Abstract
222Rn is a radioactive, odorless, and colorless element which has a half-life of 3.83 days. One of 222Rn main resources are Groundwater (wells, springs, etc.). Hence, the use of groundwater with high concentration of 222Rn can increase the risk of lung and stomach cancers. Concentration of 222Rn in tap water of Minab city in two temperatures 5 and 15 ºC was measured by radon meter model RTM1668-2. The effective dose was calculated by equations proposed by UNSCEAR. Geometric mean concentration of 222Rn in drinking water was found to be 0.78±0.06 and 0.46±0.04 Bq/l at 5 and 15 °C (p value<0.05), respectively. The effective doses were 0.006 and 0.003 mSv/y for adults, and 0.011 and 0.007 mSv/y for the children, respectively (p value<0.05). Besides, the effective dose for adult through inhaling 222Rn at 5 and 15 °C were estimated 0.0021 and 0.0012mSv/y, respectively. Geometric mean concentration in 222Rn drinking water and effective dose received from drinking water and inhalation of 222Rn is lower than WHO and EPA standard limits. Increasing temperature of drinking water will decrease the effective dose received. Annual Effective dose received from inhalation and consumption of 222Rn in drinking water in children is more than adults.
Keywords: Radon 222, Effective dose, tap water, child and adults humans
1. Introduction
Radon 222 (222Rn) is produced as a result of decay of Radium 226 (226Ra) in Uranium 235 (235U) chain. This element is radioactive, odorless, colorless, and water soluble and has a half-life of 3.83 days (Al-Khateeb, Al-Qudah, Alzoubi, Alqadi, & Aljarrah, 2012; Ju, Ryu, & Jang, 2012). Several studies indicate that 222Rn indoor air concentration have a significant relationship with lung cancer (Torres-Durán, Barros-Dios, Fernández, & Ruano-Ravina, 2014). Indoor air death rate from 222Rn has been announced approximately 21,000 people a year, 10 times more than air pollution deaths (Environmental Protection Agency, 2010). Studies have shown that 222Rn, received the annual effective dose 1.3mSv/y due to natural exposure (2.4 mSv/y) to dedicate (Over 50%) (Magill & Galy, 2005). United Nations Scientific Committee on the effects of atomic radiation (UNSCEAR) has expressed exposure standard effective dose received from natural radioactive 2.5 mSv/y, which is 1 mSv/y related to 222Rn (Mehra and Bala, 2013; Radiation, 2000). 222Rn in drinking water can enter the internal organs such as the stomach and cause cancer (Somlai, Tokonami, Ishikaw, Vancsur, Gáspár, 2007; Auvinen, Salonen, Pekkanen, Pukkala, & Ilus, 2005; Alizadeh, Mahvi, & Fakhri, 2014). Also 222Rn inhalation can cause damage to DNA lung cells and leads to lung cancer in the population (Todorovic, Nikolov, Forkapic, Bikit, & Mrdja, 2012; Motesaddi, Fakhri, Alizadeh, Mohseni, & Jafarzadeh, 2014). European Commission and the World Health Organization has proposed concentration of 222Rn in the drinking water, 100 Bq/l as the standard limit (WHO, 2006). EPA 11 Bq/l, has been suggested as the maximum concentration Level (MCL) of 222Rn in drinking water (Environmental Protection Agency, may 2012). Many studies have shown that groundwater resources rather than surface water resources have much higher concentration of radioactive materials such as 222Rn (Amin, 2013; Rožmarić, Rogi, Benedik, & Štrok, 2012). The total indicative dose (TID) induced by radioactive substances (3H, 40K, 222Rn) as well as those produced through 222Rn decayed in drinking water is reported to be 0.1 mSv/y by WHO and the European committee (Somlai at al., 2007; Todorovic at al., 2012; WHO, 2004). Due to the exit of 222Rn during water transfer in distribution network, water transfer from one container to another, storing and boiling water, determining the standardized effective dose induced by 222Rn is difficult (Ishikawa, Tokonami, Yoshinaga, & Narazaki, 2005). Hence, the effective standard level dose 0.1 mSv/y is used for the analysis. In the present study, the effective dose of 222Rn received by children and adult age groups in Minab drinking water was calculated.
