Abstract
Background
Natural killer (NK) cells are potent cytotoxic lymphocytes that play a critical role in tumor immunosurveillance and control. Cancer stem cells (CSC) initiate and sustain tumor cell growth, mediate drug refractory cancer relapse and express the well-known surface marker CD133.
Methods
DNA fragments from two fully humanized single chain fragment variable (scFv) antibody recognizing CD16 on NK-cells and CD133 on CSC were genetically spliced forming a novel drug, 16 × 133 BiKE that simultaneously recognizes these antigen to facilitate an immunologic synapse. The anti-CD133 was created using a fusion protein prepared by fusing DNA fragments encoding the two extracellular domains of CD133. Immunization of mice with the resulting fusion protein generated an unique antibody that recognized the molecular framework and was species cross-reactive.
Results
In vitro 51chromium release cytotoxicity assays at both high and low effector:target ratios demonstrated the ability of the heterodimeric biological drug to greatly enhance NK-cell killing of human Caco-2 colorectal carcinoma cells known to overexpress CD133. The tumor associated antigen specificity of the drug for CD133 even enhanced NK-cell cytotoxicity against the NK-resistant human Burkitt's lymphoma Daudi cell line, which has less than 5% CD133 surface expression. Flow cytometry analysis revealed increases in NK-cell degranulation and Interferon-γ production upon co-culture with Caco-2 targets in the presence of the drug.
Conclusion
These studies demonstrate that the innate immune system can be effectively recruited to kill CSC using bispecific antibodies targeting CD133, and that this anti-CD133 scFv may be useful in this bispecific platform or, perhaps, in the design of more complex trispecific molecules for carcinoma therapy.
1 INTRODUCTION
CD133 has been identified as a marker of cancer stem cells (CSC) in different tumors [1-3] including colorectal cancer [4]. CD133 is a 5-transmembrane glycoprotein that localizes to membrane protrusions and shares 60% homology at the amino acid level between human and mouse [5, 6]. It is known to be expressed on normal stem cells [7-9], but may be expressed in reduced copy number compared to CSC [10]. CD133 epitopes bound by commercial anti-CD133 antibodies are not lost, but rather masked or shielded through changes in the structure of CD133, likely through differential glycosylation [11]. We developed an anti-CD133 single chain fragment variable (scFv) reagent that circumvented the problems of differential glycosylation in targeting CD133 [12].
Natural killer (NK) cells are large granular lymphocytes that serve as potent effectors of the innate immune system [reviewed in [13]]. The ability of NK-cells to recognize and kill targets is regulated by a sophisticated repertoire of inhibitory and activating cell surface receptors. NK-cell cytotoxicity can occur by natural cytotoxicity, mediated via the engagement of the natural cytotoxicity receptors, or by antibodies that trigger antibody dependent cell-mediated cytotoxicity (ADCC) through CD16, the potent activating Fcγ receptor that is highly expressed by the CD56dim subpopulation of NK-cells.
Studies have shown the ability of scFv reagents to effectively harness the therapeutic potential of NK-cell-mediated tumor cytotoxicity through the simultaneous engagement of the potent NK-cell activating receptor CD16 and tumor associated antigen (TAA) of interest [14, 15]. Thus, we developed a novel bispecific killer cell engager (BiKE) capable of targeting CSCs by splicing a gene encoding a human anti-CD16 scFv to a scFv recognizing human CD133. Anti-CD16 was derived from a human phage display library [16]. The purpose of generating the 16 × 133 BiKE was to facilitate the immunological synapse between effector and target cells to enhance NK-mediated killing of CSCs.
In this paper, we show that 16 × 133 BiKE specifically binds and activates resting NK-cells inducing degranulation and interferon-γ (IFN-γ)production against CD133+ human colorectal cancer cells. In effect, we show that anti-CSC scFv can be genetically modified to render them capable of ADCC, engaging and enhancing the ability of the innate immune system to kill cancer cells. Thus, these new bispecific agents could possibly be used to selectively engage the innate immune system as new alternative therapeutic modalities for treating various carcinomas including colon, breast, lung, prostate, ovarian, and others.
2 Methods
2.1 Construction of 16 × 133 BiKE
Synthesis and assembly of hybrid genes encoding the single chain bispecific scFv 16 × 133 BiKE was accomplished using DNA shuffling and DNA ligation techniques. The construct is illustrated in Figure 1A. The fully assembled gene (from 5’ end to 3’ end) consisted of an NcoI restriction site, an ATG initiation codon, the VH and VL regions of anti-human CD16 (NM3E2) derived from a phage display library produced by McCall et al. [16], a 20 amino acid segment of human muscle aldolase (PSGQAGAAASESLFVSNHAY) (HMA), the VH and VL regions of CD133, and finally, a SalI restriction site. The resultant 1526bp NcoI/SalI fragment gene was spliced into the pET21d expression vector under control of an isopropyl-β-D-thiogalactopyranoside (IPTG) inducible T7 promoter (Figure 1A). DNA sequencing analysis (Biomedical Genomics Center, University of Minnesota) was used to verify that the gene was correct in sequence and had been cloned in frame. Genes for monospecific anti-CD16 scFv and anti-CD133 were created in the same manner.
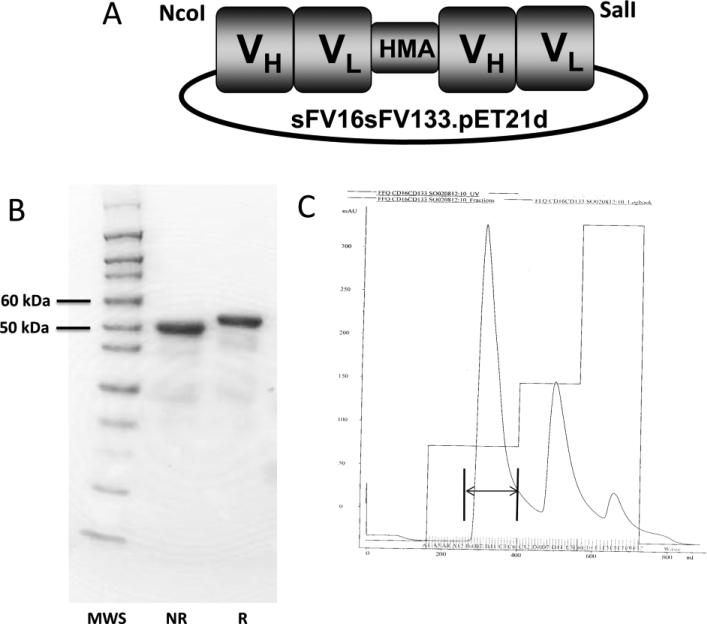
Figure 1.
