Abstract
Background
Previous studies have indicated that patients on maintenance hemodialysis(HD) have worse survival compared to kidney transplant(KTx) recipients. However, none of these studies have compared mortality of the United States(US) patients using alternative dialysis modalities such as home HD with KTx recipients.
Methods
Comparing patients who started home HD with those who received kidney transplantation in the US between 2007–2011, we created a 1:1 propensity-matched(PS) cohort of 4,000 patients and examined the association between treatment modality and all-cause mortality using Cox proportional hazard models.
Results
The mean±SD age of the PS-matched home HD and KTx patients at baseline were 54±15 years and 54±14 years, 65% were male(both groups), 70% and 72% of patients were whites and 19% were African American(both groups), respectively. Over 5-years of follow-up, home HD patients had 4-times higher mortality risk compared to KTx recipients in the entire patient population (hazard ratio(HR):4.06,95%confidence interval(CI):3.27–5.04);total event number=411), and similar difference was found across each race stratum. However, during the first year of therapy, while the white home HD patients had higher mortality risk (HR:4.21,95%CI:3.10–5.73;total event number=332) compared to their KTx counterparts, there was no significant difference in mortality risk between African American home HD and KTx patients (HR:1.62,95%CI:0.77–3.39;total event number=55). This result was consistent across different types of kidney donors.
Conclusions
Home HD patients appear to have 4 times higher mortality compared to KTx recipients regardless of the type of kidney donor. Further studies are needed to understand the reasons underlying racial differenes during the first year of therapy.
Introduction
Kidney transplantation (KTx) is the treatment of choice for patients with end stage renal disease (ESRD). Several studies have compared survival of waitlisted dialysis patients with KTx recipients.1–8 One of the largest study of 230,000 dialysis patients showed that mortality was significantly lower among patients who received a KTx compared with transplant wait-listed dialysis patients (3.8 vs. 6.3/100 patient-years).8
Previous studies showed that home hemodialysis (home HD) provides better survival than in-center HD. Marshall et al9 examined 865 home HD patients and 21,184 in-center HD patients over 72,052 patient-years and found that home HD was associated with 47% lower mortality risk, and Weinhandl et al10 reported 13% lower mortality risk in 1,873 home HD patients compared with 9,365 matched (1:5) patients. In addition, in a recent 1:10 propensity score (PS) matched analysis of patients from the United States, France and Canada, home HD patients had a 45% lower mortality risk compared to in-center HD patients.11
It is currently unknown whether KTx provides better survival than home HD in ESRD patients. To the best of our knowledge, only two Canadian studies compared the survival of home HD patients with KTx recipients.12–14 Pauly et al12 used data from two regional programs in Canada to compare survival between patients treated with nocturnal home HD and patients who received a KTx from either a deceased or a living donor as reported in the US Renal Data System. Nocturnal home HD patients were randomly matched to patients who received either of the 2 transplant modalities in a 1:3:3 ratio; 177 nocturnal home HD patients were matched to 531 deceased and 531 living transplant recipients and followed for up to 12.4 years. In adjusted models, there was no difference in survival between nocturnal home HD patients and deceased transplant recipients (HR=0.87, 95% CI: 0.50–1.51); however, patients who received a living donor transplant had a 41% better survival compared to nocturnal home HD patients (HR=0.51, 95% CI: 0.28–0.91).12 Furthermore, in another study which compared 173 Canadian nocturnal home HD patients to 1,517 Canadian KTx recipients from the same institution, patients with a KTx had a reduced risk of treatment failure and death compared with home HD patients.14
There are several reasons why the comparative effectiveness of the two modalities may differ in the United States; to our knowledge there are no such studies comparing home HD with KTx in the United States. There is a high prevalence of low-flow systems such as NxStage in the United States, which provide lower solute clearances than conventional HD machines used in Canada. In addition, none of the platforms in the United States have been approved for nocturnal HD, and hence most home HD patients undergo short, daily dialysis. Finally, the risk of death in patients undergoing HD or with KTx is in general higher in the United States than in Canada.15,16
We examined the survival of incident patients in a large, nationally representative contemporary cohort of patients from the United States to test the hypothesis that patients undergoing home HD have a higher risk of mortality compared to those receiving a KTx.
Materials and Methods
Data Source and Cohort Definition
The study cohort comprised incident home HD patients treated by one of the largest dialysis providers in the United States, and incident KTx recipients transplanted between January 1st 2007 and December 31st 2011 in the entire US. Pertinent data for the two groups were obtained from electronic medical records from the large dialysis provider and from the United States Renal Data System, respectively. The study was approved by the Institutional Review Committees of the Los Angeles Biomedical Research Institute at Harbor-UCLA, University of California Irvine Medical Center, University of Washington and University of Tennessee Health Science Center.
Home Hemodialysis cohort
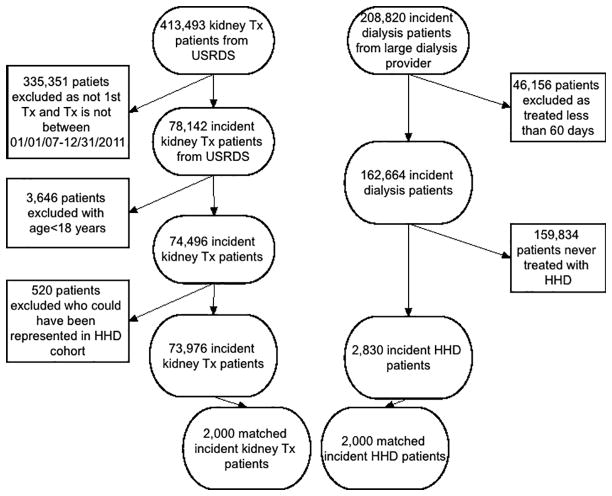
The original source population was a cohort of 208,820 incident (newly initiated) dialysis patients. Patients were included in the cohort if they were ≥ 18 years old. Patients were excluded if they did not receive dialysis treatment for at least 60 days or did not have any treatment with home HD over their duration of follow up. The detailed description of dialysis modality assignment is discussed elsewhere.17 Our final home HD cohort included 2,830 patients (Figure 1).
