, and when pharmaceutical companies had access to data.
Genet Med 18 7, 663–671.
Conclusions:
Additional research is needed to understand factors affecting willingness to give broad consent for biobank research and data sharing in order to address concerns to enhance acceptability.
Genet Med 18 7, 663–671.
Keywords: biobank, broad consent, data sharing, systematic review, tiered consent
Vast amounts of genomic and phenotypic data are needed for many types of research. Frequently, data must be aggregated from several sites to achieve the necessary sample size. These data are often placed in biobanks or biorepositories, which may exist at both the site(s) of collection and in aggregated or centralized sites, such as the database of Genotypes and Phenotypes. These data, which often were collected for one purpose—whether for clinical use or a specific research project—frequently can be studied for other research. These facts raise two distinct, but related, questions. The first is under what conditions data can and should be repurposed for other research in order to increase what can be learned from them. The second is whether data can and should be shared with other investigators in academic institutions, the government, and the commercial sector.
Currently, regulations for the protection of research participants and the Health Information Technology for Economic and Clinical Health Act amendments to the Health Insurance Portability and Accessibility Act Privacy Rule1 permit the sharing and repurposing of data under certain conditions without the consent of the individual from whom the data were obtained. However, the regulatory landscape is rapidly changing. The Advanced Notice of Proposed Rule Making (ANPRM) issued in 2011 by the Office of Human Research Protections deemed all biospecimens “identifiable per se” and so would require that individuals sign a “standard, brief general consent form” that would provide to participants an opportunity to say no to all future research.2 In 2014 the National Institutes of Health (NIH) required that investigators obtain broad consent for research and data sharing as a condition of funding for genomics research, with very few exceptions.3
Nonetheless, questions remain about the ethical and practical desirability and acceptability of broad consent for research and data sharing. Approaches to obtain permission for use of genomic samples and data include no consent, opt-out, opt-in, case-by-case, tiered or categorical,4 and broad or blanket consent. Many have argued that blanket consent for unanticipated future research uses is unethical5 or unworkable,6 whereas others argue that such consent is acceptable as long as additional protections are in place,7 especially since broad data sharing promotes discovery related to health and disease. Debates have also addressed what sort of control, if any, individuals ought to have over the sharing of data obtained from them, with a similar array of options.6,8,9 Each option has proponents who present ethical, legal, and social arguments for their positions, often citing studies of public opinion.10,11 This raises the question of what impact public opinion should have on the development of public policy in this arena.
In 2013, the NIH asked the Consent, Education, Regulation, and Consultation (CERC) Working Group of the Electronic Medical Records and Genomics (eMERGE) Network to conduct a population-based survey of public opinion about the acceptability of both broad consent for research and wide data sharing. To inform the development of this survey and to synthesize the existing literature, we conducted a systematic literature review of empirical research that has been conducted on these topics, the results and policy implications of which are reported here.
Materials and Methods
Definitions
We defined “broad consent” as a process in which participants agree prospectively to have their samples, genomic data, and health information retained for use in any future research deemed appropriate by a biobank and/or relevant oversight bodies. Studies of broad consent may use an opt-in or an opt-out model. “Categorical consent,” by contrast, is a process in which participants agree prospectively to future use of their samples and data for particular types of research, usually by categories of disease (e.g., cardiac diseases, diabetes). “Data sharing” refers to the transfer of biospecimens with their associated genotypic and/or phenotypic information, data derived from biospecimens, and/or health information to researchers at institutions that are not directly affiliated with the biobanks or to other biorepositories.
Literature search strategy
We systematically searched the literature on broad consent and data sharing for biobank research using the following databases: MEDLINE via the PubMed interface, Web of Science, National Reference Center for Bioethics Literature databases (EthxWeb, GenETHX), and Dissertation Abstracts International. Search strategies used subject heading terms appropriate for each database and key words relevant to biobanking, consent, and data sharing (Supplementary Table S1 online). Searches were limited to the literature published since 1990 to capture current views about biobanking. We also manually searched the reference lists of included studies and of recent narrative and systematic reviews addressing the topic. Our initial searches were done between October and December 2013 and were updated in March 2015. All citations were imported into DistillerSR systematic review software.
Two reviewers (N.A.S. and a colleague) initially screened titles and abstracts, and two investigators (N.A.G. and E.W.C.) reviewed the full text of the included articles. Articles were included if they reported empirical data with sufficient detail to enable use and aggregation of the data and results about individuals in the United States regarding one or more of the following: participant perceptions of broad consent or data sharing for biobank research, preferences for different consent models for biobank research, information about people's opinions about participating in biobank research, or providing broad consent for biobank research. Disagreements between reviewers were resolved by discussion that included a third reviewer (A.H.M.A.) to reach consensus.
