Abstract
Increased activity of the tryptophan-metabolizing enzyme indoleamine 2,3-dioxygenase (IDO) is associated with immunological and neurological disorders, and inhibition of its enzyme activity could be a therapeutic approach for treatment of these disorders. The aim of the present study was to establish a large animal model to study the accumulation of the potential IDO inhibitor 1-methyltryptophan (1-MT) in blood and different organs of domestic pigs (Sus scrofa domestica). Because 1-MT has not been previously evaluated in pigs, the pharmacokinetics of a single subcutaneous 1-MT application was investigated. Based on this kinetic study, a profile for repeated 1-MT applications over a period of five days was simulated and tested. The results show that a single administration of 1-MT increases its concentrations in blood, with the maximum concentration being obtained at 12 h. Repeated daily injections of 1‑MT generated increasing plasma concentrations followed by a steady-state after two days. Twelve hours after the final application, accumulation of 1-MT was observed in the brain and other organs, with a substantial variability among various tissues. The concentrations of 1-MT measured in plasma and tissues were similar to, or even higher, than those of tryptophan. Our data indicate that repeated subcutaneous injections of 1-MT provide a suitable model for accumulation of 1-MT in plasma and tissues of domestic pigs. These findings provide a basis for further research on the immunoregulatory functions of IDO in a large animal model.
Keywords: indoleamine 2, 3-dioxygenase, methyltryptophan, pharmacokinetics, pig, tryptophan
Introduction
Activation of the enzyme indoleamine 2,3-dioxygenase (IDO) is a mechanism of innate immunity and has different physiological functions with a highly cell type-specific pattern of inducibility [8]. Depletion of tryptophan (TRP) by IDO induction leads to suppression of intracellular pathogens and T-cell proliferation [28, 32]. Increased activity of IDO has been observed in a number of immunological disorders. It has been shown that IDO is highly expressed in malignant human tumors, leading to effective immune escape by inhibiting T-cell responses [33]. In patients with sepsis, increased IDO activity is associated with a higher mortality risk [12].
Inhibition of IDO may be a promising strategy to restore host immunity and enhance the antitumor potential of chemotherapy. The application of IDO-inhibiting drugs to enhance the therapeutic effects of the standard treatment for patients with malignant tumors is currently under investigation in several clinical trials [9]. One competitive IDO inhibitor, 1-methyltryptophan (1-MT), has been tested for treatment of malignant tumors [20]. 1-MT binds to the ferrous IDO-enzyme, but the additional methyl group prevents its degradation along the kynurenine pathway [2]. There are two stereoisomers, 1-methyl-D-tryptophan and 1-methyl-L-tryptophan, which exhibit different properties for IDO inhibition depending on the cell type. In studies with recombinant IDO enzyme in cell-free assay systems, the L-isomer of 1-MT (Ki = 19 µM/l) was found to be a more potent inhibitor of IDO than the D-isomer (Ki > 100 µM/l) [11]. Furthermore, recent studies indicate that 1-methyl-L-tryptophan abrogates IDO activity in dendritic cells, whereas the D-isomer is largely inactive against the IDO enzyme [21, 22].
Administration of 1-MT to mice induced rejection of a fetus, suggesting that 1-MT reverses the immunosuppression in the placenta by inhibition of IDO [28]. Furthermore, in vivo administration of 1-MT in murine tumor models leads to retardation, but not to total arrest of tumor growth [27, 33]. Studies in mice have revealed that 1-MT enhances the inhibitory effect of other enzyme inhibitors on tumor growth, assuming reactivation of the immune system by IDO inhibition [35]. In in vivo studies, 1-MT was effective in reversing the suppression of T-cell proliferation and showed therapeutic effects in murine tumor models, especially in combination with chemotherapy [11]. Our studies in mice showed that in vivo treatment with 1-MT attenuates TRP depletion, restores the antibacterial defense, and simultaneously reduces depression-like alterations in repeatedly stressed mice [17].
Because of the high biomedical relevance of IDO-associated disorders, it is important to verify the findings from mouse models in animal models that are more physiologically similar to humans. Compared with rodents, pigs (Sus scrofa domestica) have obvious advantages with respect to the relevance for human pathophysiology. It is a species that closely resembles humans in anatomy, genetics, and physiology and is increasingly used as a model for humans in biomedical research [1, 26, 30]. In a previous study, we established a pig model for IDO activation by lipopolysaccharide stimulation that led to IDO protein expression in blood and different organs, depletion of TRP, and increase of TRP metabolites in plasma [34]. The aim of the present study was to establish a model for the systemic accumulation of the IDO inhibitor 1-MT in domestic pigs as a basis for further research on the immunoregulatory function of IDO. Accumulation of 1-MT was examined in blood plasma and different organs because IDO activity is inducible in many cell types and tissues [8]. However, because 1-MT was applied for the first time in pigs in this study, its pharmacokinetics after a single subcutaneous (s.c.) injection was investigated, and based on the results, a profile for repeated 1-MT applications was simulated and verified. Thus, the concentrations of 1-MT and TRP were measured in blood plasma at different time points and in various regions of the brain and other organs after repeated administration of 1-MT.