Figure 1.
Flow chart of patients’ selection
Kidney transplant cohort
The United States Renal Data System includes all patients who received a kidney transplant between 1963 and 2012. Patients ≥ 18 years old at time of transplant and who received their first kidney between January 1st 2007 and December 31st 2011 were included in our cohort. Patients were excluded if they were represented in the home HD cohort yielding a cohort with 73,976 KTx patients (Figure 1).
For the main analyses we created a PS (1:1) matched cohort consisting of 2,000 home HD and 2,000 KTx patients (Figure 1).
Exposure, Covariates, Outcome
Data on age, gender, race/ethnicity, ESRD etiology and vintage, access type, insurance, body mass index, serum albumin, blood hemoglobin and coexisting conditions was obtained from the two data sources and refined. The following nine coexisting conditions were considered: diabetes mellitus, hypertension, alcohol abuse, atherosclerotic heart disease, other cardiac disease (pericarditis and cardiac arrhythmia), congestive heart failure, cerebrovascular disease, chronic obstructive pulmonary disease, and malignancy. Exposure was defined as home HD versus KTx, and the outcome was all-cause mortality.
Statistical Analysis
Data were summarized using proportions, means ± SD, or median (interquartile range (IQR)) as appropriate. PS matching was used to account for baseline differences arising from dissimilarities in clinical and demographic characteristics of home HD and KTx patients. We created PS matched cohorts for all patients and in race strata (African-American and white). We additionally PS matched home HD patients to KTx recipients of specific KTx donor type [living, deceased, standard criterion donor (SCD), extended criterion donor (ECD), donor after cardiac death (DCD), non-DCD, or donor with Kidney Donor Profile Index18 (KDPI)>20 or ≤20]. STATA’s “psmatch2” command suite was used to generate the 1:1 PS-matched cohorts using nearest neighbor matching without replacement. The following variables were included in a logistic regression model to calculate the propensity scores: age, gender, race/ethnicity, primary insurance, type of vascular access at the time of transplantation/home HD, cause of ESRD, previous time on ESRD, body mass index, blood hemoglobin, serum albumin, comorbidities at baseline (diabetes, hypertension, cardiovascular disease, atherosclerotic heart disease, heart failure, cerebrovascular disease, chronic obstructive pulmonary disease, malignancy, and alcohol abuse). Home HD and KTx patients before and after matching were compared using standardized differences in all PS matched cohorts.19 Figure S1 shows the receiver operating characteristic curve of the calculated propensity score compared to actual modality in the entire cohort.
Associations between renal replacement modalities (home HD vs KTx) and all-cause mortality were assessed using the Kaplan-Meier method, and Cox proportional hazards models (for time to event analyses). Models in PS-matched cohorts were not additionally adjusted for covariates. For the main analyses, the start of the follow-up period was the start date of home HD modality or the date of kidney transplantation. Patients were followed until date of death, date of censoring [transfer to a different dialysis modality, kidney transplantation, transfer to a different facility or other reason for home HD patients, or date of allograft loss (re-transplantation, first date of dialysis) for KTx patients], or the end of the follow-up period (December 31st, 2011) (Table S17). In sensitivity analyses, we used an alternative censoring method where home HD patients were not censored at time of transfer to a different dialysis modality and continued to be followed until death, end of follow-up, or for other causes of censoring. As a significant proportion of home HD patients were transplanted during the follow-up period, there is a significant degree of informative censoring due to the selective removal of a healthier group of transplant-eligible home HD patients. We performed a competing risk model analyses to take this into account. Our event of interest was all-cause mortality and the competing event was kidney transplantation in the home HD group and graft loss in the KTx group. In our study we used the Fine and Gray model,20 which extends the Cox proportional hazards model to competing-risks data by considering the subdistribution hazard.
Effect modification by race was tested for the association of treatment modality with all-cause mortality. As the first-order interaction was statistically significant, we performed stratified analysis in African American and white patients. Statistical analyses were performed using Stata MP version 13 (Stata Corporation, College Station, TX, USA) and SAS, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
Baseline characteristics of home HD and KTx patients before and after matching are shown in Table 1. Home HD patients were older, more likely to be male, diabetic, and white, had a higher prevalence of hypertension, atherosclerotic heart disease, congestive heart failure, and other cardiovascular disease and had higher serum albumin. After PS matching, all baseline variables were well balanced between home HD and KTx patients (Table 1).
Table 1.