Data extraction and analysis
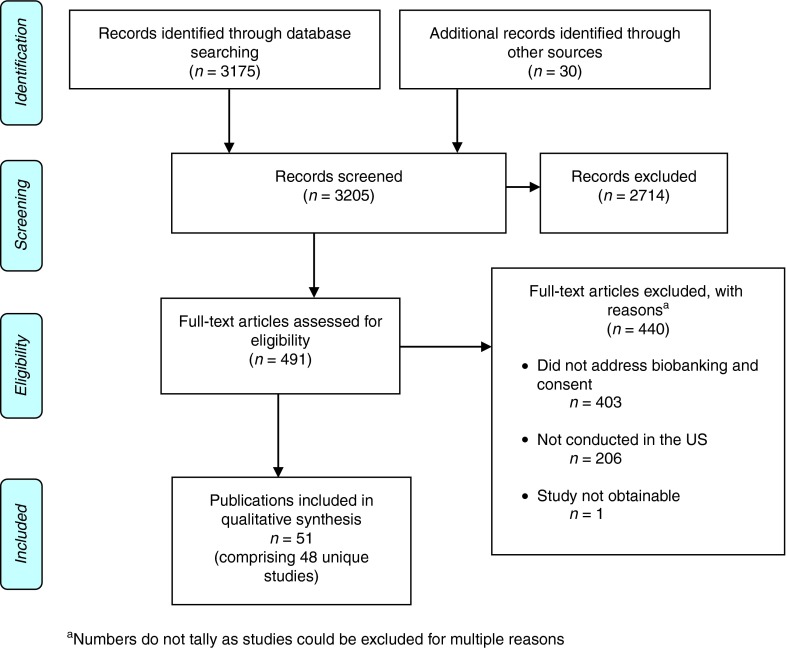
We identified and screened a total of 3,205 citations and abstracts through the electronic database searches and manual review of articles and bibliographies (Figure 1). After reviewing titles and abstracts, we excluded 2,714 studies that did not meet our criteria. We assessed the full text of the 491 remaining studies and excluded another 440 articles because they (i) did not address biobanking, consent, or data sharing (n = 403); (ii) were not conducted in the United States (n = 206); or (iii) were not obtainable (n = 1). Fifty-one publications comprising 48 unique cohorts met our inclusion criteria.
Figure 1.
Disposition of studies identified for this review.
Two investigators (N.A.G. and E.W.C.) assessed the quality of studies using questions adapted from published criteria for the quality assessment of survey and focus group studies.12,13,14 Scoring criteria fell into the following broad domains: (i) description of the methods, (ii) participant recruitment from a representative pool and response rates, (iii) appropriateness of objective study questions, and (iv) data analysis lending to reproducible results. Articles that adequately defined criteria in all four domains were rated as “good.” Articles containing information that had adequate descriptions of the methods but did not fulfill the criteria for all of the other domains received a rating of “fair.” Articles that failed to adequately define their methods, thus preventing an evaluation of representativeness, bias, or reproducibility, received a rating of “poor.” Each study was evaluated based on published and Web-accessible information. The questions used in the quality review are contained in Supplementary Table S2 online. Two investigators (N.A.G. and E.W.C.) also characterized the studies as conducted in urban, rural, or combined settings. The reviewers independently assessed each article and resolved disagreements via discussion to reach consensus.
Data were extracted into summary tables (Supplementary Table S3 online) by outlining the study population and biobank focus, methods, quality assessment, urban/rural residency, and key outcomes related to consent and data sharing. We report the relevant findings based on the terminology, percentages, and number of significant digits as presented in the publications. We qualitatively analyzed results of studies using summary tables and descriptive synthesis. The heterogeneity of study methods and populations precluded performing a meta-analysis.
Results
Article selection
A total of 51 publications comprising 48 studies were included in this review.15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65 Most studies involved surveys (n = 23), followed by focus groups (n = 8), mixed methods (n = 14), interviews (n = 1), and analyses of consent forms (n = 2) (Supplementary Table S3 online). Two publications used a mixed-methods approach that included qualitative studies that informed the development and implementation of a survey.35,45 Nineteen studies were of good quality, 27 of fair quality, and 2 of poor quality. Regardless of the assigned quality score, we included all studies in this review. Roughly one-third of the studies (n = 20) were written and published after the Office of Human Research Protections issued the ANPRM in July 2011.46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65 The number of studies published per year from 2008 to 2014 ranged from five to seven, with no notable difference after the ANPRM was issued. Some of the studies published after 2011 mention the ANPRM.46,56,59,65 Although we examined studies published since 1990, no studies that met our inclusion criteria were published before 2001.
Participant demographics
Studies included a total of 35,969 individuals. Race and/or ethnicity were available for 78.8% of the participants (Table 1). Of these, just over half (51.3%) of participants identified as white, and 13.6% were African American and 6.3% were Hispanic/Latino. Native-American, Alaska Native, Native Hawaiian, and Pacific Islander participants made up 2.2% of the sample. Representation of Asian participants was particularly low at 1.4%. Details for gender were available for 93.3% of participants. Women made up 54.2% of the total sample.
Table 1. Sociodemographic characteristics.