2. Materials and Methods
2.1 Study Area
Minab city with a population of approximately 90 thousand people is located in geographic coordinates 27º06’40N and 57º05’52, at an elevation of 45 meters above sea level (Figure 1). The city is located in a hot and humid region and the water consumption per capital was high. The only water resources in this town are three deep wells (Groundwater source), the water of which is pumped out and distributed with no purification process.
Figure 1.

sampling regions of Minab in the East province of Hormozgan, Iran (2)
2.2 Sample Collection
Since the retention time of water in the distribution network is effective on the concentration of 222Rn (Ishikawa, Tokonami, Yoshinaga, & Narazaki, 2005), thus, the sampled locations were determined from the beginning to the end of the distribution network. For 4 consecutive months, the sampling was done in 10 regions of the town. Meanwhile, 25 samples were selected from each region. During each stage of time, a total of 250 samples, each containing 2l of city tap water were obtained from 10 regions Sampling was conducted according to the proposed method (EPA).
2.3 Measurement Concentration of 222Rn
Measurement of radioactive substances in water, soil and air are done in various ways, such as alpha spectrophotometry, inductively coupled plasma/mass spectrophotometer, gamma spectrophotometry and liquid Scintillation (Rožmarić, Rogi, Benedik, & Štrok, 2012). Recently, many studies measure the concentration of 222Rn portable devices, such as RAD7 RTM (Mehra and Bala, 2013; Ju, Ryu, Jang, Dong, & Chung, 2012; Todorovic, Jovana Nikolov, Sofija Forkapic, Istvan Bikit, & Dusan Mrdja, 2012; Lee & Kim, 2006). Hence, in this study a model of portable alpha spectrophotometry RTM1688-2 was used to measure 222Rn in drinking water. To determine the effect of water temperature on the diffusion rate 222Rn of water, measurements was done at 5 and 15ºC temperatures. According to measurement of 300 mL, after the sample size reached the intended temperature, the device was placed in a closed cycle (Figure 2). The time for balance between concentration of 222Rn and its decay products (daughters 222Rn) is 4 hour approximately (Ju, Ryu, & Jang, 2012; Mehra & Bala, 2013; Lee & Kim, 2006). Hence, the 4 hour mean concentration of 222Rn (Bq/l) and the initial temperature (ºC) was recorded.
Figure. 2.

Measurement water 222Rn levels by RTM 1688-2 device, manufactured by Sarad corporation in Germany.
2.4 Calculation of effective dose (Ingestion)
To determine 222Rn annual effective dose received in the stomach from water consumption, Equation 1 was used (Sarad, 2009). In this equation E: the annual effective dose received by mSv/y, K: Coefficient conversion concentration of 222Rn to effective dose According to mSv/Bq, KM: Annual water consumed l/y, C: the concentration of 222Rn depending on Bq/l and T: the period of water consumption in the study, here was 365 days (Somlai, Tokonami, Ishikawa, Vancsura, & Gáspár, 2007).

Conversion factor for adults and children were 1×10-8 Sv/Bq and 2×10-8 Sv/Bq, respectively (Amin, 2013; WHO, 2004). KM is daily consumption which is considered 2 l/d.
2.5 Calculation of the Effective Dose (Inhalation)
In order to estimate the effective dose received annually through inhaling 222Rn of underground water, the conversion coefficient of 2.8 µSv.lit/Bq was used (Radiation, 2000). The annual geometric mean concentration of 222Rn (Bq/l) was multiplied by the coefficient 2.8×10-3, and the effective dose received annually through inhaling 222Rn was estimated in mSv/y.
2.6 Statistical Analyses
Statistical analyses were done via SPSS 16, using One-way ANOVA method and correlation coefficient. The results were also stated in mean and standard deviation forms.