Baseline characteristics of the unmatched and the 1:1 propensity score-matched cohort
| Unmatched | Matched | |||||
|---|---|---|---|---|---|---|
| Home HD | KTx | Std. Diff. | Home HD | KTx | Std. Diff. | |
| (n =2,830) | (n = 73,976) | (n=2,000) | (n=2,000) | |||
| Age (years) | 53 ± 15 | 51 ± 13 | 0.129 | 54 ± 15 | 54 ± 14 | −0.015 |
| Female (%) | 34 | 39 | −0.091 | 35 | 35 | 0.016 |
| Diabetes mellitus (%) | 60 | 36 | 0.502 | 57 | 57 | 0 |
| Race/Ethnicity (%) | ||||||
| Whites | 70 | 51 | 0.125 | 70 | 72 | −0.044 |
| African-American | 21 | 26 | −0.127 | 19 | 19 | −0.007 |
| Asian | 2 | 8 | −0.263 | 3 | 2 | 0.039 |
| Hispanic | 5 | 13 | −0.313 | 6 | 5 | 0.033 |
| Other | 2 | 2 | −0.007 | 2 | 2 | 0.010 |
| Primary insurance (%) | ||||||
| Medicare | 40 | 19 | 0.614 | 38 | 40 | −0.041 |
| Medicaid | 3 | 14 | −0.282 | 4 | 4 | 0.008 |
| Other | 57 | 66 | 0.171 | 58 | 57 | 0.037 |
| Comorbid States (%) | ||||||
| Alcohol abuse | 0.2 | 0.7 | −0.070 | 0.3 | 0.3 | −0.008 |
| History of cancer | 4 | 2 | 0.126 | 4 | 4 | 0.010 |
| Hypertension | 72 | 61 | 0.238 | 74 | 74 | 0.008 |
| Cerebrovascular disease | 1 | 2 | −0.066 | 2 | 2 | −0.046 |
| Artherosclerotic Heart Disease | 26 | 6 | 0.591 | 21 | 21 | −0.001 |
| Congestive heart failure | 49 | 6 | 1.089 | 36 | 40 | −0.079 |
| Other cardiovascular disease | 22 | 5 | 0.524 | 18 | 20 | −0.046 |
| Chronic Obstructive Pulmonary Disease | 6 | 1 | 0.253 | 4 | 4 | 0.005 |
| Access Type at time of Home HD initiation/time of KTx (%) | ||||||
| AV Fistula | 57 | 11 | 1.114 | 49 | 48 | 0.010 |
| AV Graft | 8 | 2 | 0.315 | 7 | 7 | −0.006 |
| CVC Catheter | 19 | 31 | −0.267 | 24 | 23 | 0.024 |
| Other | 0 | 0.6 | −0.097 | 0.1 | 0 | 0.032 |
| Unknown | 15 | 56 | −0.947 | 20 | 22 | −0.034 |
| Cause of ESRD (%) | ||||||
| Diabetes | 35 | 31 | 0.101 | 37 | 37 | 0.002 |
| Hypertension | 22 | 20 | 0.042 | 21 | 21 | 0.002 |
| Glomerulonephritis | 18 | 19 | −0.036 | 18 | 17 | 0.018 |
| Cystic kidney disease | 6 | 9 | −0.107 | 7 | 7 | −0.006 |
| Other urologic reason | 18 | 18 | −0.006 | 18 | 18 | −0.019 |
| Unknown | 0 | 2 | −0.201 | 0 | 0 | 0 |
| Laboratory Tests at time of Home HD initiation/time of KTx | ||||||
| Serum albumin (g/dL) | 3.9 ± 0.5 | 3.5 ± 0.6 | 0.785 | 3.9 ± 0.5 | 3.9 ± 0.6 | 0.007 |
| Blood hemoglobin (g/dL) | 11.1 ± 1.3 | 10.3 ± 1.8 | 0.540 | 11.0 ±1.3 | 11.0 ± 1.6 | 0.034 |
| Other | ||||||
| Total ESRD time before modality initiation (days) | 387 ± 374 | 1012 ± 1065 | −0.782 | 409 ± 383 | 396 ± 391 | −0.035 |
| Body Mass Index (kg/m2) | 30 ± 7 | 28 ± 6 | 0.190 | 29 ± 7 | 29 ± 6 | −0.001 |
Similar differences in baseline characteristics between home HD and KTx patients were seen in African American and white cohorts before matching, but became well balanced after PS matching (Table 2).
Table 2.
Baseline characteristics of the unmatched and the 1:1 propensity score-matched African Americans and Whites
| African Americans | Whites | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unmatched | Matched | Unmatched | Matched | |||||||||
| Home HD | KTx | Std. Diff. | Home HD | KTx | Std. Diff. | Home HD | KTx | Std. Diff. | Home HD | KTx | Std. Diff. | |
| (n =585) | (n = 19,268) | (n=364) | (n=364) | (n =1,961) | (n = 46,884) | (n=1,447) | (n=1,447) | |||||
| Age (years) | 47 ± 13 | 50 ± 13 | −0.241 | 49 ± 13 | 50 ± 13 | −0.027 | 55 ± 14 | 52 ± 14 | 0.244 | 56 ± 14 | 56 ± 13 | −0.036 |
| Female (%) | 33 | 41 | −0.153 | 34 | 34 | 0.006 | 35 | 38 | −0.057 | 35 | 34 | 0.032 |
| Diabetes mellitus (%) | 59 | 36 | 0.493 | 59 | 59 | 0 | 60 | 37 | 0.471 | 56 | 56 | 0 |
| Primary insurance (%) | ||||||||||||
| Medicare | 34 | 17 | 0.614 | 31 | 31 | −0.002 | 42 | 22 | 0.558 | 39 | 41 | −0.041 |