(A) Construction of bispecific hybrid protein 16 × 133 bispecific NK-cell engager (BiKE). From left to right, the plasmid contains VH and VL regions of anti-CD16 spliced to VH and VL regions of anti-CD133 by the linker, human muscle aldolase (HMA), to form 16 × 133 bispecific engager. (B) The protein is about 95% pure and is shown by SDS-PAGE and Coomasie Blue staining (MWS=molecular weight standard; NR=non-reduced; R= reduced). (C) Size exclusion data from the fast flow sepharose procedure (arrow marks appropriate drug size).
2.2 Inclusion Body Isolation
For bacterial protein expression, plasmids were transformed into Escherichia coli strain BL21(DE3) (Novagen, Madison WI). Following overnight culture, bacteria were grown in 800 ml Luria broth containing 50 mg/ml carbenicillin. Gene expression was induced when culture media reached an OD600 of 0.65 with the addition of isopropyl-β-D-thiogalactopyranoside (FischerBiotech, Fair Lawn, NJ). Two hours after induction, bacteria were harvested (from 4 liters cultured media we isolated 25g bacterial pellet) and then homogenized in a buffer solution (50 mM tris, 50 mM NaCl, and 5 mM EDTA pH 8.0). Following sonication and centrifugation, the pellets were extracted with 0.3% sodium deoxycholate, 5% Triton X-100, 10% glycerin, 50 mmol/L Tris, 50 mmol/L NaCl, 5 mmol/L EDTA (pH 8.0) and washed (final pellet weight was 2.4g).
2.3 Refolding and Purification
For refolding proteins from inclusion bodies (IB), IB were dissolved at 20:1 (mg wet weight/mL) in solubilization buffer (7 M Guanidine Hydrochloride, 50 mM tris, 50 mM NaCl, 5 mM EDTA and 50mM DTT, pH 8.0). Following a 1-hour incubation at 37°C, pellets were removed by centrifugation. The supernatant was diluted 20-fold with refolding buffer and incubated at 4°C for 2 days. Refolding buffer consisted of 50 mM Tris-HCl, 50 mM NaCl, 0.8 mM L-arginine, 20% glycerin, 5 mM EDTA and 1 mM GSSG, pH 8.0. The buffer was removed by 10-fold dialysis against 20 mM Tris-HCl, pH 9.0 in 20mM Tris-HCl, pH 9.0 over four column volumes. SDS-PAGE analysis was performed and the fusion proteins stained with Simply Blue life Stain (Invitrogen, Carlsbad, CA). The final yield was 4.27mg.
2.4 NK-cell Isolation and Purification
Peripheral blood mononuclear cells (PBMC) were isolated from adult blood (Memorial Blood Center, Minneapolis, MN) by centrifugation, using a Histopaque gradient (Sigma-Aldrich, St. Louis, MO), and NK-cells were purified by removing T-cells, B-cells, stem cells, dendritic cells, monocytes, granulocytes and erythroid cells via magnetic beads per the manufacturer's protocol (Miltenyi Biotec, Auburn, CA). Samples were obtained after informed consent and in accordance with the University of Minnesota human subjects Institutional Review Board and the Declaration of Helsinki.
2.5 Cell Lines
Caco-2 (human colorectal carcinoma cell line) was obtained from American Type Culture Collection (ATCC, Rockville, MD) and grown as a monolayer in tissue culture flasks [17, 18]. Cells were maintained in DMEM media supplemented with 20% fetal bovine serum, 2 mmol/L L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin. Cell cultures were incubated in a humidified 37°C atmosphere containing 5% CO2. When cells were 80-90% confluent, they were passaged, using trypsin-EDTA for detachment. All cell counts were conducted using a standard hemacytometer, and only cells with viability >95%, as determined by trypan blue exclusion, were used for experiments. Daudi (NK-resistant human Burkitt's cell line) was also obtained from ATCC and grown in suspension culture [19].
2.6 Flow cytometry
For NK-cell analysis, single cell suspensions were stained with antibodies: PE/Cy7-conjugated CD56 (HCD56; BioLegend, San Diego, CA), PerCP/Cy5.5-conjugated anti-human CD107a (LAMP-1) (H4A3; BioLegend), Pacific Blue-conjugated anti-human IFN-γ (4S.B3; BioLegend, San Diego, CA). Phenotypic acquisition of cells was performed on the LSRII (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (Tree Star Inc., Ashland, OR). To determine CD133 specificity of 16 × 133 BiKE, the drug was labeled with fluorescein isothiocyanate (FITC). CD133 specific binding was evaluated by staining of Caco-2 cells with and without fluorochrome-coupled CD133 or AHN-12 (a negative control monoclonal antibody binding to CD45 on human leukocytes) in order to measure staining intensity on the Acuri™ 6 (BD Biosciences, San Jose, CA).