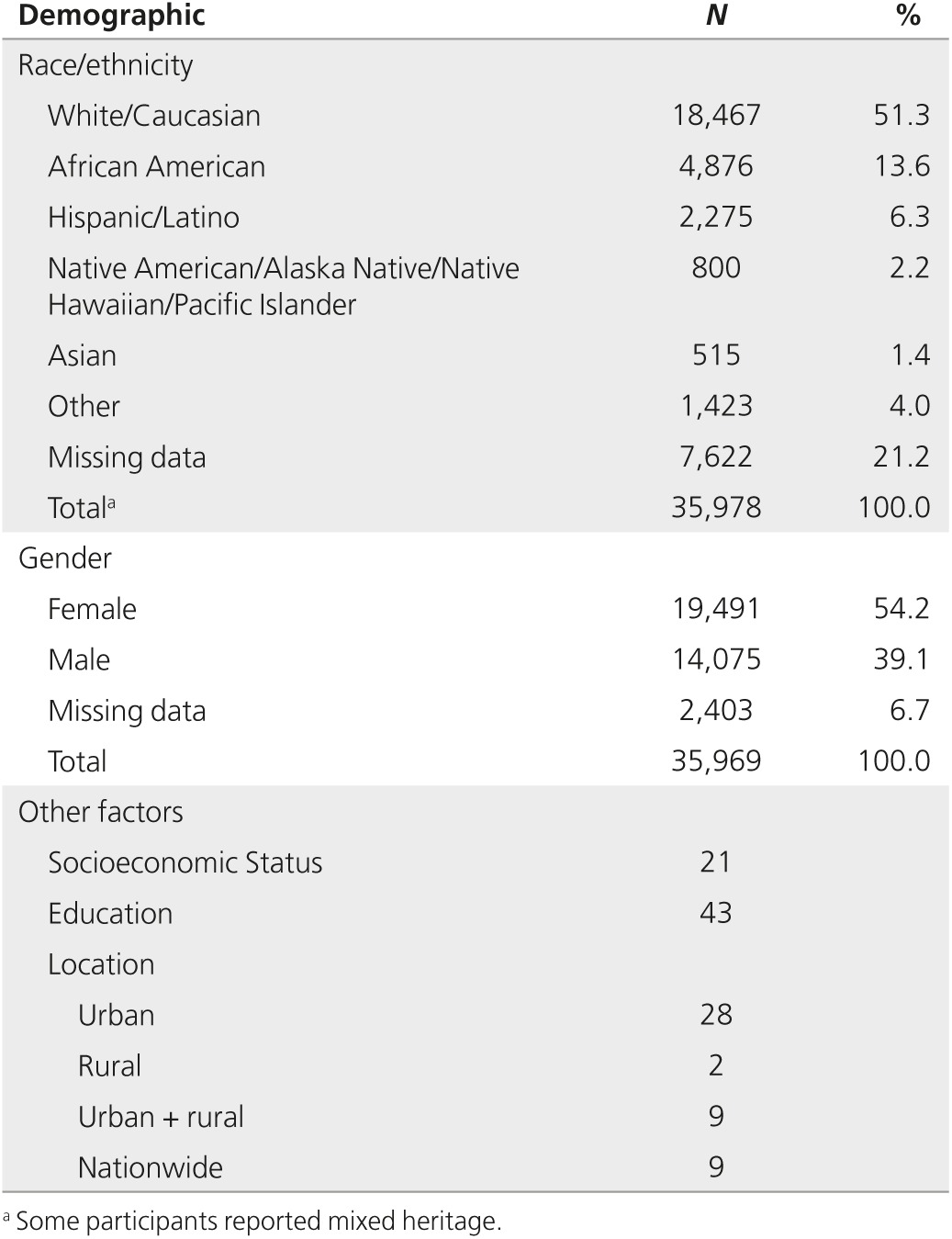
Many studies did not report other demographic data. Only 21 studies reported socioeconomic status, and 43 reported educational level. Twenty-eight studies were conducted primarily in urban settings, two were conducted in rural settings, nine were conducted in both urban and rural settings, and nine studies were conducted nationwide.
Studies of broad consent
We identified 48 unique studies that focused on different approaches to obtaining consent.15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,38,39,41,42,44,45,46,47,49,50,51,52,53,56,57,58,59,60,61,62,63,64,65 Three papers each reported two unique studies;35,42,45 other studies were reported in multiple papers.19,21,34,35,48,49,59
Willingness to provide broad consent. Investigators used a variety of approaches to ascertain support for broad consent. Some analyzed the actual choice that participants made when enrolling in research. For example, a retrospective analysis of signed informed consent forms found that 87.1% of 1,298 research participants at the NIH authorized all future research.20 In a different large national study, the National Health and Nutrition Examination Survey (NHANES), 84.8% of 4,480 overall participants recruited in 1999 and 2000 agreed to DNA specimen collection for inclusion in a national repository for genetic research.18
Many studies asked participants hypothetical questions about their willingness to provide broad consent for research. In Indiana, 88.4% of 273 cancer patients agreed that they would be “willing to permit their tissue sample to be used in research on any condition.”24 After time for deliberation, 85% of 40 focus group participants in North Carolina reported that they would agree to have blood and information stored indefinitely in a biorepository for future research.25 Similarly, 78% of 49 focus group participants in Chicago were interested in participating in a biobank, and the majority stated they would give broad consent.37 Of 30 patients who were interviewed at a Hawaiian cancer center, 77% endorsed broad consent.57 One representative nationwide survey found that 68% of 1,593 respondents were willing to give broad consent for research, although their enthusiasm waned if they had a moral objection to certain types of studies for which their samples might be used.65 Two studies examined patients' willingness to participate in biobanks managed by Kaiser Permanente: 69% of 500 Kaiser patients in the Northwest30 and 69% of 203 in Colorado54 agreed to participate in a biobank. In a focus group study in Boston, patients with breast cancer were generally positive about having their samples used for secondary studies that were not planned at the time they gave consent.22 One older survey deserves special comment. Scott et al.39 reported the results of a 1998 survey of blood donors that asked about their views regarding storage and use of the blood for research. Of the 49,775 respondents, 60.3% said that “testing stored blood for any research” was acceptable with the donor's permission, and 35.5% would not require permission for research use. These studies reported substantial acceptance for broad consent.