Materials and Methods
Animals
A total of 28 male German Landrace pigs, bred and raised in the experimental pig unit of the Leibniz Institute for Farm Animal Biology, were used in two experiments. All pigs received standard processing (oral iron supplementation and castration) within the first 3 days of life. At the beginning of the experiments, the pigs were 7 weeks old and weighed between 12 and 18 kg. The pigs were fed a commercial diet and had free access to water. All procedures involving animal handling and treatment were in accordance with the German animal protection law and were approved by the relevant authorities (Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei, Mecklenburg-Vorpommern, Germany; LALLF M-V/TSD/7221.3-1.1-027/10).
Preparation of 1-MT
We purchased 1-methyl-L-tryptophan (purity 95%) from Sigma-Aldrich (Deisenhofen, Germany) and used the triglyceride Myritol®318 (MYR) from Caesar und Loretz GmbH (Hilden, Germany) as an excipient. MYR was filtered using sterile 0.2 μm polytetrafluoroethylene filters (ReZist, Whatman GmbH, Dassel, Germany) prior to its addition to the separately supplied 1-MT. Pre-weighed 1-MT was provided in injection vials, and suspensions were prepared immediately prior to administration. MYR was added to the 1-MT via injection through the septum of the vial, followed by careful mixing and withdrawal of 4.2 ml of the suspension (corresponding to 0.5 g of 1-MT and 4.0 ml of MYR). The suspension was again gently mixed immediately before s.c. application to avoid sedimentation of 1-MT.
Experimental design
1) Pharmacokinetic study following a single administration of 1-MT
Six days prior to the administration of 1-MT, four singly housed pigs were surgically fitted with an indwelling jugular vein catheter to allow repeated blood sampling. Implantation of the jugular vein catheter was performed according to the method of Rodriguez and Kunavongkrit [29] and is described in detail by Metges et al. [25]. After the surgical procedure, the animals were allowed a 5-day convalescence period that included a 2-day treatment with the antibiotics Trimethosel (Selectavet Dr. Otto Fischer GmbH, Weyarn-Holzolling, Germany) and Metapyrin (Serumwerk Bernburg, Bernburg, Germany). At 8.00 a.m. on day 6 post operation, three pigs received 1 g of 1-MT that was given in two s.c. injections of 0.5 g of 1-MT in 4.0 ml of MYR. This dose was chosen because it corresponds approximately to the daily dietary uptake of TRP by the animals [4], and because we aimed to achieve 1-MT concentrations similar to or higher than TRP to obtain an effective inhibition of IDO activity in further experiments. While the animals were in a supine position, injections were given in the popliteal fossa of two hind legs until a wheal appeared on the skin surface. One control pig received an equivalent volume of the MYR solution. The entire s.c. application procedure lasted less than 4 min. For the analysis of 1-MT and TRP, blood was withdrawn from the catheter prior to s.c. administration and after administration at intervals of 4 h until 12 h post injection and then at intervals of 6 h until 7 days post injection. For the extraction of plasma, blood samples were collected in ice-cooled tubes containing EDTA and centrifuged at 2000 × g for 10 min at 4°C. The blood plasma was stored at −80°C until analysis. The health status of the animals was continuously checked by visual inspection twice daily.
2) Pharmacokinetic study following repeated administration of 1-MT
One week prior to the administration of 1-MT, 24 animals were housed in single pens. Twelve animals received a daily administration of 1 g of 1-MT at 8.00 p.m. over a period of five days. The daily dose was partitioned into two s.c. injections of 0.5 g of 1-MT in 4.0 ml of MYR that were given in the popliteal fossa while the animals were in a supine position and were alternated between the two hind legs and forelegs. The control group received an equivalent volume of MYR solution (n = 12). Blood samples were taken by anterior vena cava puncture before every injection, and additionally twelve hours after the fourth and fifth injections. The entire blood sampling procedure lasted < 1 min. Samples were treated as described above. For collecting tissues, six 1-MT and six MYR pigs were euthanized by i.v. injection of 3 ml of T61 (embutramide, 200 mg/ml; mebezonium iodide, 50 mg/ml; tetracaine hydrochloride, 5 mg/ml; Intervet, Unterschleißheim, Germany) 12 h after the final administration, and the body weights were then measured. After euthanasia, the livers, lungs, muscles, spleens, adrenal glands, hearts, kidneys, and brains were quickly removed. The amygdala, hippocampus, hypothalamus, and prefrontal cortex were dissected from the brain [6]. Tissues were frozen in liquid nitrogen and stored at −80°C until analysis. The remaining 12 animals were used for other investigations after the treatment period (data not shown). During the experimental period, the health status of the animals was monitored by visual inspection twice daily. Furthermore, skin temperature was measured at the inguinal region with an infrared thermometer (ThermoScan IRT 4020, Braun, Kronberg, Germany) before blood sampling and s.c. injection. Feed uptake was recorded daily.