| Medicaid | 4 | 19 | −0.258 | 7 | 7 | −0.022 | 3 | 12 | −0.330 | 3 | 3 | −0.011 |
| Other | 61 | 64 | 0.487 | 63 | 61 | 0.023 | 56 | 66 | 0.066 | 58 | 56 | 0.045 |
| Comorbid States (%) | ||||||||||||
| Alcohol abuse | 0.2 | 0.5 | −0.050 | 0.3 | 0 | 0.074 | 0.3 | 0.8 | −0.077 | 0.3 | 0.3 | 0 |
| History of cancer | 4 | 1 | 0.186 | 4 | 2 | 0.082 | 4 | 2 | 0.116 | 5 | 5 | −0.013 |
| Hypertension | 83 | 52 | 0.705 | 82 | 84 | −0.058 | 68 | 66 | 0.070 | 72 | 72 | −0.008 |
| Cerebrovascular disease | 0.2 | 2.1 | −0.185 | 0.3 | 0 | 0.074 | 2 | 2 | −0.039 | 2 | 3 | −0.023 |
| Artherosclerotic Heart Disease | 24 | 4 | 0.623 | 16 | 19 | −0.080 | 27 | 7 | 0.558 | 22 | 22 | −0.010 |
| Congestive heart failure | 50 | 7 | 1.085 | 34 | 37 | −0.063 | 49 | 7 | 1.084 | 37 | 40 | −0.053 |
| Other cardiovascular disease | 20 | 3 | 0.532 | 13 | 24 | −0.008 | 23 | 6 | 0.509 | 19 | 20 | −0.017 |
| Chronic Obstructive Pulmonary Disease | 6 | 0.7 | 0.282 | 3 | 4 | −0.015 | 6 | 1 | 0.252 | 5 | 5 | −0.003 |
| Access Type at time of Home HD initiation/time of KTx (%) | ||||||||||||
| AV Fistula | 57 | 10 | 1.168 | 46 | 46 | 0 | 56 | 12 | 1.042 | 48 | 48 | 0.007 |
| AV Graft | 13 | 2 | 0.411 | 13 | 13 | −0.016 | 7 | 1 | 0.287 | 5 | 6 | −0.018 |
| CVC Catheter | 16 | 32 | −0.365 | 22 | 22 | −0.013 | 20 | 32 | −0.270 | 24 | 24 | 0.013 |
| Other | 0 | 0.6 | −0.109 | 0 | 0 | 0 | 0.6 | 0.05 | −0.093 | 0.07 | 0 | 0.037 |
| Unknown | 13 | 55 | −1.001 | 19 | 18 | 0.028 | 16 | 54 | −0.850 | 21 | 22 | −0.013 |
| Cause of ESRD (%) | ||||||||||||
| Diabetes | 31 | 30 | 0.024 | 37 | 36 | 0.023 | 36 | 32 | 0.094 | 37 | 35 | 0.032 |
| Hypertension | 37 | 35 | 0.039 | 32 | 36 | −0.081 | 18 | 15 | 0.078 | 17 | 19 | −0.038 |
| Glomerulonephritis | 20 | 17 | 0.084 | 20 | 19 | 0.021 | 17 | 20 | −0.058 | 17 | 16 | 0.026 |
| Cystic kidney disease | 2 | 3 | −0.060 | 3 | 2 | 0.054 | 8 | 12 | −0.143 | 8 | 8 | 0.015 |
| Other urologic reason | 9 | 14 | −0.133 | 8 | 7 | 0.041 | 21 | 21 | −0.008 | 21 | 22 | −0.035 |
| Laboratory Tests at time of Home HD initiation/time of KTx | ||||||||||||
| Serum albumin (g/dL) | 4.0 ± 0.5 | 3.4 ± 0.6 | 1.082 | 3.9 ± 0.5 | 3.9 ± 0.5 | 0.016 | 3.9 ± 0.5 | 3.5 ± 0.6 | 0.659 | 3.8 ± 0.5 | 3.8± 0.6 | −0.011 |
| Blood hemoglobin (g/dL) | 10.9 ± 1.3 | 9.9 ± 1.8 | 0.670 | 10.7 ±1.4 | 10.7 ± 1.6 | −0.010 | 11.1 ± 1.3 | 10.4 ± 1.7 | 0.467 | 11.0 ±1.3 | 11.0 ± 1.7 | 0.039 |
| Other | ||||||||||||
| Total ESRD time before modality initiation (days) | 485 ± 423 | 1489 ± 1171 | −1.141 | 505 ± 449 | 509 ± 459 | −0.010 | 350 ± 342 | 829 ± 951 | −0.671 | 362 ± 357 | 379 ± 347 | −0.048 |
| Body Mass Index (kg/m2) | 30 ± 7 | 29 ± 7 | 0.131 | 29 ± 7 | 29 ± 6 | −0.034 | 30 ± 7 | 28 ± 6 | 0.205 | 29 ± 7 | 29 ± 6 | 0.009 |
Mortality in the entire PS matched cohort
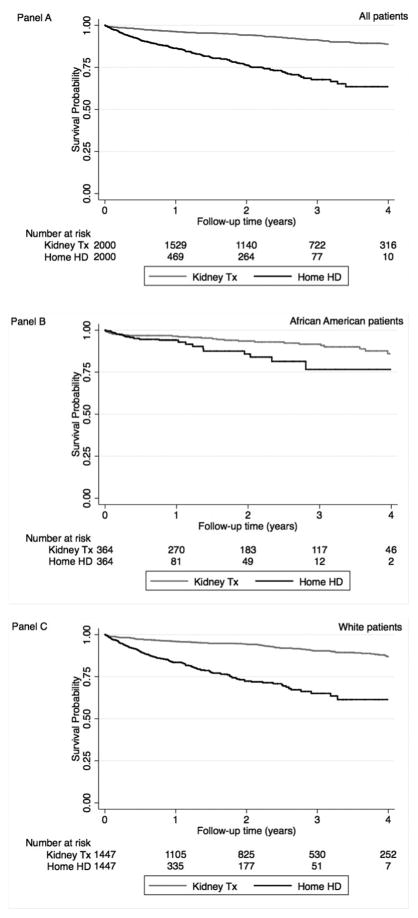
Median follow-up time was 246 days (IQR: 99–365 days) for home HD patients and 845 days (IQR: 400–1,278 days) for KTx recipients. There were 261 deaths (13%, mortality rate 145; 95%CI: 128–164/1000 patient-years) in the home HD group, and 150 deaths (7.5%, 32; 95%CI: 27–38/1000 patient-years) in the KTx group. Figure 2 Panel A shows the probability of 5-year survival of home HD and KTx patients in the PS matched group. Home HD patients had a higher mortality risk compared to KTx patients (hazard ratio (HR): 4.06, 95% confidence interval (CI): 3.27–5.04) over the entire 5-year follow-up period (Table 3).
Figure 2.
Association between renal replacement type (home hemodialysis (Home HD) versus kidney transplantation (Kidney Tx)) and mortality using Kaplan-Meier curves in propensity score matched cohorts in all patients (Panel A), African Americans (Panel B) and Whites (Panel C)
Table 3.