2.7 Interferon-γ Production and CD107a Degranulation Assay
Our use of this assay has been reported [20]. Purified peripherial blood NK-cells were incubated overnight at 37 °C, 5% CO2 in basal medium (RPMI supplemented with 10% fetal calf serum and 1% penicillin/streptomycin). Cells were washed in 1X PBS, treated with 10μg/mL of 16 × 133 BiKE or anti-CD16 scFv (negative control) or anti-CD133 scFv (negative control) and incubated for 15 minutes at 37°C. Anti-human CD107a mAb was added and further incubated for 1 hour, after which target cells, BD GolgiStop (1:1500) and BD GolgiPlug (1:1000; both from BD Biosciences, San Jose, CA) were added, and cells were further incubated for 5 hours. Cells were then harvested and stained with mAb CD56 and CD3, before fixation and permeabilization. Permeabilized cells were then stained for intracellular IFN-γ using anti-human IFN-γ mAb. IFN-γ and CD107a expression was evaluated by FACS analysis. Recombinant IL-12 (Peprotech, Rocky Hill, NJ) was used at 10 ng/ml for NK-cells. IL-18 (R&D Systems, Minneapolis, MN) was used at 100 ng/ml.
2.8 Chromium-51 Release Cytotoxicity Assay
Caco-2 target cells were labeled for 1 hour with 1μCi of 51Cr per 1×105 target cells at 37°C 5% CO2. Target cells were washed to remove excess 51Cr, and 5×103 labeled target cells were added to the wells of 96-well round-bottom plates. Resting effector NK-cells treated with 16 × 133 BiKE or negative controls were added to the plates at E:T cell ratios ranging between 20:1 and 0.08:1. Effector-target cells were incubated for 4 hours in a 37°C 5% CO2 incubator. The amount of 51Cr released, which corresponds to target cell death, was measured by a gamma scintillation counter, and the percent target cell lysis was calculated as follows: [(experimental lysis - spontaneous lysis)/(maximal lysis - spontaneous lysis)] × 100. To determine maximal lysis, 51Cr-labeled target cells were treated with 3% Triton X for 4 hours.
2.9 Statistical Analyses
Data are presented as mean +/− standard deviation. Differences between two groups were analyzed by Student's t test. Analysis and presentation of data was done with Graphpad prism 5 (GraphPad Software, Inc., La Jolla, CA, USA)
3 RESULTS
3.1 16 × 133 BiKE enhances resting NK-cell cytotoxicity of Caco-2 tumor targets
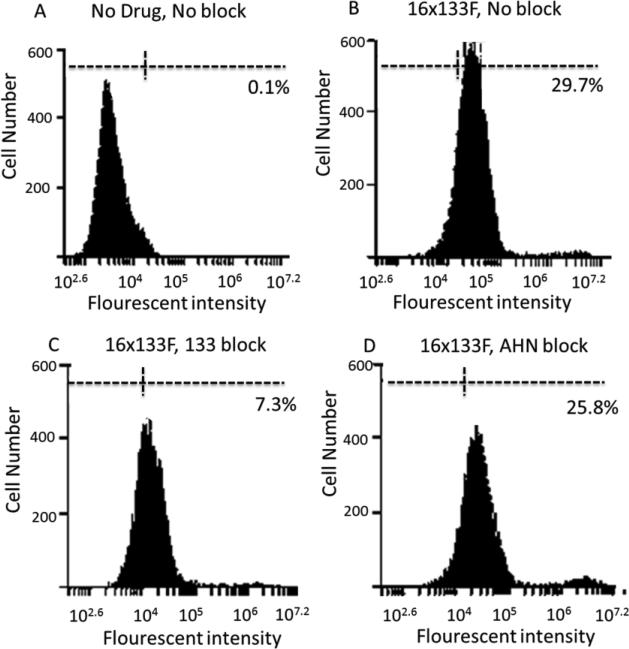
The design of the hybrid protein is shown in Figure 1A and the densitometric purity of all purified proteins was about 95% when analyzed by SDS-PAGE (Figure 1B). Also, flow cytometry studies in which 16 × 133 BiKE was directly labeled with FITC showed that the protein bound a very high percentage of the enriched NK-cell population in excess of 80% (data not shown). Additionally, we performed a flow cytometry blocking assay (Figure 2) with 16 × 133-BiKE-FITC bound Caco-2 cells, but the addition of anti-CD133 antibody prevented CD133 specific binding. Addition of control AHN12 antibody showed no ability to block.
Figure 2.
Blocking assay shows specificity of the 16 × 133 bispecific NK-cell engager (BiKE). Flow cytometry blocking assay was performed. Graph shows Caco-2 cells (human colorectal Carcinoma cell line) without staining (A), with fluorescein isothiocyanate (FITC)-labeled 16 × 133 BiKE (B), with FITC-labeled 16 × 133 BiKE, blocked with anti-CD133 antibody (C) and with FITC-labeled CD16 × CD133 BiKE blocked with negative control AHN12 antibody (D). Histograms showed positive staining with Caco-2 cells alone and with 16 × 133 BiKE- (FITC) blocked with AHN12. The anti-CD133 antibody selectively blocked 16 × 133 BiKE binding.