Quantification of 1-MT and TRP in plasma and tissue
The determination of TRP and 1-MT in plasma and tissues was performed using methods that were established at the University Medicine of Greifswald and utilizing an HPLC system (Series 200, PerkinElmer, Darmstadt, Germany) and an API2000 tandem mass spectrometer equipped with an electrospray ion source (AB Sciex, Darmstadt, Germany). Chromatography was performed using an Atlantis HILIC® RP 18 column (Waters GmbH, Eschborn, Germany) and gradient elution over 12 min with 0.02% formic acid (A; pH 3) and acetonitrile (B) in the following manner: After 2 min isocratic elution (40% B), the organic part was increased in one minute up to 60% and then held constant for 7 min. Thereafter the organic part was decreased in one minute down to 40% followed by isocratic elution for 2 min. Phenylalanine (Sigma-Aldrich, Deisenhofen, Germany) was used as an internal standard (IS) for quantification. Analytes and the internal standard were detected in the positive MRM mode (multiple reaction monitoring) with following mass transitions for quantification: TRP, 205.2→188.2; 1-MT, 219.2→202.2; and the IS, 171.3→125.1. Because of the unavailability of TRP-free plasma and tissue, all calibrations for TRP and 1-MT were conducted in distilled water. Calculations were performed online using the mass spectrometry software Analyst 1.4, with a linear regression model with 1/x weighting (x = concentration) and a lower limit of quantification of 2.3 µM for 1-MT and 1.2 µM for TRP. The main quality parameters of accuracy (–7.3% to 6.6% for TRP and ‑9.8% to 7.1% for 1‑MT) and precision (1.7% to 13.1% for TRP and 0.9% to 12.2% for 1‑MT) over the analytical spans (concentration range for TRP of 1.2 to 48 µM with r = 0.9986–0.9995 and concentration range for 1-MT from 2.3 to 69 µM with r = 0.9968–0.9989) met the international recommendations [5, 7]. For liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses, plasma samples were prepared by precipitation of protein with perchloric acid, and the obtained supernatants were adjusted to neutral pH (7–7.5) with ammonium hydroxide. Tissue samples were homogenized and centrifuged (Ultra-Turrax, 10 min, 40.000 × g, 4°C). The protein content of the tissue supernatants was determined [23], and the samples were analyzed as described above. Finally, 15-µl aliquots of the clear supernatants were injected into the analytical system. The ratio of 1-MT to TRP was calculated as an indicator of the accumulation of the competitive inhibitor 1-MT compared with TRP in tissues.
Statistical analyses
After single 1-MT administration, the maximum plasma concentration (Cmax) and time of the maximum concentration (Tmax) were obtained from the concentration-time curve in each animal. Calculated pharmacokinetic parameters were as follows: first-order kinetic rate constant (k), half-life (T1/2), and area under the concentration-time curve from time zero to 168 h (AUC0→t) using the linear trapezoidal rule. The data obtained with blood samples after a single administration of 1-MT were fitted by numerical simulation (Axum 6.0, MathSoft, Cambridge, MA, USA) assuming a pharmacokinetic one-compartment model using first-order kinetic rate constants with kelimination = 0.001 min-1, krelease = 0.0018 min-1, and kabsorption = 0.08 min-1. Data from the second experiment were evaluated by analysis of variance (ANOVA) using the MIXED procedure of SAS, Version 9.2 (SAS Institute Inc., Cary, NC, USA). Repeated measurements on the same animal were taken into account using the repeated statement in the MIXED procedure and a compound symmetry block diagonal structure of the residual covariance matrix. The model for plasma parameters comprised the fixed effects treatment (1-MT, MYR), time (0, 24, 48, 72, 84, 96, and 108 h), number of replicates (3), their two way interactions, and the random litter effect, which takes into account if the animals were full siblings. The model for the tissue parameters comprised the fixed effects (1-MT, MYR) and replicate numbers (3). In addition, the least square means and their standard errors were calculated and tested for each effect in the model using the Tukey-Kramer procedure for all pairwise multiple comparisons. Differences were considered significant if P ≤ 0.05.