Mortality risk of home hemodialysis patients compared to kidney transplant recipients in group of patients with different donor characteristics using propensity score matched cohorts in the first year and thereafter
| Entire follow-up period | 0–365 days | >365 days | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Patients/Events/% of Events | HR | 95% CI of HR | Number of Events/% of Events | HR | 95% CI of HR | Number of Events/% of Events | HR | 95% CI of HR | ||
| All donors | All patients | 4,000/411/10.3% | 4.06 | 3.27–5.04 | 264/6.6% | 3.77 | 2.86–4.97 | 147/3.7% | 4.53 | 3.23–6.34 |
| African American | 728/55/7.6% | 2.06 | 1.16–3.67 | 29/4.0% | 1.62 | 0.77–3.39 | 26/3.6% | 2.84 | 1.23–6.55 | |
| White | 2,894/332/11.5% | 4.38 | 3.43–5.59 | 224/7.7% | 4.21 | 3.10–5.73 | 108/3.8% | 4.66 | 3.14–6.92 | |
| Living donors - All patients | 3,262/308/9.4% | 6.45 | 4.93–8.43 | 202/6.2% | 7.56 | 5.15–11.09 | 106/3.2% | 5.42 | 3.66–8.01 | |
| Deceased donors - All patients | 3,354/375/11.2% | 3.45 | 2.75–4.31 | 261/7.8% | 2.85 | 2.20–3.71 | 114/3.4% | 5.16 | 3.50–7.60 | |
| ECD donors - All patients | 2,212/287/13.0% | 3.90 | 3.00–5.07 | 202/9.1% | 3.38 | 2.48–4.60 | 85/3.9% | 5.25 | 3.36–8.22 | |
| SCD donors - All patients | 3,810/378/9.9% | 4.64 | 3.69–5.84 | 249/6.5% | 4.25 | 3.17–5.69 | 129/3.4% | 5.30 | 3.69–7.60 | |
| DCD donors - All patients | 1,702/198/11.6% | 3.70 | 2.75–5.21 | 152/8.9% | 3.05 | 2.15–4.32 | 46/2.7% | 7.67 | 4.06–14.51 | |
| Non DCD donors - All patients | 3,240/357/11.0% | 3.40 | 2.70–4.27 | 248/7.7% | 3.00 | 2.28–3.93 | 109/3.3% | 4.42 | 2.98–6.56 | |
| KDPI>20 - All patients | 3,176/344/10.8% | 4.30 | 3.38–5.47 | 241/7.6% | 3.80 | 2.85–5.07 | 103/3.2% | 5.46 | 3.64–8.19 | |
| KDPI≤20 - All patients | 1,940/205/10.6% | 5.40 | 3.89–7.49 | 144/7.4% | 5.04 | 3.38–7.51 | 61/3.2% | 6.14 | 3.55–10.61 | |
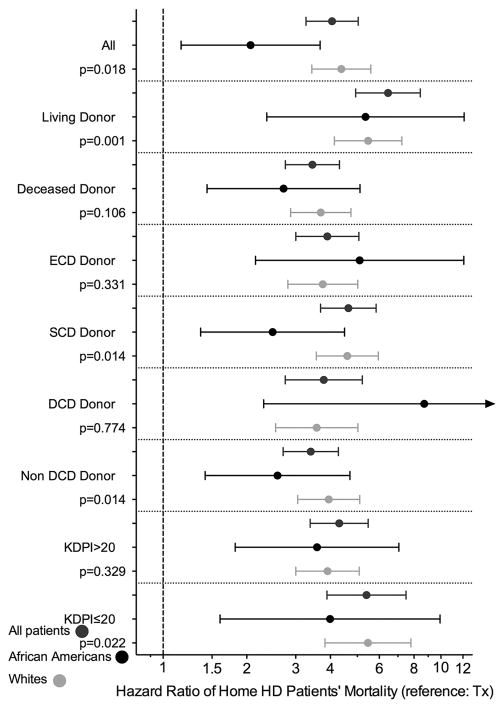
Associations of home HD vs. KTx with higher risk of mortality were found in both African Americans (Figure 2 Panel B) and whites (Figure 2 Panel C). There was a significant interaction with race for the association of treatment modality with all-cause mortality (p=0.018): mortality risk was over 4-fold higher (HR: 4.38, 95%CI: 3.43–5.59) in whites but 2-fold higher (HR: 2.06, 95%CI: 1.16–3.67) in African Americans (Figure 3). In African Americans, mortality risk increased after the first year as the survival lines were separated only after this timepoint (p<0.001 for interaction with time), while in whites the survival lines were separated from the beginning of the follow-up. In addition, there was no difference between home HD and KTx for first-year mortality risk in African American patients (HR: 1.62, 95%CI: 0.77–3.39), while white home HD patients had higher mortality risk (HR: 4.21, 95%CI: 3.10–5.73) even during the first year (Table 3 and Table S19).
Figure 3.
Mortality risk of home hemodialysis patients compared to kidney transplant recipients in group of patients with different donor characteristics using propensity score matched cohorts
Similar results were found when we used alternative censoring in our sensitivity analyses (Figures S2–S3) or competing risk regression analyses (Table S18).
Mortality in the PS matched subcohorts matching by different type of KTx donors
Baseline characteristics of subcohorts comparing home HD patients to KTx patients by different type of KTx donors, before and after matching are shown in Tables S1–S16. Significant differences in baseline characteristics of home HD and KTx patients were detected before matching, however, after PS matching all baseline variables became well balanced. Home HD patients had higher mortality risk compared to KTx patients in all PS matched subcohorts Figures S4–S11. Similar associations were found among African Americans and whites in all subcohorts, and there was effect modification by race, with a higher mortality risk observed in whites. Additionally, in most of the subcohorts (deceased-, SCD-, non-DCD-, KDPI>20 or KDPI≤20 donors) there was no difference between African American home HD and KTx patients in first-year mortality risk, while white home HD patients had higher mortality risk than their KTx counterparts even during the first year.
Discussion
In this contemporary cohort of incident home HD patients and KTx recipients in the United States, we examined the association between type of renal replacement modality with all-cause mortality. Patients who received KTx had significantly better survival regardless of the type of kidney donor type. In addition, we also detected an interaction between race and renal replacement type for mortality outcomes, in that KTx had a stronger protective effect on survival in white recipients. In contrast, home HD was associated with similar first year survival as KTx in African Americans.