Asking participants for their preference among different types of consent—broad, study-by-study, or categorical consent—revealed more mixed support for broad consent. For example, 47% of 931 veterans preferred to give broad consent over other types of consent for all research approved by an oversight board.33 After adjusting for missing data, a national survey of 4,569 adults found that 52% preferred broad consent, whereas 48% preferred study-by-study consent.59 In a survey of 751 Iowans, 42% preferred broad consent and 29% favored study-specific consent, compared with 25% who favored categorical consent.45 In another study of 315 cancer patients at two hospitals in Atlanta, 92 and 97% were willing to allow their samples to be used for research on other diseases; when asked to specify a preference, 56% preferred one-time broad consent and 11% preferred study-by-study consent over no consent or no preference.23 In a 2001 nationwide survey, 43% of 2,621 participants were willing to donate blood for genetic research and to allow it to be stored for future research.16 Similarly, only 39.3% of 30 patients who had already donated samples preferred broad consent over consent for specific studies.41 By contrast, 77.7% of 1,276 people recruited through a crowd-sourced Internet marketplace were willing to donate to biobanks, even after receiving disclosures about potentially objectionable research; however, 40.8% of participants still felt that specific consent was necessary, even if it might inhibit research progress.58 A similar nationwide survey of 1,599 individuals conducted through a probability-based online panel of adults found a wide range of opinions, with broad consent and real-time study-by-study consent considered the “worst” of five options.65
Several studies showed that participants preferred to give informed consent for each study rather than a broad consent, with preferences ranging from 42 to 72%: 42% of a national sample of 4,700 US adults34,35 (which rose to 48% after adjusting for missing data59), 43 to 50% of 931 veterans nationwide,33,50 and 60.7% of adults recruited in New York.41 Of 393 parents, 72% reported that they would want to consent each time to allow their child's dried bloodspots to be used for research.56 In focus groups of 92 Native Hawaiians, respondents repeatedly expressed desire to re-consent, although some stated that they would be content if they trusted the researcher or the biobank's governance.63 In one study of 273 Jewish individuals, 60–75% believed that consent should be required regardless of whether the DNA was collected in a research or clinical setting.15
In a focus group study of 178 Alaska Native participants, some indicated a preference to have consent options for a variety of specimen uses, storage duration, and destruction of the sample at the completion of the study.48,49 In the same group, some wanted re-contact each time, whereas others felt that a one-time consent was appropriate for new studies. In Chicago, 239 postpartum women were asked about their willingness to enroll their children into a pediatric biobank: 48% of women would enroll their child, but 24% would not; of the latter, 82% of the participants were African American.28 In another focus group study, 11 of 15 participants preferred tiered consent over other methods to exert the greatest level of control regarding how they wanted their data to be shared; however, participants who were willing to provide broad consent also appreciated the option to opt in or opt out of DNA data sharing.27
Preferences for opt-out or opt-in. Some studies reported that most respondents favored an opt-in approach,15,26,29,45,56 whereas others found that opt-out was acceptable or even preferred by the majority.23,38,42,44,53 A majority of participants—67% of 751 survey respondents and 63% of 57 focus group participants—who were asked about biobank participation in Iowa preferred opt-in, whereas 18% of survey respondents and 25% of focus group participants in the same study preferred opt-out.45 In a study of 451 nonactive military veterans, 82% thought it would be acceptable for the proposed Million Veterans biobank to use an opt-in approach, and 75% thought that an opt-out approach was acceptable; 80% said that they would take part if the biobank were opt-in as opposed to 69% who would participate if it were an opt-out approach.50 When asked to choose which option they would prefer, 29% of respondents chose the opt-in method, 14% chose opt-out, 50% said either would be acceptable, and 7% would not want to participate.
In some cases, biobank participants were re-contacted to inquire about their thoughts regarding proposed changes to the biobank in which they participated. Thirty-two biobank participants who attended focus groups in Wisconsin regarding proposed minimal-risk protocol changes were comfortable with using an opt-out model for future studies because of the initial broad consent given at the beginning of the study and their trust in the institution.44 A study of 365 participants who were re-contacted about their ongoing participation in a biobank in Seattle showed that 55% thought that opt-out would be acceptable, compared with 40% who thought it would be unacceptable.38
Similarly, several studies explored perspectives on the acceptability of an opt-out biobank at Vanderbilt University. First, 91% of 1,003 participants surveyed in the community thought leftover blood and tissues should be used for anonymous medical research under an opt-out model; these preferences varied by population, with 76% of African Americans supporting this model compared with 93% of whites.29 In later studies of community members, approval rates for the opt-out biobank were generally high (around 90% or more) in all demographic groups surveyed, including university employees, adult cohorts, and parents of pediatric patients.42,53
Three studies explored community perspectives on using newborn screening blood spots for research through the Michigan BioTrust for Health program. First, 77% of 393 parents agreed that parents should be able to opt out of having their child's blood stored for research.56 Second, 87 participants were asked to indicate a preference: 55% preferred an opt-out model, 29% preferred to opt-in, and 16% felt that either option was acceptable.47 Finally, 39% of 856 college students reported that they would give broad consent to research with their newborn blood spots, whereas 39% would want to give consent for each use for research.60 In a nationwide telephone survey regarding the use of samples collected from newborns, 46% of 1,186 adults believed that researchers should re-consent participants when they turn 18 years old.31
Identifiability of samples influences the acceptability of broad consent. Some studies examined the differences in participants' willingness to provide broad consent for samples that were de-identified or anonymous as compared with identifiable. Respondents generally preferred to give consent if their samples were identifiable. In two studies involving 429 primarily Native Hawaiian participants, 78% of Native Hawaiians and 66% of whites indicated that they would require consent for research if the specimens were identifiable and collected in the clinical setting.19 For genetics research, 81% Native Hawaiians and 78% of whites indicated that they would require consent if the specimens were identifiable.21 In a US-wide telephone survey, 81% of 1,193 respondents stated that they would want to be informed about research being done with their sample if it were identifiable; additionally, 57% said they would require permission to use their samples if they were identifiable.26 De-identification tended to allay concerns. For example, 65.8% of 504 adults who participated in a telephone survey across the United States reported that they would require consent for samples collected in the clinic if they were identifiable, compared with 27.3% who reported they would require consent if samples were anonymized.17 In the research setting, fewer people thought consent was required for identifiable (29.0%) or anonymized (12.1%) samples.17 In a study utilizing a hypothetical biobank scenario, 43% of 565 government and medical employees in New Mexico indicated that they would donate their sample for future genetic testing if it could not be traced to them.32 Not all studies found that people were worried about identifiability. In one survey of 144 clinicians, 86% said that they would donate a DNA sample to a hypothetical biobank in New York regardless of whether it was linked to or unlinked from their identity.36 In the study in New Mexico, 36% of 565 respondents found it acceptable for broadly consented samples to be used by their local university, even if the samples were linked to them.32
Factors associated with views about broad consent. Few studies reported the correlation between demographic variables and respondents' opinions. Characteristics associated with favoring broad consent included being male,30,34,59 white/Caucasian,30,34 older,30,50 and more affluent.30,50 By contrast, Asians,34 black non-Hispanics,33,50 African Americans,20 and others19,21,50 (who represented 14.3% of the total) were less likely than whites to believe that research without explicit permission was acceptable. One study of consent forms showed that 75.0% of African Americans gave broad consent compared with 88.4% of whites (P = 0.002).20 Similarly, in the NHANES data, 78.7% of African Americans and 87.1% of whites consented to genetics research.18
A few studies looked at other factors that correlated with preferring broad consent. One study reported that participants who were significantly more likely to prefer broad consent also believed that participating would “make me feel like I was contributing to society” (odds ratio = 1.85; P = 0.001), that the study would accelerate medical treatments and cures (odds ratio = 2.20; P = 0.001), and that participating in the cohort study would be easy (odds ratio = 1.59; P < 0.001).59 Other investigators reported that the large majority (97.7%) of respondents said “yes” or “maybe” to the idea that it is a “gift” to society when an individual takes part in medical research.46 Many other studies cited the benefit of research to improve health as a reason to favor broad consent.