Results
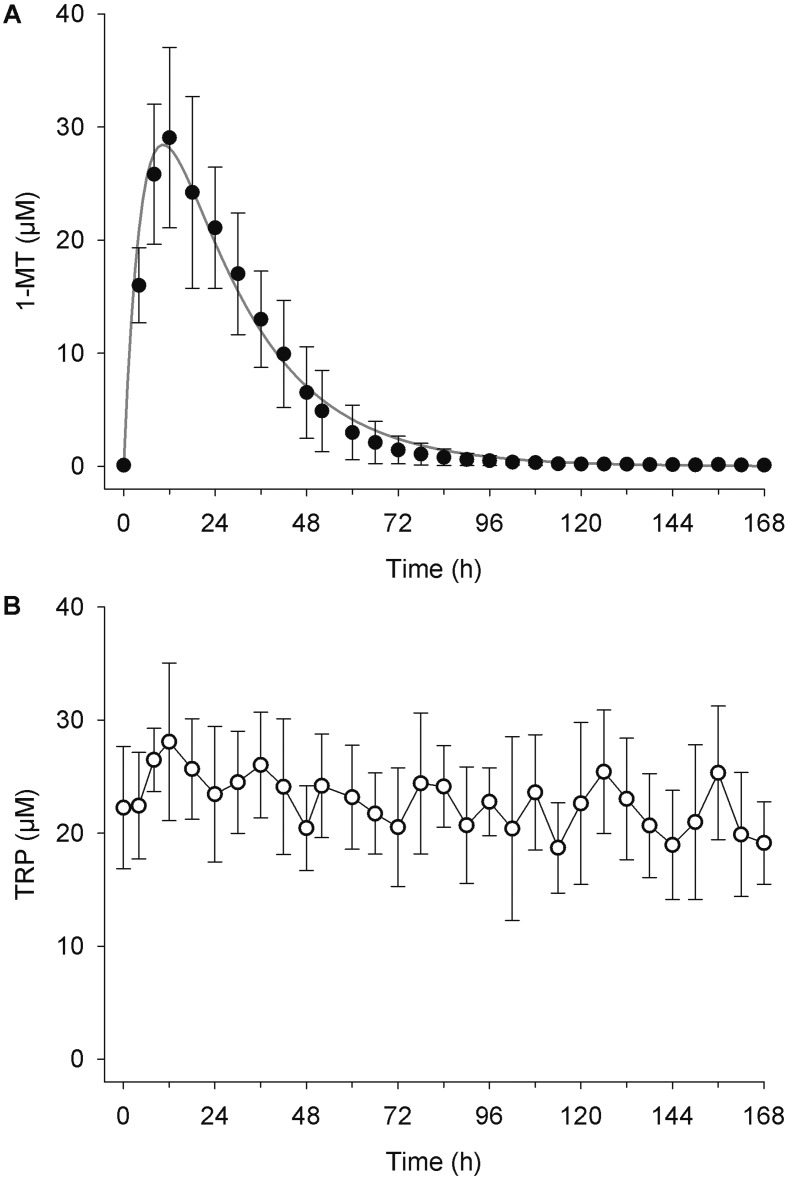
Pharmacokinetic parameters of 1-MT are shown in Table 1 and the plasma concentration versus time curve in Fig. 1A. After a single s.c. injection, the plasma concentrations of 1-MT reached a maximum of 29.1 ± 8.0 µM after 12 h, and 24 h after the injection, the plasma concentrations of 1-MT remained elevated (21.1 ± 5.3 µM). The half-life (T½) of 1-MT was 10.9 ± 2.0 h. In the control animal, which received MYR, 1-MT was not detectable in plasma. The average TRP concentration in plasma over the entire 7-day period was 22.8 ± 5.1 µM (min, 13.1 µM; max, 37.7 µM; Fig. 1B). No impairment of the health status could be observed after a single s.c. injection of either 1-MT or MYR.
Table 1. Pharmacokinetic parameters of 1-MT after a single s.c. administration of 1 g 1-MT to pigs.
| Parameters | Units | 1-MT |
|---|---|---|
| Cmax | µM | 29.07 ± 7.97 |
| Tmax | h | 12 ± 0 |
| k | h-1 | 0.065 ± 0.011 |
| T1/2 | h | 10.93 ± 2.03 |
| AUC0→t | µM h | 959.9 ± 345.8 |
Cmax, maximum concentration in pigs plasma; Tmax, time to reach maximum plasma concentration; k, first-order kinetic rate constant; T1/2, half-life; AUC0→t, area under the concentration-time curve from time zero to 168 h. means ± standard deviation, n= 3.
Fig. 1.
Plasma concentrations of 1-MT (A) and TRP (B) after a single s.c. administration of 1 g of 1-MT. Concentrations of 1-MT are shown as the means ± the standard deviation for three animals receiving 1-MT. TRP concentrations are shown as the means ± the standard deviation for all four animals.
Figure 2 shows the simulated profile of plasma 1-MT concentrations based on five repeated daily administrations and the actual plasma concentrations that were measured at different time points. Immediately prior to the first administration, 1-MT was not detectable in the plasma. Repeated daily applications increased the plasma concentrations of 1-MT, with significantly higher levels occurring at 48 h than at 24 h. The plasma concentrations of 1-MT at 48 h did not differ from the concentrations after 96 h, indicating that steady state conditions of trough values were reached after two subcutaneous administrations. The maximum values between two administrations were observed by significant increased 1-MT concentrations 12 h after the fourth and fifth injections (Fig. 2). In the MYR-treated control animals, the plasma concentrations of 1-MT were not detectable.
Fig. 2.
Simulated profile for plasma 1-MT concentrations after five repetitive s.c. administrations once a day (dashed line) deduced from the single dose experiment and plasma 1-MT concentrations after five s.c. administrations at 0, 24, 48, 72, and 96 h. Samples were taken immediately prior to the first administration of 1-MT and 24 h (time points 24, 48, 72, and 96 h) or 12 h (time points 84 and 108 h) after the preceding administration of 1-MT. 1-MT concentrations are presented as the least square means ± the standard error (n=12 for time points 0, 24, 48, 72, and 84 h; n=6 for time points 96 and 108 h). Significant differences (P < 0.05) between time points are shown by different letters. At 0 h, 1-MT was not detectable in plasma.