Our results confirm that KTx is the treatment of choice for end stage renal disease, even when compared to home HD. Home HD patients had 4-times higher risk of mortality compared to their KTx counterparts. These findings are similar to those observed in studies examining nocturnal home HD from Ontario, Canada.14 Conversely, results from previous studies have shown benefits of home HD and may have led to the hypothesis that home HD patients had similar survival to KTx patients. Home HD may provide certain advantages in ESRD patients, including improved blood pressure control21–24 despite increased extracellular fluid volume.21,25 Nocturnal home HD patients have shown reductions in total peripheral resistance and plasma norepinephrine levels.26 In addition, randomized trials and meta-analyses suggest that home HD is associated with reduction in left ventricular mass compared to conventioal in-center HD.21,24,27,28 Home HD treatment improves anemia without altering erythropoietin requirements or iron status.29 Additonally, home HD provides better phosphorus control than in-center HD.24,30–32 Sleep disorders such as sleep apnea and restless legs syndrome, which are predictors of mortality in ESRD patients,33,34 improve in home HD patients,35 but are still very prevalent in KTx population.36,37 Conversion from in-center to home HD reduces the number of apnea or hypopnea events in patients with obstructive sleep apnea.35 However, despite these previous findings, our data confirm that regardless of the type of kidney donor, KTx provides better survival than home HD for patients with ESRD.
Interestingly, clinically and statistically significant interactions were found between race (African American and white patients) and renal replacement therapy type for mortality outcomes. Among whites, home HD patients had significantly higher mortality risk in the first year and thereafter. However, the first-year survival was similar with home HD and KTx in African Americans except when the kidney was donated from a living donor, and the mortality risk associated with home HD increased only after the first year. This finding should be interpreted with caution. The subgroup of African Americans in our study was relatively small (n=728), consequently the observed event number was also small (n=55), so this subgroup analysis may be underpowered. Further studies are needed to confirm or reject our results. These results may suggest that while waiting for a transplant all patients requiring renal replacement therapy could benefit from home HD. Furthermore, it is possible that home HD is the best dialysis option for African American patients, although our study didn't compare it directly with conventional in-centre hemodialysis or with peritoneal dialysis, and hence this may need further examination in future studies. There are several potential explanations for the lack of first-year survival benefit in KTx living donor versus home HD in African American patients. First, African Americans compared to whites have a higher probability of developing ESRD, secondary to genetic predisposition even in strata of patients with a history of diabetes and hypertension.38 In addition, the patient and renal allograft survival is significantly lower in African Americans than in whites,15,39,40 which might be due to higher immunologic risk, lower medication adherence, or decreased access to pre- and posttransplantation care.41–43 African American patients on maintenance dialysis also have better survival rates than whites44 secondary to several factors such as higher muscle mass,45 and better nutritional and inflammatory status.46 A similar survival advantage can be detected among home HD patients. In a recent study, we examined patients who initiated maintenance dialysis between 2007 and 2011 in any of 2217 dialysis facilities operated by a single large dialysis organization, with follow-up through Dec. 31, 2011. Compared to whites, African Americans undergoing home HD had a lower risk for death even after adjustment for important confounders.47 Such discrepant associations of African American race with survival in in home HD vs. in KTx could explain the effect modificaiton by race described in our study. Higher survival chance in home HD and lower survival in KTx and the economic and social inequality between whites and African Americans can also explain the result of this study.
Our study has number of strengths. It is the first comparison of mortality for home HD patients and KTx recipients from the United States. This is the first study using a PS matched approach to balance measured confounders. Moreover, we were able to compare the mortality risk of home HD with KTx from different types of kidney donors. In addition, we performed sensitivity analyses with an alternate censoring method by continuing to follow patients after home HD therapy ended, which confirmed our results. Furthermore, we also performed sensitivity analyses using competing risk regression analyses to take into account informative censoring due to the selective removal of a healthier group of transplant eligible home HD patients. The results of these analyses were qualitatively similar to our main results. Finally, we assessed the effect modification of race in the association of modality type with the mortality outcome.
The results of our study should be interpreted in light of some potential limitations. First, home HD data were derived from facilities operated by a single dialysis provider. However, this constitutes almost one-third of all patients undergoing maintenance dialysis in the US. Second, we acknowledge that our sample size and event numbers in home HD patients are small. The relatively small sample size may have a bearing on the proportion of African American patients (n=728) given small number of events (n=55), where the effect size exhibited large variability, i.e., as low as 16% but as high as 367% for higher mortality risk. Hence, our result should be qualified in this context and need to be confirmed in larger studies involving more African American patients and more events. However, to the best of our knowledge ours is still the largest cohort of home HD patients assembled to date and compares outcomes to a large cohort of KTx recipients. In addition, our result may not be applicable to populations outside the US, as the nocturnal home hemodialysis practice is significantly different in Canada or Europe. Furthermore, median follow-up time in home HD patients was relatively short. The main reason for this was that our home HD patients were transferred to other dialysis modalities after a relatively short period of time. Further studies are needed to identify the cause of this phenomenon. Third, we did not have data regarding the home HD patients’ waitlist status, consequently we were not able to perform subgroup analysis in this subcohort. Lastly, despite the fact that we were able to PS-match our cohorts for many confounding factors, there are likely remaining unmeasured or unknown confounders that could have affected the results of this study.
Conclusion
In conclusion, patients who received KTx had significantly better survival compared to home HD patients, regardless of kidney donor type. African American home HD patients had similar first year survival to African American KTx patients without a living donor. Further studies are needed to confirm and understand the reasons underlying racial differenes in mortality risk in home HD versus KTx patients.
Supplementary Material
Acknowledgments
Funding Sources
The work in this manuscript has been performed with the support of grant R21AG047306 and R01DK95668 (MZM, KKZ, CPK and RM).
Parts of this material were accepted for presentation at the American Society of Nephrology Kidnney Week on November 3–8, 2015 in San Diego, CA
List of abbreviations
- CI
confidence interval
- DCD
donor after cardiac death
- ECD
extended criterion donor
- ESRD
end stage renal disease
- HHD
home hemodialysis
- HD
hemodialysis
- HR
hazard ratios
- IQR
interquartile range
- KDPI
Kidney Donor Profile Index
- KTx
kidney transplant
- PS
propensity score
- SD
standard deviation
- SCD
standard criterion donor
- US
United States
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
Miklos Z Molnar contributed to data collection, contributed to analysis of the data, interpretation of data and writing the manuscript.