3. Results
Geometric mean and range of concentration of 222Rn in drinking water was measured 0.78±0.06 Bq/l and 0.19-1.7 Bq/l at 5 ºC and 0.46±0.04 Bq/l and 0.16-1.45 Bq/l at 15 ºC, respectively (p value<0.05). (Tables 1 and 2).
Table 1.
Geometric mean (GM±SE), Middle, maximum and minimum concentration of 222Rn tap water samples in the temperature of 5 ºC (Bq/l) (n=250; Note 1)
| Regions | Minimum | Maximum | Middle | Geometric mean |
|---|---|---|---|---|
| 1 | 0.96 | 1.8 | 0.24 | 1.17±0.1 |
| 2 | 0.77 | 1.71 | 0.5 | 1.14±0.1 |
| 3 | 0.68 | 1.08 | 0.98 | 0.93±0.08 |
| 4 | 0.78 | 1.15 | 0.9 | 0.92±0.08 |
| 5 | 0.48 | 0.96 | 0.87 | 0.77±0.06 |
| 6 | 0.65 | 0.89 | 0.76 | 0.72±0.06 |
| 7 | 0.42 | 0.69 | 0.56 | 0.57±0.5 |
| 8 | 0.43 | 0.85 | 0.72 | 0.65±0.5 |
| 9 | 0.26 | 0.65 | 0.46 | 0.47±0.4 |
| 10 | 0.2 | 0.65 | 0.54 | 0.49±0.4 |
Table 2.
Geometric mean (GM±SE), Middle, maximum and minimum concentration of 222Rn drinking water samples in the temperature of 15ºC (Bq/l) (n=250)
| Regions | Min | Max | Middle | Geometric mean |
|---|---|---|---|---|
| 1 | 0.6 | 1.14 | 0.78 | 0.81±0.6 |
| 2 | 0.54 | 1.45 | 0.76 | 0.86±0.7 |
| 3 | 0.26 | 0.82 | 0.59 | 0.53±0.4 |
| 4 | 0.48 | 0.88 | 0.62 | 0.62±0.5 |
| 5 | 0.17 | 0.75 | 0.53 | 0.48±0.4 |
| 6 | 0.19 | 0.75 | 0.47 | 0.43±0.3 |
| 7 | 0.18 | 0.49 | 0.32 | 0.33±0.2 |
| 8 | 0.16 | 0.49 | 0.39 | 0.37±0.3 |
| 9 | 0.17 | 0.54 | 0.29 | 0.27±0.2 |
| 10 | 0.2 | 0.42 | 0.26 | 0.27±0.2 |
The percent of concentration frequency distributions of 222Rn in drinking water of 10 regions of Minab city in temperatures 5 and 15 ºC are shown in Figures 3 and 4. The maximum and minimum frequency distribution concentration of 222Rn at the temperature of 5 °C was observed in the range of 0.6-0.9 Bq/l and >0.4 Bq/l, respectively. At the temperature of 15 °C, they were observed in the range of >0.4 Bq/l and 1.1-1.3 Bq/l.
Figurer 3.

Percent of frequency distributions concentration of 222Rn Drinking water temperature in 5 ºC
Figure 4.