Studies of data sharing
We identified 23 studies of data sharing.23,24,25,27,28,30,33,34,40,41,42,43,44,46,48,51,52,54,55,59,61,62,64 The earliest publications about participants' preferences on data sharing date to 2006. Most studies of data sharing were conducted with studies of consent preferences; however, six studies were conducted with the primary goal of eliciting preferences on data sharing.37,40,43,48,54,55
Willingness to share with other researchers. Participants were generally willing to have their samples and information shared with other academic institutions. Willingness to share data with academic and medical researchers was acceptable for 92% of 4,659 US adults generally,34 and 80% of 931 US veterans specifically.33 More than 70% of 100 young adults in Baltimore who were enrolled in a longitudinal study of prevention were willing to share results arising from their DNA.62 “Nearly three fourths” of 40 community members in a focus group study in North Carolina were comfortable with academic researchers having access to their samples.25 Many of 79 focus group participants in Seattle endorsed the value of sharing, agreed that sharing locally and with close collaborators was acceptable, and were comfortable with nonprofit and public-interest organizations using data from their samples.40 In one focus group study of 48 primarily white and female participants in Iowa, the majority cited positive reasons for donating their samples to help and to contribute to advancements in research, and that data sharing would not affect their decision to enroll in a biobank.44 In another focus group study of 100 African Americans in North Carolina, many recognized the benefits of data sharing but wanted the potential risks to be disclosed, and some wanted the data to be restricted.43 In another study of patients with inflammatory bowel diseases, 97.3% of 92 respondents were comfortable with sharing their biological sample with investigators in the United States, but 23.8% were uncomfortable with sharing with investigators outside the United States.64
Willingness to share in national databases or federal repositories. Some participants expressed concern over sharing their data and information with federal repositories. In one study, 18.5% of 4,050 Vanderbilt University faculty and staff were more likely to want to participate in their institution's biobank if the de-identified data were deposited into a national database; however, 12.1% were less likely to want to participate.42 In many studies, the location of the repository was often important. In two large nationwide surveys, 80% of 4,659 adults were willing to have their data shared with government researchers; however, 75% of the same sample also were concerned about “the government having [their] samples and information.”34 Similarly, another study found that 71% of 931 veterans were willing to grant database access to government researchers, but half were concerned about “the government having [their] samples and information.”33
Other studies have shown that some people are concerned about government involvement in maintaining databases containing biomedical information. More than half of the 40 participants in a focus group study of North Carolina community members were concerned about government researchers having access to their institution's biorepository.25 Despite concerns, 61% of 203 Kaiser patients in Colorado would still provide a sample even if the data would be submitted to a government database,54 and 82% of 500 Kaiser patients in Oregon agreed to have their information posted in a US government database.30 In a large metropolitan area in southwest Florida, some of 95 focus group participants believed that biospecimens were already being collected from leftover tissue, and others suspected that tissues were already being shared with researchers in other countries who lack “‘strict laws' governing research.”52 In a focus group study of 178 Alaska Native participants, some cited mistrust of the government and police having access to their samples and wanted transparency from the researchers about how their samples were used.48
Willingness to share with commercial enterprises. The majority of participants were willing to share with pharmaceutical company researchers, but the percentage was generally less than the percentage willing to share with academic researchers. Seventy-five percent of 4,659 US adults,34 54% of 931 veterans,33 55.2% of 1,599 adults responding to a nationwide survey,65 and 75.1% of members of the Crohn's and Colitis Foundation of America Partners cohort were willing to share with pharmaceutical company researchers.64 Focus group participants in Florida voiced concern about providing blanket consent because they would not benefit financially from any resulting discoveries.52
Factors associated with views about data sharing. With the exception of gender, few demographic data (e.g., about race/ethnicity, socioeconomic status, education, and urban/rural residency) were available. Even when demographic information was obtained, investigators did not always report how these variables correlated with respondents' opinions. Therefore, it was largely not possible to draw meaningful conclusions about the associations between sociodemographic factors and views on data sharing.