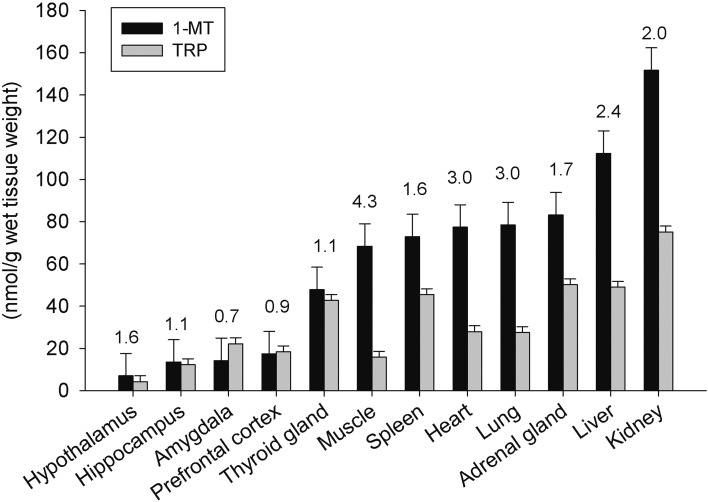
The concentrations of 1-MT and TRP in different tissues after five daily injections of 1-MT are shown in Fig. 3. Accumulation of 1-MT was observed in all tissues with a substantial variability among the different tissues. The highest concentrations of 1-MT, at greater than 100 nmol/g wet tissue weight, were found in the kidney and liver, followed by the adrenal gland, lung, heart, spleen, muscle, and thyroid gland, with the concentrations being between 48 and 83 nmol/g wet tissue weight. Concentrations lower than 20 nmol/g wet tissue weight were detected in the different brain areas. Variability of TRP concentrations between tissues was generally lower, with TRP concentrations of greater than 40 nmol/g wet tissue weight observed in the kidney, liver, adrenal gland, spleen, and thyroid gland and concentrations lower than 30 nmol/g wet tissue observed in the other tissues. A comparison of 1-MT and TRP concentrations revealed that in each tissue, 1-MT concentrations were nearly equal to or higher than TRP concentrations, as shown by 1-MT/TRP ratios ranging from 0.7 in the amygdala to 4.3 in the muscle.
Fig. 3.
Distribution of 1-MT and TRP in different tissues 12 h after the final s.c. injection of 1-MT (n=6). Concentrations of 1-MT and TRP are presented as the least square means ± the standard error. The ratio of 1-MT/TRP in each tissue is given above the respective bars as the least square mean.
The repeated s.c. administration of both MYR and 1-MT in MYR in the popliteal fossa over 5 days caused a local swelling around the puncture sites, which remained until the end of the treatment period. No significant differences were found in body weight (1-MT, 14.1 ± 1.1 kg; MYR, 14.5 ± 1.1 kg; p = 0.8), daily feed uptake (1-MT, 0.66 ± 0.02 kg; MYR, 0.66 ± 0.02 kg; P = 0.97), and skin temperature (1-MT, 39.1 ± 0.1°C; MYR, 39.0 ± 0.1°C; P = 0.2) between groups.
Discussion
The present study investigated the accumulation of 1-MT in blood and tissues after single and repeated s.c. injections of 1-MT in pigs. Previous studies in mice, rats, and dogs showed that single and repeated administrations of 1-MT were able to increase 1-MT concentrations in blood and tissues using oral, intravenous, intraperitoneal, or subcutaneous administrations [3, 14, 15, 17]. In the present study, 1-MT was applied for the first time in a porcine model. Subcutaneous injection of 1-MT was chosen because it enables reliable administration of a defined amount of 1-MT to each animal without risking vomiting or dysphagia, in contrast to oral application. For s.c administration of 1-MT, a medium chain triglyceride was used as an excipient. Lipophilic solutions have been demonstrated to be effective at enabling drug absorption from the injection site when administered by s.c. injections. The drug molecule reaches blood capillaries or lymphatic vessels by release of the agent from the oil vehicle in a tissue environment [24, 37]. The results show that a single administration of 1-MT increases the plasma concentration of 1-MT relative to that of TRP, indicating that 1-MT can accumulate sufficiently to act as a competitive inhibitor of IDO. From these data, a model for daily repetitive s.c. injections was calculated and tested in a second experiment. We found that steady state trough values were reached 24 h after the second and following administrations. Additional measurements at time points where maximum concentrations were expected, i.e., 12 h after application, confirmed the simulated profile.