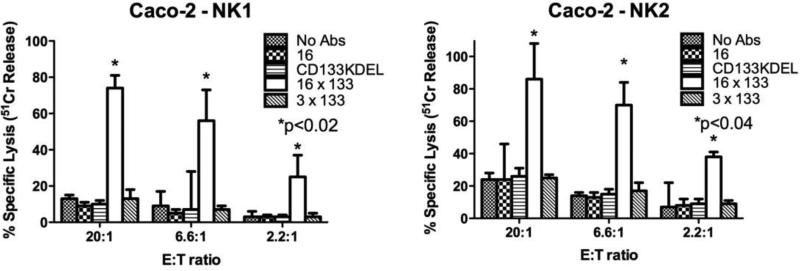
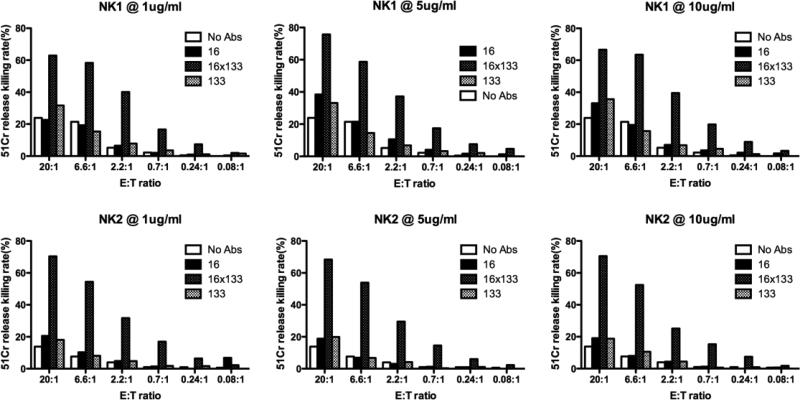
Carcinoma cell lines, including Caco-2, are known for their resistance to NK-cell mediated killing. Therefore, to determine the ability of 16 × 133 BiKE to facilitate the killing of CD133-expressing carcinoma cells, Caco-2 cells were co-cultured with resting NK-cells. Caco-2 cells tested by flow cytometry are 60-70% CD133 positive. The 51Cr release assay is typically used to measure the killing of target cells by NK-cells since the assay measures the release of 51Cr from isotope pulsed targets upon their lysis. If the 16 × 133 BiKE was able to facilitate NK killing, it would facilitate the release of 51Cr from Caco-2 cells. When Caco-2 cells were treated with 10 ug/ml of drug, killing was 77% and 83% in two different normal donors at a 20:1 E:T ratio (Figure 3). Various controls including anti-CD16 scFv alone, an anti-CD3 × anti-133 bispecific antibody and an anti-CD133 targeted toxin (CD133KDEL) did not enhance killing (CD133KDEL did not kill because of the short duration of the assay). Additionally we found out that washing out the drug during the four hour 51Cr release assay diminished drug activity (data not shown). Perhaps these in vitro data indicate that sustained drug concentration may be an important issue in vivo. In a separate experiment, different 16 × 133 BiKE concentrations and different E:T ratios were more extensively tested in two additional NK-cell donors (Figure 4). Data show that even at lower doses (1ug/ml) and at very low effector target (E:T) ratios, the drug still enhanced NK killing. For example, at an E:T ratio of 0.7:1, about 15% of the cells were still killed. Minimal activity was registered with the controls including anti-CD133 alone.
Figure 3.
Testing the activity of the 16 × 133 bispecific NK-cell engager (BiKE) in chromium release assays. Freshly isolated Natural killer (NK)-cells were added to Caco-2 cells (human colorectal Carcinoma cell line) at the indicated effector:target (E:T) ratios. The engineered reagents were added at the concentration of 10 nM. Shown are the results from two donors, NK1 and NK2. NK-cells and targets incubated with 16 × 133 BiKE showed precipitously higher killing compared to negative controls including NK-cells with no reagent (no Abs), the anti-CD16 single chain fragment variable (scFv) alone, CD133 × CD3, or CD133KDEL (a targeted toxin has the potential for killing CD133+ tumor cells) (mean specific lysis and standard deviation (SD) is shown).
Figure 4.
Testing the activity of the 16 × 133 bispecific NK-cell engager (BiKE) at various drug concentrations and effector:target (E:T) ratios. Freshly isolated Natural killer (NK)-cells were added to Caco-2 cells (human colorectal Carcinoma cell line) at different E:T ratios, 20:1, 6.6:1, 2.2:1, 0.7:1, 0.24:1, and 0.08:1. The engineered reagents were added at the concentration of 1, 5, or 10 nM. The results are shown from two donors, NK1 and NK2. NK-cells and targets incubated with 16 × 133 bispecific engagers showed higher killing compared to negative controls including NK-cells with no reagent (no Abs), CD16 alone, or CD133 alone (mean specific lysis is shown).
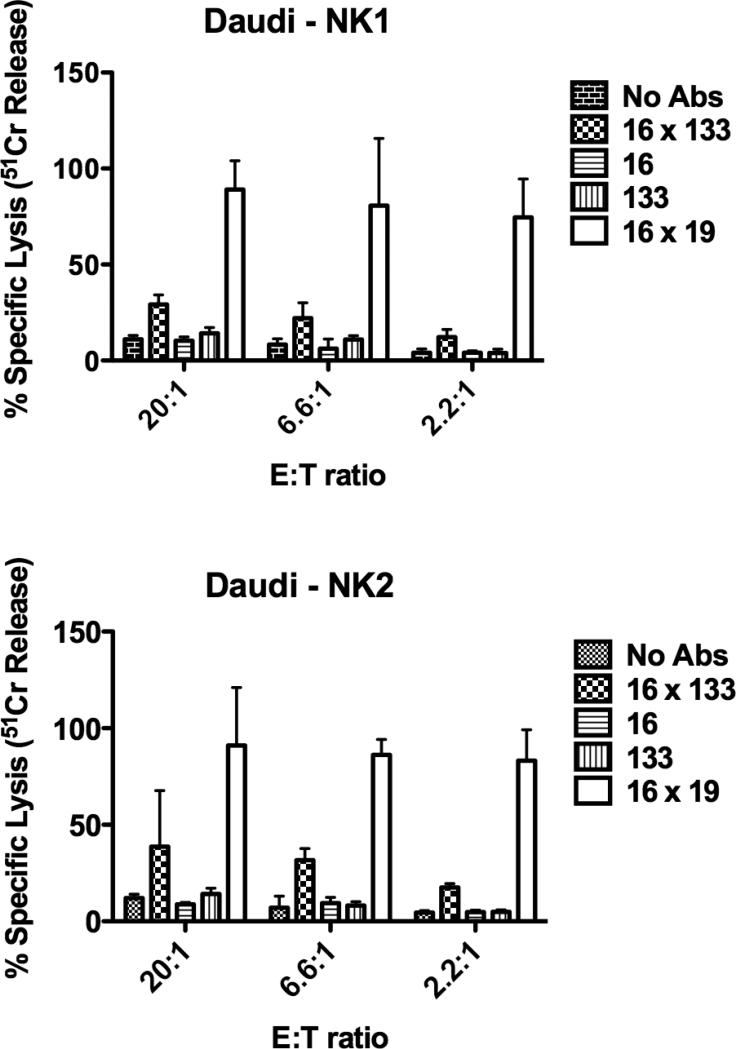
3.2 16 × 133 BiKE is selective and has an effect against Daudi B-cells
Another experiment was performed in which 16 × 133 BiKE treated resting NK-cells were tested for their ability to kill Daudi B-cell lymphoma targets. Flow cytometry analysis of Daudi, revealed only ~4-6% CD133 expression. In contrast, Caco-2 has high CD133 expression. NK-cell cytotoxicity against Daudi targets was limited in the presence of 16 × 133 BiKE. Despite this low expression, about 39% of the Daudi targets were killed at a 20:1 E:T ratio demonstrating the effectiveness with which this drug facilitates effector-target cell interactions and mediates tumor cell cytotoxicity (Figure 5). Negative controls including anti-CD16 scFv, anti-CD133 scFv and no drug did not enhance NK activity. As expected, the positive control 16 × 19 greatly enhanced NK-cell targeting of Daudi targets because CD19 is widely expressed on B-cells and has 95% expression on Daudi targets.