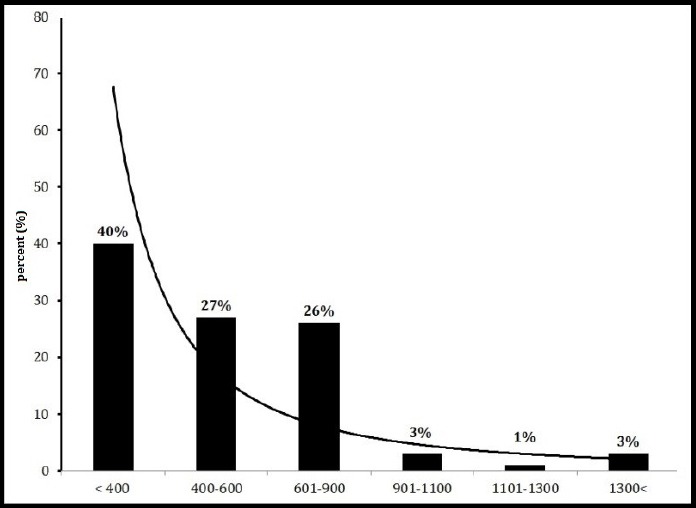
Percent of frequency distribution concentration of 222Rn in drinking water at 15 ºC
The effective dose received annually through drinking water at the temperature of 5 °C in the children and adult groups was 0.011 and 0.007 mSv/y, respectively. At the temperature of 15 °C it was 0.007 and 0.003 mSv/y (p value<0.05). The effective dose received annually through inhaling 222Rn in drinking water at the temperatures of 5 °C and 15 °C were 0.0021 and 0.0012 mSv/y, respectively (p value<0.05).
Figure 5.

Geometric mean concentration of 222Rn drinking water in 10 regions of Minab at the temperatures of 5 °C and 15 °C
4. Discussion
Geometric mean concentration of 222Rn of drinking water at 5 ºC (0.78±0.06 Bq/l) is greater than of 15 ºC (0.46±0.04 Bq/l). P value<0.05 between concentration of 222Rn of drinking water in temperatures 5 and 15 ºC, indicate a significant difference. Consistent with our results, several studies reduce emissions 222Rn where the effect of reduced water solubility was observed as the temperature increased (Yalcin, Gurler, Akar, Incirci, & Kaynak, 2011; GalipYucea & Gasparonb, 2013; Oner, Yalim, Akkurt, & Orbay, 2009).
As it can be seen in Table 3, the range concentration of 222Rn in drinking water of Amasiya (0.39-1.17 Bq/l) is within the range concentration of 222Rn drinking water of Minab (0.16-1.7 Bq/l). The range concentration of 222Rn in drinking water of Tehran (27.7-74.3 Bq/l), Buvaji (12-41 Bq/l), Uberiya (5.9-65.7 Bq/l), and Islamabad (25.9-158.4 Bq/l) are much greater than Minab and Amasiya cities. Difference concentration of 222Rn in these towns (Amasiyia and Minab) may be due to different factors such as concentration of 222Rn in the water source, geological substrate type, water retention time and temperature during the measurement (Yiğitoğlu, Öner, Yalim, Akkurt, Okur, 2010). Groundwater resources (springs, wells, etc.) due to contact with the various layers of the earth, has more Total Dissolved Solid (TDS), including radioactive materials relative to surface waters (rivers, lakes, etc.). Layers of earth containing igneous rocks (granite) are larger, with higher concentration of radioactive material 235U (Rožmarić, Rogi, Benedik, & Štrok, 2012; Rezaei & Jalili-Majareshin, 2011). Since the 222Rn is the product of series of 235U decay, it can be expected that concentration in groundwater which cross from substrate type, are higher (Przylibski, Mamont-Cies´la, Kusyk, Dorda, & Kozlowska, 2004). Drinking water sources in Tehran, Buage, Umbria and Islamabad are of underground type similar to Minab and Amasia. However, concentration of 222Rn in the drinking water of Minab and Amasia is different. This could be due to differences in geological structure, measuring temperature and retention time of water. The effective dose received by children age group at the temperature of 5 °C (0.011 mSv/y) was 1.57 times more than of that at 15 °C (0.006 mSv/y). For adults it was 2 times bigger. Since the mean concentration of 222Rn at 5 °C (0.78±.06 Bq/l) is more than that of 15 °C (0.46±.04 Bql-1), the effective dose received is higher at this temperature. The effective dose received annually by adults and children from drinking water at 5 and 15 °C was below the standard 0.1 mSv/y. The activated coefficient of converting 222Rn to effective dose is higher in children group than adults (Somlai at al., 2007). The effective dose received from drinking water in this age group at the temperatures of 5 and 15 °C are 1.83 and 2.33 times bigger than adults’.