Vanessa Ravel contributed to data collection and analysis of the data.
Elani Streja contributed to data collection and analysis of the data.
Csaba P Kovesdy contributed to interpretation of data and writing the manuscript.
Rajnish Mehrotra contributed to writing the manuscript.
Kamyar Kalantar-Zadeh contributed to data collection, contributed to analysis of the data, interpretation of data and writing the manuscript.
Disclosures
CPK and KKZ are employees of the Department of Veterans affairs. Opinions expressed in this paper are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs. The results of this paper have not been published previously in whole or part.
Contributor Information
Miklos Z Molnar, Email: mzmolnar@uthsc.edu.
Vanessa Ravel, Email: vravel@uci.edu.
Elani Streja, Email: estreja@uci.edu.
Csaba P Kovesdy, Email: ckovesdy@uthsc.edu.
Rajnish Mehrotra, Email: RMehrotra@Nephrology.washington.edu.
Kamyar Kalantar-Zadeh, Email: kkz@uci.edu.
References
- 1.Schnuelle P, Lorenz D, Trede M, Van Der Woude FJ. Impact of renal cadaveric transplantation on survival in end-stage renal failure: evidence for reduced mortality risk compared with hemodialysis during long-term follow-up. J Am Soc Nephrol. 1998;9(11):2135–2141. doi: 10.1681/ASN.V9112135. [DOI] [PubMed] [Google Scholar]
- 2.Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA. 1993;270(11):1339–1343. [PubMed] [Google Scholar]
- 3.Ojo AO, Port FK, Wolfe RA, Mauger EA, Williams L, Berling DP. Comparative mortality risks of chronic dialysis and cadaveric transplantation in black end-stage renal disease patients. Am J Kidney Dis. 1994;24(1):59–64. doi: 10.1016/s0272-6386(12)80160-0. [DOI] [PubMed] [Google Scholar]
- 4.Rabbat CG, Thorpe KE, Russell JD, Churchill DN. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11(5):917–922. doi: 10.1681/ASN.V115917. [DOI] [PubMed] [Google Scholar]
- 5.Meier-Kriesche HU, Ojo AO, Port FK, Arndorfer JA, Cibrik DM, Kaplan B. Survival improvement among patients with end-stage renal disease: trends over time for transplant recipients and wait-listed patients. J Am Soc Nephrol. 2001;12(6):1293–1296. doi: 10.1681/ASN.V1261293. [DOI] [PubMed] [Google Scholar]
- 6.Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol. 2005;16(6):1859–1865. doi: 10.1681/ASN.2004121092. [DOI] [PubMed] [Google Scholar]
- 7.Gill JS, Tonelli M, Johnson N, Kiberd B, Landsberg D, Pereira BJ. The impact of waiting time and comorbid conditions on the survival benefit of kidney transplantation. Kidney Int. 2005;68(5):2345–2351. doi: 10.1111/j.1523-1755.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 9.Marshall MR, Hawley CM, Kerr PG, et al. Home hemodialysis and mortality risk in Australian and New Zealand populations. Am J Kidney Dis. 2011;58(5):782–793. doi: 10.1053/j.ajkd.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Weinhandl ED, Liu J, Gilbertson DT, Arneson TJ, Collins AJ. Survival in daily home hemodialysis and matched thrice-weekly in-center hemodialysis patients. J Am Soc Nephrol. 2012;23(5):895–904. doi: 10.1681/ASN.2011080761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nesrallah GE, Lindsay RM, Cuerden MS, et al. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. J Am Soc Nephrol. 2012;23(4):696–705. doi: 10.1681/ASN.2011070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pauly RP, Gill JS, Rose CL, et al. Survival among nocturnal home haemodialysis patients compared to kidney transplant recipients. Nephrol Dial Transplant. 2009;24(9):2915–2919. doi: 10.1093/ndt/gfp295. [DOI] [PubMed] [Google Scholar]
- 13.Pauly RP. Nocturnal home hemodialysis and short daily hemodialysis compared with kidney transplantation: emerging data in a new era. Adv Chronic Kidney Dis. 2009;16(3):169–172. doi: 10.1053/j.ackd.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Tennankore KK, Kim SJ, Baer HJ, Chan CT. Survival and hospitalization for intensive home hemodialysis compared with kidney transplantation. J Am Soc Nephrol. 2014;25(9):2113–2120. doi: 10.1681/ASN.2013111180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SJ, Schaubel DE, Fenton SS, Leichtman AB, Port FK. Mortality after kidney transplantation: a comparison between the United States and Canada. Am J Transplant. 2006;6(1):109–114. doi: 10.1111/j.1600-6143.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 16.Robinson BM, Zhang J, Morgenstern H, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85(1):158–165. doi: 10.1038/ki.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, et al. Predictors of Treatment with Dialysis Modalities in Observational Studies for Comparative Effectiveness Research. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv097. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88(2):231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine J, Gray R. A proportional hazards model for subdistribution of a competing risk. Journal of American Statistical Association. 1999;94:496–509. [Google Scholar]
- 21.Chan CT, Floras JS, Miller JA, Richardson RM, Pierratos A. Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int. 2002;61(6):2235–2239. doi: 10.1046/j.1523-1755.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- 22.Lockridge RS, Jr, Spencer M, Craft V, et al. Nightly home hemodialysis: five and one-half years of experience in Lynchburg, Virginia. Hemodial Int. 2004;8(1):61–69. doi: 10.1111/j.1492-7535.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 23.McGregor DO, Buttimore AL, Lynn KL, Nicholls MG, Jardine DL. A Comparative Study of Blood Pressure Control with Short In-Center versus Long Home Hemodialysis. Blood Purif. 2001;19(3):293–300. doi: 10.1159/000046957. [DOI] [PubMed] [Google Scholar]