Figure 5.
Testing the activity of the 16 × 133 bispecific NK-cell engager (BiKE) against Daudi targets (NK-resistant human Burkitt's lymphoma cell line). Daudi expresses fewer than 5% CD133+ cells. Freshly isolated NK-cells were added to Daudi-cells at different effector:target (E:T) ratios. The engineered reagents were added at the concentration of 10 nM. The results are shown from two donors, NK1 and NK2. NK-cells and targets incubated with 16 × 133 BiKE showed higher killing compared to negative controls (mean specific lysis and SD is shown).
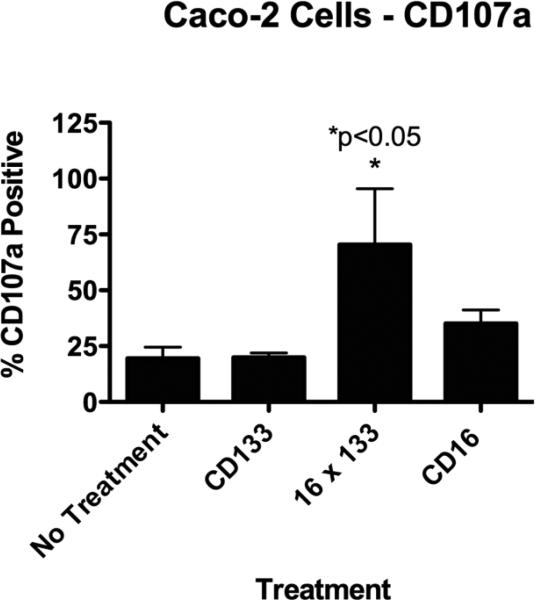
3.3 Resting NK-cell degranulation against Caco-2 targets is enhanced in the presence of 16 × 133 BiKEs
To determine if 16 × 133 BiKE induced NK-cell degranulation, resting NK-cells were co-cultured with Caco-2 targets in the presence or absence of the 16 × 133 BiKE (10 ug/ml) and CD107a expression, a marker of degranulation, was analyzed by flow cytometry. Figure 6 shows that 16 × 133 BiKE, but not anti-CD133, caused a precipitous elevation in CD107a expression. Treatment with anti-CD16 slightly elevated CD107a expression levels, which is not unexpected give that CD16 ligation alone can deliver a mild activation signal. However, 16 × 133 BiKE induced superior degranulation that combined with 51Cr release data in Figures 3 and 4, further confirming the ability of the drug to mediate ADCC. In a second experiment (not shown), we found that the activity of 16 × 133 BiKE did not diminish until the drug was diluted to 0.5 ug/ml correlating with our findings in the 51chromium release assay.
Figure 6.
Lytic Degranulation. The 16 × 133 bispecific NK-cell engager (BIKE) was evaluated for lytic degranulation using a flow cytometry assay measuring lytic degranulation and CD107a. Enriched Natural killer (NK) cells were incubated with Caco-2 targets (human colorectal carcinoma cell line). CD107a expressing cells were evaluated within the gated CD56+/CD3− NK-cell population. Cells treated with 16 × 133 BiKE showed elevated degranulation while cells treated with the negative controls did not (mean percentage of 107a+ NK-cell population with SD is shown). When the no treatment group was compared to the 16 X 133 group, significantly enhanced degranulation was shown.
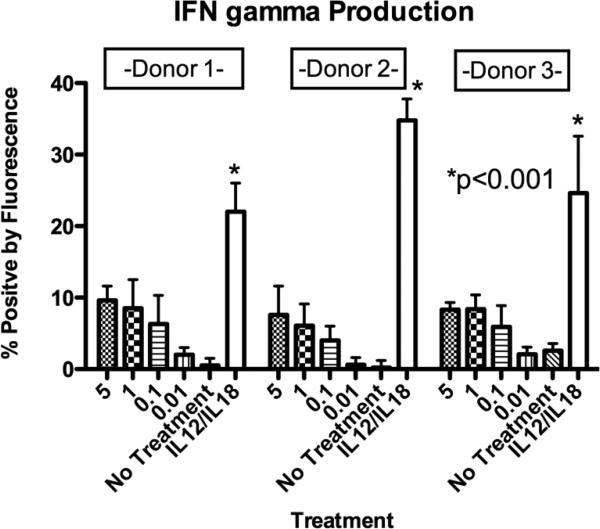
3.4 16 × 133 BiKE enhances resting NK-cell IFN-γ production against Caco-2 targets
NK-cells mediate their activity by direct cell-cell mediated killing but also by their ability to produce cytokines after target cell recognition. Therefore the ability of the 16 × 133 BiKE to induce IFN-γ production in resting NK-cells when co-cultured with Caco-2 targets was next evaluated. As expected, Interleukin (IL)-12 and IL-18 activation induced high levels of IFN-γ production. In contrast, NK-cells exposed to targets and drug (16 × 133 BiKE) had only moderate increases of IFN-γ production in a dose dependent manner compared to untreated controls, shown in Figure 7.
Figure 7.