Table 3.
Range concentration of 222Rn in tap water of Minab city compared with some other cities
| Country / City | Water source | Range | References |
|---|---|---|---|
| Pakistan/Islamabad | Groundwater | 25.9-158.4 | (Ali, Khan, Akhter, Khan and Waheed, 2010) |
| Italy/Umberia | Groundwater | 5.9-65.7 | (Borio, Rongoni, Saetta, Desideri and Roselli, 2005) |
| Turkey/Amasiya | Groundwater | 0.39-1.17 | (Oner, Yalim, Akkurt and Orbay, 2009) |
| China/Bovaji | Groundwater | 12-41 | (Xinwei, 2006) |
| Iran/Tehran | Groundwater | 27.7-74.3 | (N.Alirezazadeh, 2005) |
| Iran/Minab | Groundwater | 0.16-1.7 | This Study |
Effective dose due to inhalation of 222Rn from drinking water is much lower than the standard 1mSv/y effective dose due to 222Rn inhalation (Villalba, Sujoa, Cabrera, Jime´neza, & Villalobos, 2005). Effective dose received from 222Rn inhalation from drinking water at 5 ºC, is 1.82 times bigger than that of temperature 15 ºC. As can be seen in Table 4, the effective dose received by children (0.011 and 0.007 mSv/y) and adults (0.006 and 0.003 mSv/y) at a temperature 5 and 15 ºC of drinking water of Minab city is greater than Tehran (0.000129 and 0.00066 mSv/y), Balaton (0.0004 and 0.0002 mSv/y), Mashhad (0.00029 mSv/y for adult), Australia (0.005 mSv/y for adult), Gotaya (0.000122-0.0003 mSv/y) and Kastomono (0.00032-0.00093 mSv/y in summer and 0.00049-0.0008 mSv/y in the spring). 222Rn concentration range in drinking water of Tehran (27.7-74.3 Bq/l) is more than Minab City (0.16-1.7 Bq/l). However, due to the low effective dose conversion factor activity (0.35×10-8Sv/Bq) and capital annual consumption of water (children 75lit and adults 100lit), a lower effective dose is received by people of Tehran (N. Alirezazadeh, 2005). Lower effective dose in other cities can be due to low concentration of 222Rn, conversion factors and capital water consumption. Effective dose of induced inhalation in Minab (0.0021 and 0.0012 mSv/y) is higher than cities of Mashhad (0.0004 mSv/y), Gotiya (0.00003-0.00014 mSv/y) and is lower than cities of Tehran (0.01mSv/y), and Bovaji (0.03-0.14 mSv/y). However, effective dose of inhalation activity conversion factor of Minab (2.8 µSv/y) is higher than Tehran cities and Bovaji (1.8 µSvy-1) (Xinwei, 2006; N. Alirezazadeh, 2005), but due to the higher concentration of 222Rn in tap water, inhalation effective dose is higher in Minab city.
Table 4.
Annual effective dose received by age groups of children and adults caused by the inhalation 222Rn and ingestion of tap water Minab (Iran) and other cities
| City / Country | Annual effective dose | |||
|---|---|---|---|---|
| Drinking water (stomach) mSv/y | Inhalation (lung) mSv/y | References | ||
| Childs | adults | |||
| Minab/Iran1 | 0.011 | 0.006 | 0.0021 | This study |
| Minab/Iran2 | 0.007 | 0.003 | 0.0012 | This study |
| Mashhad/Iran | - | 0.00029 | 0.0004 | (Binesh, Mohammadi, Mowlavi, & Parvaresh, 2010) |
| Tehran/Iran | 0.000129 | 0.00066 | 0.01 | (N. Alirezazadeh, 2005) |
| Bovaji/China | 0.03-0.14 | (Xinwei, 2006) | ||
| Balaton/Netherlands | 0.0004 | 0.0002 | (Somlai at al., 2007) | |
| Australia | 0.005 | (Kralik, Friedrich and Vojir, 2003) | ||
| Gotaya/Turkey | - | 0.000122-0.0003 | 0.00014-0.00003 | (Sahin, Çetinkaya, Saç, & Içhedef, 2013) |
| Kastomono/Turkey | - | 0.00032-0.000933 0.00049-0.00084 |
- | (Yalcin at al., 2011) |
5 ºC Temperatures water;
15 ºC Temperatures water;
Summer season;
Spring season.