The willingness of patients with cancer to share seemed to be shaped by their devotion to the institution at which they were receiving care. For example, patients with cancer in Indiana who agreed to participate in a biobank were less likely to be willing to allow their tissue samples to be used by researchers who were not affiliated with the local researchers (89.7%), compared with 96.3% who were willing to share with local university researchers (P < 0.01).24 Half of 100 patients with breast cancer at MD Anderson Cancer Center preferred to allow only their physician (24%) or other researchers at their hospital (26%) to use their de-identified genetic data for research; fewer patients were willing to share their de-identified data with any cancer researcher (25%) or any researcher (18%).61 In another study, 95% of 315 patients with cancer in Atlanta were willing to allow researchers to share samples with other local researchers, but only 85% and 92% of participants at two different sites were willing to have their samples shared elsewhere in the United States (P < 0.05).23
Discussion and Conclusion
In 2013, NIH funded the eMERGE consortium to perform a broad population-based survey to assess public opinion about broad consent for research and data sharing. This systematic literature review, which ultimately contained 48 studies involving 35,969 participants, was conducted to identify gaps and issues that needed to be addressed in this survey.
The most notable finding is that many people do not favor broad consent for either research itself or for research and subsequent wide data sharing. While the majority often expressed support for broad consent when that was the only choice offered, only a minority of respondents favored broad consent when other options, such as tiered or study-by-study consent, were offered. Furthermore, earlier studies focused on the importance of obtaining consent for research, whereas later studies focused on the preferences for different consent options. Willingness to give broad consent increased if data were de-identified. While individuals were generally willing for data or biospecimens to be shared with other academic researchers, individuals were less willing for their data to be shared in federal databases or with commercial enterprises. These findings differ from recent assertions that the public generally supports broad consent.66,67
What is equally striking are the large gaps in what is known about factors that affect people's decisions. Gender is the only demographic for which there is essentially complete information. Yet while a few studies generally found that men were more likely to support broad consent, most investigators did not examine the impact of gender on attitudes. Although data about race/ethnicity are incomplete, it seems that minorities often have more concerns about broad consent, although existing evidence suggests that these concerns can be ameliorated in some cases by discussion and education. Much less is known about the impact of sociodemographic factors—such as socioeconomic status, education, and whether people live in urban or rural environments—on attitudes toward broad consent and data sharing. Building on these findings, the eMERGE CERC survey developed a sampling strategy, experimental study design, and survey questions to ascertain more uniformly the views of individuals throughout society in order to identify and address concerns.
This study had several limitations. First, we used broad search terms to capture the existing literature on broad consent and data sharing. The literature addressing these concepts is not well indexed. Thus, while we used multiple approaches (e.g., searching multiple sources, reviewing reference lists, and searching the unpublished, “gray” literature, such as dissertations and reports) to comprehensively identify studies, we may not have identified all salient research. We excluded commentaries and one dissertation from which data could not be extracted. Second, we adapted existing metrics of quality scores to our study. For many studies, we were unable to ascertain the appropriateness of study questions or an analysis plan, thus limiting our ability to thoroughly assess the quality of the studies. Third, the studies that have been conducted to date have a number of limitations, which in turn limit the generalizability of this literature review. Several methodologies were used across studies, often in ways that limit direct comparability. Many of the surveys focus what people say they think, rather what they actually do, even though opinions may differ from action. Definitions of consent were not always consistent and have changed over time, which not only limits our ability to compare studies but also may affect our evaluation of older studies given today's ethical standards for biobanking governance. However, all studies were sufficiently focused on broad consent for research or for data sharing to permit some comparison. Most of the surveys heavily oversampled whites, whereas the qualitative studies disproportionately involved minority participants. Studies that incorporated an educational component may have influenced respondents compared with those studies that did not involve education around biobanking practices. This review also was limited to the United States, which is warranted given the different policy preferences in other countries.
The ultimate goal of this literature review and the eMERGE CERC survey is to obtain a more comprehensive understanding of public opinion about broad consent for data sharing and use. The studies included here typically noted a general acceptance for broad consent and endorsement of data sharing, but with notable privacy and governance concerns, especially by minority participants. The policy question will be what to do if some people, particularly from certain demographics, express a desire for more granular control over the use of data obtained from them in light of the policy trend toward requiring individual consent for broad data use and sharing. At a minimum, it suggests the need to engage those who are skeptical, even if it is decided that the public good of research to improve health outweighs honoring individual objections in some cases or the risk that some people will choose not to participate.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The eMERGE Network was initiated and funded by National Human Genome Research Institute through the following grants: U01HG006828 (Cincinnati Children's Hospital Medical Center/Boston Children's Hospital), U01HG006830 (Children's Hospital of Philadelphia), U01HG006389 (Essentia Institute of Rural Health), U01HG006382 (Geisinger Clinic), U01HG006375 (Group Health Cooperative), U01HG006379 (Mayo Clinic), U01HG006380 (Icahn School of Medicine at Mount Sinai), U01HG006388 (Northwestern University), U01HG006378 (Vanderbilt University Medical Center), U01HG006385 (Vanderbilt University Medical Center serving as the Coordinating Center), and the eMERGE Consent, Education, Regulation, and Consultation (CERC) working group supplement grant.
The authors thank Rachel R. Walden, previously at Vanderbilt's Eskind Biomedical Library, for conducting the electronic searches and assisting with the initial screening of titles and abstracts.
Supplementary Material
References
- HITECH Act amendments to the HIPAA Privacy Rule. 45 C.F.R. §164.508 (c)(2015).
- Office of Human Research Protections. Advanced Notice of Proposed Rule Making, 76 Fed. Reg. 44512–31. 26 July 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-07-26/html/2011–18792.htm. Accessed 17 April, 2015.
- National Institutes of Health. Genomic Data Sharing Policy, 79 Fed. Reg. 51345–54. 28 August 2014. https://www.federalregister.gov/articles/2014/08/28/2014–20385/final-nih-genomic-data-sharing-policy. Accessed 17 April, 2015.
- National Cancer Institute/National Action Plan on Breast Cancer. NCI/NAPBC Model Informed Consent Documents. http://oprs.usc.edu/files/2013/01/modelconsenthandouts.pdf. Accessed 17 April 2015.