The analyses of peripheral and brain tissues showed that 1-MT accumulated in all tissues examined, with the highest concentrations found in the kidney and liver, whereas lower concentrations were found in brain areas. These results support findings in mice, where the highest concentrations of 1-MT were detected in the kidney, heart, and liver and lower concentrations were found in the brain after oral administration of 1-MT [14]. It can be assumed that 1-MT is transported to the brain by an active carrier mechanism through the blood brain barrier, similar to TRP, and that high concentrations of 1-MT in the plasma are not necessarily a reflection of high 1-MT concentrations in brain areas. In our study, the concentrations of 1-MT in most of the investigated tissues were higher than the corresponding TRP concentrations, leading to an 1-MT/TRP ratio > 1. It is known that the ability of plasma to bind drugs plays a considerable role in the transport, distribution, and metabolism of these compounds [13]. Protein-binding studies have determined that less than 15% of 1-MT was bound to plasma proteins, whereas the majority of 1-MT was found in an unbound state [14]. In contrast, 77% to 94% of the tryptophan in plasma has been reported to be bound to serum albumin or plasma proteins [31, 36], thus influencing its transport by specific amino acid transporters. Studies by Karunakaran et al. [16] showed that 1-MT is a soluble substrate for TRP specific amino acid transporters, thus permitting the transport of 1-MT in peripheral tissues in a manner similar to transport of TRP. In the present study, a higher percentage of unbound 1-MT in plasma compared with TRP may be responsible for the high 1-MT/TRP ratios in some of the tissues. This is supported by the finding that 1-MT concentrations in peripheral tissues (48–152 nmol/g wet tissue weight) were higher than the mean corresponding 1-MT concentrations in plasma (38 nmol/ml) at the time of tissue sampling. In young pigs, the blood volume in different organs varies, ranging from 32 µl/g in the brain to 408 µl/g in the liver [19]. Considering the 1-MT concentrations in plasma found in the present study, less than 5% from the 1-MT accumulated in organs was derived from “contamination” with blood. Studies in mice showed that within 48 h post dose, 35% of 1-MT was excreted via urine and 13% was excreted via feces [14]. Therefore, the high 1-MT concentrations measured in the kidney may be a reflection of this major route of excretion. To our knowledge, no information is available about whether 1-MT can be metabolized into TRP, thus interfering with enzyme inhibition. For the L-isomer of 1-MT, a Ki value of 19 µM has been reported [11], which is lower than the plasma concentrations found in the present study, and therefore it can be assumed that inhibition is likely [18].
In the present study, repetitive s.c. administration of MYR and 1-MT suspended in MYR caused local swelling around the puncture sites, which remained until the end of the treatment period. This indicates a prolonged absorption time for MYR, which may impede repeated administrations over longer periods. The animals exhibited no fever response, and they exhibited no significant changes in feed uptake or body weight at the end of the five-day period of 1-MT administration. Currently, there are few data available regarding the safety and side effects of treatment with 1-MT. Our findings support the results of a previous study in which repeated oral administration of 1-MT at a saturating dose was well tolerated, with no toxic effects and no significant changes of physiologically relevant parameters in rats and dogs [14]. Clinical studies in patients with metastatic breast cancer, aimed at the determination of the drug safety of 1-MT, describe a maximum tolerated dose of 1.6 g/day given by mouth over a time frame of up to 4 weeks [10].
In summary, the present study established a model for the accumulation of 1-MT in domestic pigs. We administered 1-MT by repetitive daily s.c. injections over five days, leading to steady state conditions in plasma after two days. At the end of the administration period, 1-MT was shown to accumulate in peripheral and brain tissues with similar or higher concentrations compared with those of TRP. Thus, accumulation in blood and tissues demonstrates that repetitive s.c. administration of 1-MT is an appropriate model that can be used in studies of 1-MT as a potential inhibitor of IDO for biomedical research in this large animal model.
Conflict of Interests
The authors declare that there are no conflicts of interest with respect to this work.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (www.dfg.de; grant numbers: KA 1266/5-1, SCHU 853/7-1).
Acknowledgments
The authors gratefully acknowledge Silvia Langhoff, Dagmar Mähling, Petra Müntzel, Martina Pohlmann, Birgit Sobczak, and Regina Wal from the Institute of Behavioural Physiology, Leibniz Institute for Farm Animal Biology (FBN), and Thomas Brand and Sabine Ristow from the Institute of Pharmacy, University of Greifswald, for excellent technical assistance. The authors are also grateful to Cornelia Müller from the Institute of Clinical Chemistry and Laboratory Medicine, University of Greifswald, for assisting with the quantification of 1-MT and TRP. In addition, we thank Olaf Bellmann, Evelin Normann, Heidi Sievert, and colleagues from the experimental pig unit for excellent animal care.