- 24.Rocco MV, Lockridge RS, Jr, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80(10):1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nesrallah G, Suri R, Moist L, Kortas C, Lindsay RM. Volume control and blood pressure management in patients undergoing quotidian hemodialysis. Am J Kidney Dis. 2003;42(1 Suppl):13–17. doi: 10.1016/s0272-6386(03)00532-8. [DOI] [PubMed] [Google Scholar]
- 26.Chan CT, Harvey PJ, Picton P, Pierratos A, Miller JA, Floras JS. Short-term blood pressure, noradrenergic, and vascular effects of nocturnal home hemodialysis. Hypertension. 2003;42(5):925–931. doi: 10.1161/01.HYP.0000097605.35343.64. [DOI] [PubMed] [Google Scholar]
- 27.Susantitaphong P, Koulouridis I, Balk EM, Madias NE, Jaber BL. Effect of frequent or extended hemodialysis on cardiovascular parameters: a meta-analysis. Am J Kidney Dis. 2012;59(5):689–699. doi: 10.1053/j.ajkd.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Culleton BF, Walsh M, Klarenbach SW, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298(11):1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 29.Chan CT, Liu PP, Arab S, Jamal N, Messner HA. Nocturnal hemodialysis improves erythropoietin responsiveness and growth of hematopoietic stem cells. J Am Soc Nephrol. 2009;20(3):665–671. doi: 10.1681/ASN.2008050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaber BL, Lee Y, Collins AJ, et al. Effect of daily hemodialysis on depressive symptoms and postdialysis recovery time: interim report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. Am J Kidney Dis. 2010;56(3):531–539. doi: 10.1053/j.ajkd.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Walsh M, Manns BJ, Klarenbach S, Tonelli M, Hemmelgarn B, Culleton B. The effects of nocturnal compared with conventional hemodialysis on mineral metabolism: A randomized-controlled trial. Hemodial Int. 2010;14(2):174–181. doi: 10.1111/j.1542-4758.2009.00418.x. [DOI] [PubMed] [Google Scholar]
- 32.Pellicano R, Strauss BJ, Polkinghorne KR, Kerr PG. Body composition in home haemodialysis versus conventional haemodialysis: a cross-sectional, matched, comparative study. Nephrol Dial Transplant. 2010;25(2):568–573. doi: 10.1093/ndt/gfp490. [DOI] [PubMed] [Google Scholar]
- 33.Unruh ML, Sanders MH, Redline S, et al. Subjective and objective sleep quality in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the sleep heart health study. Am J Kidney Dis. 2008;52(2):305–313. doi: 10.1053/j.ajkd.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unruh ML, Levey AS, D'Ambrosio C, Fink NE, Powe NR, Meyer KB. Restless legs symptoms among incident dialysis patients: association with lower quality of life and shorter survival. Am J Kidney Dis. 2004;43(5):900–909. doi: 10.1053/j.ajkd.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102–107. doi: 10.1056/NEJM200101113440204. [DOI] [PubMed] [Google Scholar]
- 36.Szentkiralyi A, Czira ME, Molnar MZ, et al. High risk of obstructive sleep apnea is a risk factor of death censored graft loss in kidney transplant recipients: an observational cohort study. Sleep Med. 2011;12(3):267–273. doi: 10.1016/j.sleep.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Molnar MZ, Lazar AS, Lindner A, et al. Sleep apnea is associated with cardiovascular risk factors among kidney transplant patients. Clin J Am Soc Nephrol. 2010;5(1):125–132. doi: 10.2215/CJN.04030609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ. Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged medicare beneficiaries. J Am Soc Nephrol. 2007;18(4):1299–1306. doi: 10.1681/ASN.2006050524. [DOI] [PubMed] [Google Scholar]
- 39.Press R, Carrasquillo O, Nickolas T, Radhakrishnan J, Shea S, Barr RG. Race/ethnicity, poverty status, and renal transplant outcomes. Transplantation. 2005;80(7):917–924. doi: 10.1097/01.tp.0000173379.53347.31. [DOI] [PubMed] [Google Scholar]
- 40.Kasiske BL, Neylan JF, 3rd, Riggio RR, et al. The effect of race on access and outcome in transplantation. N Engl J Med. 1991;324(5):302–307. doi: 10.1056/NEJM199101313240505. [DOI] [PubMed] [Google Scholar]
- 41.Hardinger KL, Stratta RJ, Egidi MF, et al. Renal allograft outcomes in African American versus Caucasian transplant recipients in the tacrolimus era. Surgery. 2001;130(4):738–745. doi: 10.1067/msy.2001.116922. discussion 745-737. [DOI] [PubMed] [Google Scholar]
- 42.Butkus DE, Meydrech EF, Raju SS. Racial differences in the survival of cadaveric renal allografts. Overriding effects of HLA matching and socioeconomic factors. N Engl J Med. 1992;327(12):840–845. doi: 10.1056/NEJM199209173271203. [DOI] [PubMed] [Google Scholar]
- 43.Weng FL, Israni AK, Joffe MM, et al. Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. J Am Soc Nephrol. 2005;16(6):1839–1848. doi: 10.1681/ASN.2004121059. [DOI] [PubMed] [Google Scholar]
- 44.Kucirka LM, Grams ME, Lessler J, et al. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306(6):620–626. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noori N, Kovesdy CP, Dukkipati R, et al. Racial and ethnic differences in mortality of hemodialysis patients: role of dietary and nutritional status and inflammation. Am J Nephrol. 2011;33(2):157–167. doi: 10.1159/000323972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Streja E, Kovesdy CP, Molnar MZ, et al. Role of nutritional status and inflammation in higher survival of African American and Hispanic hemodialysis patients. Am J Kidney Dis. 2011;57(6):883–893. doi: 10.1053/j.ajkd.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehrotra R, Soohoo M, Rivara M, et al. Racial and Ethnic Disparities in Utilization of and Outcomes with Home Dialysis in the United States. 2015 under review. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.