- Tomlinson T. Respecting donors to biobank research. Hastings Cent Rep 2013;43:41–47. [DOI] [PubMed] [Google Scholar]
- Stein DT, Terry SF. Reforming biobank consent policy: a necessary move away from broad consent toward dynamic consent. Genet Test Mol Biomarkers 2013;17:855–856. [DOI] [PubMed] [Google Scholar]
- Hofmann B. Broadening consent–and diluting ethics? J Med Ethics 2009;35:125–129. [DOI] [PubMed] [Google Scholar]
- Williams H, Spencer K, Sanders C, et al. Dynamic consent: a possible solution to improve patient confidence and trust in how electronic patient records are used in medical research. JMIR Med Inform 2015;3:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. NIH, Lacks family reach understanding to share genomic data of HeLa cells. Press release, 7 August 2013. http://www.nih.gov/news/health/aug2013/nih-07.htm. Accessed 17 April 2015.
- Pereira S, Gibbs RA, McGuire AL. Open access data sharing in genomic research. Genes (Basel) 2014;5:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master Z, Nelson E, Murdoch B, Caulfield T. Biobanks, consent and claims of consensus. Nat Methods 2012;9:885–888. [DOI] [PubMed] [Google Scholar]
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–357. [DOI] [PubMed] [Google Scholar]
- Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. Int J Qual Health Care 2003;15:261–266. [DOI] [PubMed] [Google Scholar]
- Huston P. Reporting on surveys: information for authors and peer reviewers. CMAJ 1996;154:1695–1704. [PMC free article] [PubMed] [Google Scholar]
- Schwartz MD, Rothenberg K, Joseph L, Benkendorf J, Lerman C. Consent to the use of stored DNA for genetics research: a survey of attitudes in the Jewish population. Am J Med Genet 2001;98:336–342. [DOI] [PubMed] [Google Scholar]
- Wang SS, Fridinger F, Sheedy KM, Khoury MJ. Public attitudes regarding the donation and storage of blood specimens for genetic research. Community Genet 2001;4:18–26. [DOI] [PubMed] [Google Scholar]
- Wendler D, Emanuel E. The debate over research on stored biological samples: what do sources think? Arch Intern Med 2002;162:1457–1462. [DOI] [PubMed] [Google Scholar]
- McQuillan GM, Porter KS, Agelli M, Kington R. Consent for genetic research in a general population: the NHANES experience. Genet Med 2003;5:35–42. [DOI] [PubMed] [Google Scholar]
- Fong M, Braun KL, Chang RM. Native Hawaiian preferences for informed consent and disclosure of results from research using stored biological specimens. Pac Health Dialog 2004;11:154–159. [PubMed] [Google Scholar]
- Chen DT, Rosenstein DL, Muthappan P, et al. Research with stored biological samples: what do research participants want? Arch Intern Med 2005;165:652–655. [DOI] [PubMed] [Google Scholar]
- Fong M, Braun KL, Chang RM. Native Hawaiian preferences for informed consent and disclosure of results from genetic research. J Cancer Educ 2006;21(suppl 1):S47–S52. [DOI] [PubMed] [Google Scholar]
- Kaphingst KA, Janoff JM, Harris LN, Emmons KM. Views of female breast cancer patients who donated biologic samples regarding storage and use of samples for genetic research. Clin Genet 2006;69:393–398. [DOI] [PubMed] [Google Scholar]
- Pentz RD, Billot L, Wendler D. Research on stored biological samples: views of African American and White American cancer patients. Am J Med Genet A 2006;140:733–739. [DOI] [PubMed] [Google Scholar]
- Helft PR, Champion VL, Eckles R, Johnson CS, Meslin EM. Cancer patients' attitudes toward future research uses of stored human biological materials. J Empir Res Hum Res Ethics 2007;2:15–22. [DOI] [PubMed] [Google Scholar]
- Beskow LM, Dean E. Informed consent for biorepositories: assessing prospective participants' understanding and opinions. Cancer Epidemiol Biomarkers Prev 2008;17:1440–1451. [DOI] [PubMed] [Google Scholar]
- Hull SC, Sharp RR, Botkin JR, et al. Patients' views on identifiability of samples and informed consent for genetic research. Am J Bioeth 2008;8:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AL, Hamilton JA, Lunstroth R, McCullough LB, Goldman A. DNA data sharing: research participants' perspectives. Genet Med 2008;10:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidich AB, Joseph JW, Ober C, Ross LF. Empirical data about women's attitudes towards a hypothetical pediatric biobank. Am J Med Genet A 2008;146A:297–304. [DOI] [PubMed] [Google Scholar]
- Pulley JM, Brace MM, Bernard GR, Masys DR. Attitudes and perceptions of patients towards methods of establishing a DNA biobank. Cell Tissue Bank 2008;9:55–65. [DOI] [PubMed] [Google Scholar]
- Goddard KA, Smith KS, Chen C, McMullen C, Johnson C. Biobank recruitment: motivations for nonparticipation. Biopreserv Biobank 2009;7:119–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg AJ, Hull SC, Botkin JR, Wilfond BS. Pediatric biobanks: approaching informed consent for continuing research after children grow up. J Pediatr 2009;155:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoop JG, Roberts LW, Hammond KA. Genetic testing of stored biological samples: views of 570 U.S. workers. Genet Test Mol Biomarkers 2009;13:331–337. [DOI] [PubMed] [Google Scholar]
- Kaufman D, Murphy J, Erby L, Hudson K, Scott J. Veterans' attitudes regarding a database for genomic research. Genet Med 2009;11:329–337. [DOI] [PubMed] [Google Scholar]
- Kaufman DJ, Murphy-Bollinger J, Scott J, Hudson KL. Public opinion about the importance of privacy in biobank research. Am J Hum Genet 2009;85:643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, Hudson K. Public perspectives on informed consent for biobanking. Am J Public Health 2009;99:2128–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern MH. Clinician attitudes and intentions toward participation in a DNA biobank. Thesis, Case Western Reserve University, 2010. [Google Scholar]