Interferon-γ (IFN-γ) production. 16 × 133 bispecific NK-cell engager (BIKE) treated cells were evaluated for IFN-γ production using a flow cytometry assay. Enriched Natural killer (NK)-cells were incubated with Caco-2 targets (human colorectal carcinoma cell line). IFN-γ producing cells were evaluated within the gated CD56+/CD3− NK-cell population. Cells were treated with varying concentrations (μg/ml) of the 16 × 133 BiKE and showed moderate IFN-γ elevation. In contrast, cells treated with supraphysiologic levels of Interleukin (IL)-12/IL18 as a control showed high levels of IFN-γ production (mean percentage of INFγ+ NK-cell population with SD is shown). Comparisons via t-test were drawn to the 5μg group.
4 Discussion
Engaging T cells with bispecific antibodies to kill cancer cells by signaling through the CD3 complex shows promise but also NK-cells show potential in cancer defense binding them through CD16.
There are several opportunities achieving the goal of activation of immunological effector cells and at the same time targeting a cancer cell. Tetravalent diabodies as well as duovalent triplebodies show high anti-cancer reactivity [21-23]. Evidence grows revealing beneficial properties in treatment with scFv including lower immunogenicy, rapid blood clearance and improved tumor penetration compared to larger molecules with high efficacy in cancer therapy when targeting immune effectors like T-cells or NK-cells [21, 22].
Here we studied NK-cells because they contain the same toxic granzyme and perforin granules and can also be recruited to kill tumor cells but by themselves have been marginally effective against carcinomas.
By using a bispecific scFv platform that we call BiKEs, engaging CD16 on NK-cells can be effectively recruited to kill carcinomas that have shed Major Histocompatibility Complex (MHC) class I markers to avoid T-cell killing [24]. Our institution is particularly interested in enhancing NK-cell killing of carcinomas because of our experience in NK-cell adoptive transfer were hundreds of patients have been treated over the past 15 years. Although NK-cells are triggered through ligands expressed on tumors, their activity is potentially limited by lack of specificity. Our goal is to increase efficacy of NK-cell therapy by making them antigen specific.
The original contribution of this work is the engineering of a new anti-cancer molecule that selectively engages NK-cells to attack what might be one of the most important targets in carcinoma therapy, the drug resistant CSC. CD133 is a well-established marker on CSC verified by sorting CD133+ cells from various tumor types and then demonstrating that these sorted cells have enhanced tumor initiation and self-renewal capabilities. Furthermore, our group has used this same anti-CD133 scFv in the construction of targeted toxins that have impressive anti-tumor affects in treating human breast [25], head and neck [26] and ovarian cancer [27] in xenograft models. Tumor free status in these studies was achieved despite the fact that only 5% of the tumor cells express CD133. Additionally relevance of CD133 targeting was seen in a murine model were pancreatic (SW1990) hepatic (Hep3B) cancer as well as glioblastoma bearing mice were treated with anti-CD3/anti-CD133 bispecific antibody. Data showed the drug caused significantly enhanced tumor inhibition [28, 29].
Evidence shows that CSC play a key role in tumor development and progression in a variety of different cancers [30, 31]. It is now believed that CSC comprise a minority population within a tumor that is able to self-renew and produce the heterogeneous lineages of cancer cells that develops into the bulk of the tumor mass [25]. Tumorigenic CSCs have proven to be highly resistant to conventional chemotherapy, so these cells are believed to be at the root cause of tumor relapse. Thus, 16 × 133 BiKE may provide a powerful method of directing non-selective NK-cells to specifically kill CSCs associated with drug resistance in a variety of different cancers.
An important mechanism by which immune engagers function involves the facilitation of an efficient immunological synapse between the effector NK-cell and the tumor target of interest. The direct triggering through CD16 mediates an ADCC-like response. In additional, the juxtaposition of NK-cells and targets likely facilitates other activating receptors to enhance CD16 signaling such as LFA-1/ICAM interactions. A better understanding of these events at the molecular level will undoubtedly help us to better use this drug and show whether dual targeting to the NK-cell is of additional value.
Besides expression on CSC, CD133 is known to be expressed on normal stem cells [7-9]. One unique aspect of our anti-CD133 is that it crosses different species because it recognizes framework elements common to mouse and human CD133 [32]. This is attributed to the unique manner in which the monoclonal antibody was produced. CD133 consists of two extracellular domains and one intracellular domain. A fusion protein was prepared by fusing DNA fragments encoding the two extracellular domains. The resulting protein was used to immunize mice [12]. In a different study by our group, targeted toxin made with this same anti-CD133 scFv was very well tolerated in mice arguing that there is little lethal non-target toxicity [27]. In fact, safety studies of this drug in a mouse model revealed no significant CD133 related toxicity in either the nervous nor vascular systems despite the fact that anti-tumor efficacy was achieved. Furthermore, studies showed no drug-related toxicity against human pluripotent stem cells in colony assays [26]. Interestingly, despite low CD133 expression (4-6%), 39% of Daudi cells were killed. We believe this bystander effect is related to the ability of our drug to form an immunological synapse and activate NK-cells triggering the release of cytotoxic granules and inflammatory cytokines. Perhaps, loss of HLA upon cancer cell transformation causes NK-cell activation as dictated by the “missing self theory”. Together, these findings indicate that a biologically active dose of drug may be achieved in vivo prior to dose limiting toxicity.
Other factors may impact drug toxicity. Antibodies that activate lymphocytes are known to have the potential to cause adverse events due to sudden and extensive cytokine release. We focused on IFN-γ since it is prominent in cytokine toxicity. While we found that 16 × 133 BiKE mediated IFN-γ release by NK-cells in this study, we were encouraged by the low level induced. Papadakis et al. showed the ability of IL12/IL18 to markedly enhance production of INF-γ, a hallmark cytokine, in cell cultures with NK-cells [33]. In a different study, we compared CD56 enriched NK-cells stimulated with an anti-CD16 BiKE to cells stimulated with supraphysiologic levels of IL-12/18. BiKE coincubation led to IFN-γ release, but in a lower amount compared to IL-12/18 cultures [34]. Together, these data suggest that CD16 stimulation via 16 × 133 BiKE therapy will induce antigen specific IFN-γ release needed to eradicate tumor and this might be controlled with pharmacologic dosing by simple discontinuation of drug administration allowing rapid elimination due to short half-life of scFvs.