References
- 1.Bendixen E., Danielsen M., Larsen K., Bendixen C.2010. Advances in porcine genomics and proteomics—a toolbox for developing the pig as a model organism for molecular biomedical research. Brief. Funct. Genomics 9: 208–219. doi: 10.1093/bfgp/elq004 [DOI] [PubMed] [Google Scholar]
- 2.Cady S.G., Sono M.1991. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch. Biochem. Biophys. 291: 326–333. doi: 10.1016/0003-9861(91)90142-6 [DOI] [PubMed] [Google Scholar]
- 3.Criado G., Šimelyte E., Inglis J.J., Essex D., Williams R.O.2009. Indoleamine 2,3 dioxygenase-mediated tryptophan catabolism regulates accumulation of Th1/Th17 cells in the joint in collagen-induced arthritis. Arthritis Rheum. 60: 1342–1351. doi: 10.1002/art.24446 [DOI] [PubMed] [Google Scholar]
- 4.Eder K., Nonn H., Kluge H., Peganova S.2003. Tryptophan requirement of growing pigs at various body weights. J. Anim. Physiol. Anim. Nutr. (Berl.) 87: 336–346. doi: 10.1046/j.1439-0396.2003.00442.x [DOI] [PubMed] [Google Scholar]
- 5.European Medicine Agency (EMA). 2012. Guideline on bioanalytical method validation (EMEA/CHMP/EWP/192217/2009 Rev.1 Corr.*).
- 6.Félix B., Léger M.E., Albe-Fessard D., Marcilloux J.C., Rampin O., Laplace J.P.1999. Stereotaxic atlas of the pig brain. Brain Res. Bull. 49: 1–137. doi: 10.1016/S0361-9230(99)00012-X [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration (FDA). 2013. Guidance for Industry. Bioanalytical Method Validation: Analytical methods validation.
- 8.González A., Varo N., Alegre E., Díaz A., Melero I.2008. Immunosuppression routed via the kynurenine pathway: a biochemical and pathophysiologic approach. Adv. Clin. Chem. 45: 155–197. doi: 10.1016/S0065-2423(07)00007-8 [DOI] [PubMed] [Google Scholar]
- 9.Health USNIo.https://www.clinicaltrials.gov/ct2/ results?term=1-methyl-D-Tryptophan&Search=Search date of access: 16.09.2015.
- 10.Health USNIo.https://www.clinicaltrials.gov/ct2/show/results/NCT01042535?term=1-methyl-DTryptophan& rank=1§=Xb70156 date of access: 16.09.2015.
- 11.Hou D.Y., Muller A.J., Sharma M.D., DuHadaway J., Banerjee T., Johnson M., Mellor A.L., Prendergast G.C., Munn D.H.2007. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 67: 792–801. doi: 10.1158/0008-5472.CAN-06-2925 [DOI] [PubMed] [Google Scholar]
- 12.Huttunen R., Syrjänen J., Aittoniemi J., Oja S.S., Raitala A., Laine J., Pertovaara M., Vuento R., Huhtala H., Hurme M.2010. High activity of indoleamine 2,3 dioxygenase enzyme predicts disease severity and case fatality in bacteremic patients. Shock 33: 149–154. doi: 10.1097/SHK.0b013e3181ad3195 [DOI] [PubMed] [Google Scholar]
- 13.Jia L., Wong H., Wang Y., Garza M., Weitman S.D.2003. Carbendazim: disposition, cellular permeability, metabolite identification, and pharmacokinetic comparison with its nanoparticle. J. Pharm. Sci. 92: 161–172. doi: 10.1002/jps.10272 [DOI] [PubMed] [Google Scholar]
- 14.Jia L., Schweikart K., Tomaszewski J., Page J.G., Noker P.E., Buhrow S.A., Reid J.M., Ames M.M., Munn D.H.2008. Toxicology and pharmacokinetics of 1-methyl-d-tryptophan: absence of toxicity due to saturating absorption. Food Chem. Toxicol. 46: 203–211. doi: 10.1016/j.fct.2007.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung I.D., Lee M.G., Chang J.H., Lee J.S., Jeong Y.I., Lee C.M., Park W.S., Han J., Seo S.K., Lee S.Y., Park Y.M.2009. Blockade of indoleamine 2,3-dioxygenase protects mice against lipopolysaccharide-induced endotoxin shock. J. Immunol. 182: 3146–3154. doi: 10.4049/jimmunol.0803104 [DOI] [PubMed] [Google Scholar]
- 16.Karunakaran S., Umapathy N.S., Thangaraju M., Hatanaka T., Itagaki S., Munn D.H., Prasad P.D., Ganapathy V.2008. Interaction of tryptophan derivatives with SLC6A14 (ATB0,+) reveals the potential of the transporter as a drug target for cancer chemotherapy. Biochem. J. 414: 343–355. doi: 10.1042/BJ20080622 [DOI] [PubMed] [Google Scholar]
- 17.Kiank C., Zeden J.P., Drude S., Domanska G., Fusch G., Otten W., Schuett C.2010. Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS ONE 5: e11825. doi: 10.1371/journal.pone.0011825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li A.P.2007. In vitro evaluation of metabolic drug-drug interactions: concepts and practice. pp. 1–30. In: Drug-drug interaction in pharmaceutical development (Li, A.P. ed.), Wiley, Hoboken. [Google Scholar]
- 19.Linderkamp O., Berg D., Betke K., Köferl F., Kriegel H., Riegel K.P.1980. Blood volume and hematocrit in various organs in newborn piglets. Pediatr. Res. 14: 1324–1327. doi: 10.1203/00006450-198012000-00010 [DOI] [PubMed] [Google Scholar]