- Lemke AA, Wolf WA, Hebert-Beirne J, Smith ME. Public and biobank participant attitudes toward genetic research participation and data sharing. Public Health Genomics 2010;13:368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludman EJ, Fullerton SM, Spangler L, et al. Glad you asked: participants' opinions of re-consent for dbGap data submission. J Empir Res Hum Res Ethics 2010;5:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EA, Schlumpf KS, Mathew SM, et al. Biospecimen repositories: are blood donors willing to participate? Transfusion 2010;50(9):1943–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinidad SB, Fullerton SM, Bares JM, Jarvik GP, Larson EB, Burke W. Genomic research and wide data sharing: views of prospective participants. Genet Med 2010;12:486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle-Mansilla JI, Ruiz-Canela M, Sulmasy DP. Patients' attitudes to informed consent for genomic research with donated samples. Cancer Invest 2010;28:726–734. [DOI] [PubMed] [Google Scholar]
- Brothers KB, Morrison DR, Clayton EW. Two large-scale surveys on community attitudes toward an opt-out biobank. Am J Med Genet A 2011;155A:2982–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga SB, O'Daniel J. Public perspectives regarding data-sharing practices in genomics research. Public Health Genomics 2011;14:319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty CA, Garber A, Reeser JC, Fost NC; Personalized Medicine Research Project Community Advisory Group and Ethics and Security Advisory Board. Study newsletters, community and ethics advisory boards, and focus group discussions provide ongoing feedback for a large biobank. Am J Med Genet A. 2011;155A:737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon CM, L'heureux J, Murray JC, et al. Active choice but not too active: public perspectives on biobank consent models. Genet Med 2011;13:821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Mitchell R, Cadigan RJ, Davis AM, Dobson AW, Gladden RQ. A trade secret model for genomic biobanking. J Law Med Ethics 2012;40:612–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette D, Langbo C, Bach J, Kleyn M. Michigan BioTrust for Health: public support for using residual dried blood spot samples for health research. Public Health Genomics 2012;15:146–155. [DOI] [PubMed] [Google Scholar]
- Hiratsuka V, Brown J, Dillard D. Views of biobanking research among Alaska native people: the role of community context. Prog Community Health Partnersh 2012;6:131–139. [DOI] [PubMed] [Google Scholar]
- Hiratsuka VY, Brown JK, Hoeft TJ, Dillard DA. Alaska native people's perceptions, understandings, and expectations for research involving biological specimens. Int J Circumpolar Health 2012;71:18642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D, Bollinger J, Dvoskin R, Scott J. Preferences for opt-in and opt-out enrollment and consent models in biobank research: a national survey of veterans administration patients. Genet Med 2012;14:787–794. [DOI] [PubMed] [Google Scholar]
- Lemke AA, Halverson C, Ross LF. Biobank participation and returning research results: perspectives from a deliberative engagement in South Side Chicago. Am J Med Genet A 2012;158A:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque JS, Quinn GP, Montel-Ishino FA, et al.; Tampa Bay Community Cancer Network Partners. Formative research on perceptions of biobanking: what community members think. J Cancer Educ 2012;27:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers KB, Westbrook MJ, Wright MF, et al. Patient awareness and approval for an opt-out genomic biorepository. Per Med 2013;10. doi:10.2217/pme.13.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahm AK, Wrenn M, Carroll NM, Feigelson HS. Biobanking for research: a survey of patient population attitudes and understanding. J Community Genet 2013;4:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgeway JL, Han LC, Olson JE, et al. Potential bias in the bank: what distinguishes refusers, nonresponders and participants in a clinic-based biobank? Public Health Genomics 2013;16:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel DB, Platt T, Platt J, King SB, Kardia SL. Community perspectives on public health biobanking: an analysis of community meetings on the Michigan BioTrust for Health. J Community Genet 2014;5:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun KL, Tsark JU, Powers A, et al. Cancer patient perceptions about biobanking and preferred timing of consent. Biopreserv Biobank 2014;12:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornick MC, Ryan KA, Kim SY. Impact of non-welfare interests on willingness to donate to biobanks: an experimental survey. J Empir Res Hum Res Ethics 2014;9:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J, Bollinger J, Dvoskin R, Kardia SL, Kaufman D. Public preferences regarding informed consent models for participation in population-based genomic research. Genet Med 2014;16:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T, Platt J, Thiel DB, Fisher N, Kardia SL. ‘Cool! and creepy': engaging with college student stakeholders in Michigan's biobank. J Community Genet 2014;5:349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogith D, Yusuf RA, Hovick SR, et al. Attitudes regarding privacy of genomic information in personalized cancer therapy. J Am Med Inform Assoc 2014;21(e2):e320–e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storr CL, Or F, Eaton WW, Ialongo N. Genetic research participation in a young adult community sample. J Community Genet 2014;5:363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauali i M, Davis EL, Braun KL, et al. Native Hawaiian views on biobanking. J Cancer Educ 2014;29:570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MD, Cadigan RJ, Cook SF, et al. Perceptions of patients with inflammatory bowel diseases on biobanking. Inflamm Bowel Dis 2015;21:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson T, De Vries R, Ryan K, Kim HM, Lehpamer N, Kim SY. Moral concerns and the willingness to donate to a research biobank. JAMA 2015;313:417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C, Eckstein L, Berkman B, et al. Broad consent for research with biological samples: workshop conclusions. Am J Bioeth 2015;15:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truven Health Analytics. Health Poll: Data Privacy. Ann Arbor, MI. November 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.