No one to date has created a bispecific antibody that recruits the innate immune response to kill CSC. In this paper we demonstrate that our 16 × 133 BiKE is a potent engager of the innate immune system capable of inducing NK-cell degranulation and IFN-γ production and mediating selective targeting of CD133+ tumor cells. Our data suggest the 16 × 133 BiKE may have therapeutic potential in a clinical NK-cell therapy program for carcinomas, as it could serve as an alternative therapy for drug resistant CSCs by its unique mechanism of action.
Key Points.
Bispecific single chain fragment against CD16 and CD133 (BiKE) was synthesized
BIKE treatment engages NK-cell activity against CD133+ cancer stem cells
Acknowledgements
We acknowledge the excellent technical assistance of Elizabeth Taras, Valerie McCuller, Bin Zhang, Deborah Todhunter, Andy Sicheneder, and Seunguk Oh. This work was supported by the Minnesota Ovarian Cancer Alliance, HERA, the Randy Shaver Foundation, Atwater cancer Drug Development fund, and die Deutsche Krebshilfe (J.U.S., 111548)
This study was funded in part by the US Public Health Service Grant R01-CA36725 awarded by the NCI and the NIAID, DHHS, the Mayo Partnership Award, the HERA Women's Cancer Foundation, Minnesota Ovarian Cancer Alliance, the Lion Fund, William Lawrence and Blanche Hughes Fund the Randy Shaver Foundation, the Atwater Cancer Drug Development Award, the Deutsche Krebshilfe (JU.S.) and a CETI translational award from the University of Minnesota Masonic Cancer Center.
Footnotes
7 Compliance with Ethical Standards
Schmohl JU, Dougherty PR, Miller JS, Vallera DA declare, that there are no financial conflicts in regards to this work. Gleason MK is an employee of Sandoz Inc.
References
- 1.Harper LJ, Piper K, Common J, Fortune F, Mackenzie IC. Stem cell patterns in cell lines derived from head and neck squamous cell carcinoma. J ORAL PATHOL MED. 2007;36(10):594–603. doi: 10.1111/j.1600-0714.2007.00617.x. doi:10.1111/j.1600-0714.2007.00617.x. [DOI] [PubMed] [Google Scholar]
- 2.Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. BREAST CANCER RES. 2008;10(1):R10. doi: 10.1186/bcr1855. doi:10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Z, Qin R, Wei C, Wang M, Shi C, Tian R, et al. Pancreatic cancer cells resistant to chemoradiotherapy rich in “stem-cell-like” tumor cells. BREAST CANCER RES. 2011;56(3):741–50. doi: 10.1007/s10620-010-1340-0. doi:10.1007/s10620-010-1340-0. [DOI] [PubMed] [Google Scholar]
- 4.Fabrizi E, di Martino S, Pelacchi F, Ricci-Vitiani L. Therapeutic implications of colon cancer stem cells. World journal of gastroenterology : WORLD J GASTROENTERO. 2010;16(31):3871–7. doi: 10.3748/wjg.v16.i31.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbeil D, Roper K, Weigmann A, Huttner WB. AC133 hematopoietic stem cell antigen: human homologue of mouse kidney prominin or distinct member of a novel protein family? BLOOD. 1998;91(7):2625–6. [PubMed] [Google Scholar]
- 6.Corbeil D, Roper K, Hellwig A, Tavian M, Miraglia S, Watt SM, et al. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J BIOL CHEM. 2000;275(8):5512–20. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- 7.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. BLOOD. 1997;90(12):5002–12. [PubMed] [Google Scholar]
- 8.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. BLOOD. 2000;95(3):952–8. [PubMed] [Google Scholar]
- 9.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, et al. Direct isolation of human central nervous system stem cells. P NATL ACAD SCI USA. 2000;97(26):14720–5. doi: 10.1073/pnas.97.26.14720. doi:10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, Anderson M, et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. BRIT J CANCER. 2008;99(1):100–9. doi: 10.1038/sj.bjc.6604437. doi:10.1038/sj.bjc.6604437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavon LF, Gamarra LF, Marti LC, Amaro Junior E, Moreira-Filho CA, Camargo-Mathias MI, et al. Ultrastructural characterization of CD133+ stem cells bound to superparamagnetic nanoparticles: possible biotechnological applications. J MICROSC. 2008;231(3):374–83. doi: 10.1111/j.1365-2818.2008.02049.x. doi:10.1111/j.1365-2818.2008.02049.x. [DOI] [PubMed] [Google Scholar]
- 12.Swaminathan SK, Olin MR, Forster CL, Cruz KS, Panyam J, Ohlfest JR. Identification of a novel monoclonal antibody recognizing CD133. J IMMUNOL METHODS. 2010;361(1-2):110–5. doi: 10.1016/j.jim.2010.07.007. doi:10.1016/j.jim.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. SCIENCE. 2011;331(6013):44–9. doi: 10.1126/science.1198687. doi:10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein C, Kellner C, Kugler M, Reiff N, Mentz K, Schwenkert M, et al. Novel conjugates of single-chain Fv antibody fragments specific for stem cell antigen CD123 mediate potent death of acute myeloid leukaemia cells. BRIT J HAEMATOL. 2010;148(6):879–89. doi: 10.1111/j.1365-2141.2009.08033.x. doi:10.1111/j.1365-2141.2009.08033.x. [DOI] [PubMed] [Google Scholar]
- 15.Singer H, Kellner C, Lanig H, Aigner M, Stockmeyer B, Oduncu F, et al. Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J IMMUNOTHER. 2010;33(6):599–608. doi: 10.1097/CJI.0b013e3181dda225. doi:10.1097/CJI.0b013e3181dda225. [DOI] [PubMed] [Google Scholar]