- 20.Liu X., Newton R.C., Friedman S.M., Scherle P.A.2009. Indoleamine 2,3-dioxygenase, an emerging target for anti-cancer therapy. Curr. Cancer Drug Targets 9: 938–952. doi: 10.2174/156800909790192374 [DOI] [PubMed] [Google Scholar]
- 21.Löb S., Königsrainer A., Zieker D., Brücher B.L., Rammensee H.G., Opelz G., Terness P.2009. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol. Immunother. 58: 153–157. doi: 10.1007/s00262-008-0513-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Löb S., Konigsrainer A., Schafer R., Rammensee H.G., Opelz G., Terness P.2008. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood 111: 2152–2154. doi: 10.1182/blood-2007-10-116111 [DOI] [PubMed] [Google Scholar]
- 23.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J.1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 24.Martinez M.N.2011. Factors influencing the use and interpretation of animal models in the development of parenteral drug delivery systems. AAPS J. 13: 632–649. doi: 10.1208/s12248-011-9303-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metges C.C., Lang I.S., Hennig U., Brüssow K.P., Kanitz E., Tuchscherer M., Schneider F., Weitzel J.M., Steinhoff-Ooster A., Sauerwein H., Bellmann O., Nürnberg G., Rehfeldt C., Otten W.. Intrauterine growth retarded progeny of pregnant sows fed high protein:low carbohydrate diet is related to metabolic energy deficit. PLoS ONE 7: e31390. doi: 10.1371/journal.pone.0031390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meurens F., Summerfield A., Nauwynck H., Saif L., Gerdts V.2012. The pig: a model for human infectious diseases. Trends Microbiol. 20: 50–57. doi: 10.1016/j.tim.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller A.J., DuHadaway J.B., Donover P.S., Sutanto-Ward E., Prendergast G.C.2005. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 11: 312–319. doi: 10.1038/nm1196 [DOI] [PubMed] [Google Scholar]
- 28.Munn D.H., Zhou M., Attwood J.T., Bondarev I., Conway S.J., Marshall B., Brown C., Mellor A.L.1998. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281: 1191–1193. doi: 10.1126/science.281.5380.1191 [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez H., Kunavongkrit A.1983. Chronical venous catheterization for frequent blood sampling in unrestrained pigs. Acta Vet. Scand. 24: 318–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth W.J., Kissinger C.B., McCain R.R., Cooper B.R., Marchant-Forde J.N., Vreeman R.C., Hannou S., Knipp G.T.2013. Assessment of juvenile pigs to serve as human pediatric surrogates for preclinical formulation pharmacokinetic testing. AAPS J. 15: 763–774. doi: 10.1208/s12248-013-9482-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talbert A.M., Tranter G.E., Holmes E., Francis P.L.2002. Determination of drug-plasma protein binding kinetics and equilibria by chromatographic profiling: exemplification of the method using L-tryptophan and albumin. Anal. Chem. 74: 446–452. doi: 10.1021/ac010643c [DOI] [PubMed] [Google Scholar]
- 32.Takikawa O.2005. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. Biochem. Biophys. Res. Commun. 338: 12–19. doi: 10.1016/j.bbrc.2005.09.032 [DOI] [PubMed] [Google Scholar]
- 33.Uyttenhove C., Pilotte L., Théate I., Stroobant V., Colau D., Parmentier N., Boon T., Van den Eynde B.J.2003. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 9: 1269–1274. doi: 10.1038/nm934 [DOI] [PubMed] [Google Scholar]
- 34.Wirthgen E., Tuchscherer M., Otten W., Domanska G., Wollenhaupt K., Tuchscherer A., Kanitz E.2014. Activation of indoleamine 2,3-dioxygenase by LPS in a porcine model. Innate Immun. 20: 30–39. doi: 10.1177/1753425913481252 [DOI] [PubMed] [Google Scholar]
- 35.Yang H.J., Yen M.C., Lin C.C., Lin C.M., Chen Y.L., Weng T.Y., Huang T.T., Wu C.L., Lai M.D.2010. A combination of the metabolic enzyme inhibitor APO866 and the immune adjuvant L-1-methyl tryptophan induces additive antitumor activity. Exp. Biol. Med. (Maywood) 235: 869–876. doi: 10.1258/ebm.2010.010001 [DOI] [PubMed] [Google Scholar]
- 36.Yang J., Hage D.S.1997. Effect of mobile phase composition on the binding kinetics of chiral solutes on a protein-based high-performance liquid chromatography column: interactions of D- and L-tryptophan with immobilized human serum albumin. J. Chromatogr. A 766: 15–25. doi: 10.1016/S0021-9673(96)01040-0 [DOI] [PubMed] [Google Scholar]
- 37.Zuidema J., Kadir F., Titulaer H.A.C., Oussoren C.1994. Release and absorption rates of intramuscularly and subcutaneously injected pharmaceuticals. Int. J. Pharm. 105: 189–207. doi: 10.1016/0378-5173(94)90103-1 [DOI] [Google Scholar]