- 16.McCall AM, Adams GP, Amoroso AR, Nielsen UB, Zhang L, Horak E, et al. Isolation and characterization of an anti-CD16 single-chain Fv fragment and construction of an anti-HER2/neu/anti-CD16 bispecific scFv that triggers CD16-dependent tumor cytolysis. MOL IMMUNOL. 1999;36(7):433–45. doi: 10.1016/s0161-5890(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 17.Fogh J, Fogh JM, Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. Journal of the National Cancer Institute. 1977;59(1):221–6. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 18.Jumarie C, Malo C. Caco-2 cells cultured in serum-free medium as a model for the study of enterocytic differentiation in vitro. J IMMUNOTHER. 1991;149(1):24–33. doi: 10.1002/jcp.1041490105. doi:10.1002/jcp.1041490105. [DOI] [PubMed] [Google Scholar]
- 19.Klein E, Klein G, Nadkarni JS, Nadkarni JJ, Wigzell H, Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. CANCER RES. 1968;28(7):1300–10. [PubMed] [Google Scholar]
- 20.Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, et al. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. MOL CANCER THER. 2012;11(12):2674–84. doi: 10.1158/1535-7163.MCT-12-0692. doi:10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portner LM, Schonberg K, Hejazi M, Brunnert D, Neumann F, Galonska L, et al. T and NK cells of B cell NHL patients exert cytotoxicity against lymphoma cells following binding of bispecific tetravalent antibody CD19 × CD3 or CD19 × CD16. CANCER IMMUNOL IMMUN. 2012;61(10):1869–75. doi: 10.1007/s00262-012-1339-9. doi:10.1007/s00262-012-1339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asano R, Nakayama M, Kawaguchi H, Kubota T, Nakanishi T, Umetsu M, et al. Construction and humanization of a functional bispecific EGFR × CD16 diabody using a refolding system. FEBS J. 2012;279(2):223–33. doi: 10.1111/j.1742-4658.2011.08417.x. doi:10.1111/j.1742-4658.2011.08417.x. [DOI] [PubMed] [Google Scholar]
- 23.Schubert I, Saul D, Nowecki S, Mackensen A, Fey GH, Oduncu FS. A dual-targeting triplebody mediates preferential redirected lysis of antigen double-positive over single-positive leukemic cells. MABS. 2014;6(1):286–96. doi: 10.4161/mabs.26768. doi:10.4161/mabs.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf E, Hofmeister R, Kufer P, Schlereth B, Baeuerle PA. BiTEs: bispecific antibody constructs with unique anti-tumor activity. DRUG DISCOV TODAY. 2005;10(18):1237–44. doi: 10.1016/S1359-6446(05)03554-3. doi:10.1016/S1359-6446(05)03554-3. [DOI] [PubMed] [Google Scholar]
- 25.Ohlfest JRZD, Panyam J, Swaminathan SK, Oh S, Waldron NN, Toma S, Vallera DA. Immunotoxin targeting CD133+ breast carcinoma cells. DRUG DELIV TRANSL RES. 2013;3:195–204. doi: 10.1007/s13346-012-0066-2. [DOI] [PubMed] [Google Scholar]
- 26.Waldron NN, Kaufman DS, Oh S, Inde Z, Hexum MK, Ohlfest JR, et al. Targeting tumor-initiating cancer cells with dCD133KDEL shows impressive tumor reductions in a xenotransplant model of human head and neck cancer. MOL CANCER THER. 2011;10(10):1829–38. doi: 10.1158/1535-7163.MCT-11-0206. doi:10.1158/1535-7163.MCT-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skubitz AP, Taras EP, Boylan KL, Waldron NN, Oh S, Panoskaltsis-Mortari A, et al. Targeting CD133 in an in vivo ovarian cancer model reduces ovarian cancer progression. GYNECOL ONCOL. 2013;130(3):579–87. doi: 10.1016/j.ygyno.2013.05.027. doi:10.1016/j.ygyno.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang J, Li C, Wang Y, Lv H, Guo Y, Dai H, et al. Cytokine-induced killer (CIK) cells bound with anti-CD3/anti-CD133 bispecific antibodies target CD133(high) cancer stem cells in vitro and in vivo. CLIN IMMUNOL. 2013;149(1):156–68. doi: 10.1016/j.clim.2013.07.006. doi:10.1016/j.clim.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Prasad S, Gaedicke S, Machein M, Mittler G, Braun F, Hettich M, et al. Effective Eradication of Glioblastoma Stem Cells by Local Application of an AC133/CD133-Specific T-cell-Engaging Antibody and CD8 T Cells. CANCER RES. 2015;75(11):2166–76. doi: 10.1158/0008-5472.CAN-14-2415. doi:10.1158/0008-5472.CAN-14-2415. [DOI] [PubMed] [Google Scholar]
- 30.Jordan CT, Guzman ML, Noble M. Cancer stem cells. NEW ENGL J MED. 2006;355(12):1253–61. doi: 10.1056/NEJMra061808. doi:10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 31.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. CANCER RES. 2006;66(19):9339–44. doi: 10.1158/0008-5472.CAN-06-3126. doi:10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 32.Swaminathan SKNL, Waldron NN, Kalscheuer S, Zellmer D, Olin MR, Ohlfest IR, Vallera DA, Panyam I. Identification and characterization of a novel scFv recognizing human and mouse CD133. DRUG DELIV TRANSL RES. 2013 doi: 10.1007/s13346-012-0099-6. in press. doi:10.1007/s13346-012-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papadakis KA, Prehn JL, Landers C, Han Q, Luo X, Cha SC, et al. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J IMMUNOL. 2004;172(11):7002–7. doi: 10.4049/jimmunol.172.11.7002. [DOI] [PubMed] [Google Scholar]
- 34.Vallera DA, Zhang B, Gleason MK, Oh S, Weiner LM, Kaufman DS, et al. Heterodimeric bispecific single-chain variable-fragment antibodies against EpCAM and CD16 induce effective antibody-dependent cellular cytotoxicity against human carcinoma cells. CANCER BIOTHER RADIO. 2013;28(4):274–82. doi: 10.1089/cbr.2012.1329. doi:10.1089/cbr.2012.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]