5. Conclusion
Geometric mean concentration of 222Rn in drinking water at temperatures 5 and 15 ºC (0.78±0.06 and 0.46±0.04 Bq/l) are lower than EPA and WHO standard limits. Annual Effective dose received from inhalation and consumption of 222Rn in drinking water in children is more than adults (p value<0.05). Also, effective dose received in both age groups, are much lower than EPA and WHO standard limits. Increasing the temperature reduces the effect on concentration of 222Rn in drinking water, followed by a reduction in received effective dose. Hence, it is recommended to reduce the effective dose received in the cities with high concentration of 222Rn in drinking water, water ingestion be at higher temperature.
Acknowledgments
The authors of this paper wish to express their gratitude to the chemical lab staff of Minab urban water and water-waste Company who dedicatedly cooperated in conduction of this research.
References
- Ali N, Khan E, Akhter P, Khan F, Waheed A. Estimation of mean annual effective dose through radon concentration in the water and indoor air of Islamabad and Murree. Radiation Protection Dosimetry. 2010;141:183–191. doi: 10.1093/rpd/ncq160. http://dx.doi.org/10.1093/rpd/ncq160 . [DOI] [PubMed] [Google Scholar]
- Alirezazadeh N. Radon concentrations in public water supplies in Tehran and evaluation of radiation dose. Iran. J. Radiat. Res. 2005;3(2):79–83. [Google Scholar]
- Alizadeh A, Mahvi A. H, Fakhri Y. The Effect of Tobacco Smoking On Concentration of 222 Rn Indoor Air and the Annually Received Effective Dose. Journal of Applied Sciences Research. 2014:10. [Google Scholar]
- Al-Khateeb H. M, Al-Qudah A. A, Alzoubi F. Y, Alqadi M. K, Aljarrah K. M. Radon concentration and radon effective dose rate in dwellings of some villages in the district of Ajloun. Jordan. Applied Radiation and Isotopes. 2012;70:1579–1582. doi: 10.1016/j.apradiso.2012.04.009. http://dx.doi.org/10.1016/j.apradiso.2012.04.009 . [DOI] [PubMed] [Google Scholar]
- Amin R. M. Evaluation of radon gas concentration in the drinking water and dwellings of south-west Libya, using CR-39 detectors. International Journal of Environmental Sciences. 2013. p. 4. http://dx.doi.org/10.6088/ijes.2014040400005 .
- Auvinen A, Salonen L, Pekkanen J, Pukkala E, Ilus T, Kurttio P. Radon and other natural radionuclides in drinking water and risk of stomach cancer: A case-cohort study in Finland. International Journal of Cancer. 2005;114:109–113. doi: 10.1002/ijc.20680. http://dx.doi.org/10.1002/ijc.20680 . [DOI] [PubMed] [Google Scholar]
- Binesh A, Mohammadi S, Mowlavi A. A, Parvaresh P. Evaluation of the radiation dose from radon ingestion and inhalation in drinking water. International Journal of Water Resources and Environmental Engineering. 2010;2:174–178. [Google Scholar]
- Borio R, Rongoni A, Saetta D. M. S, Desideri D, Roselli C. Radon and tritium measurements in drinking water in a region of central Italy (Umbria) Journal of Radioanalytical and Nuclear Chemistry. 2005;266:397–403. http://dx.doi.org/10.1007/s10967-005-0923-2 . [Google Scholar]
- Galip Y, Gasparonb M. Preliminary risk assessment of radon in groundwater: a case study from Eskisehir, Turkey. Isotopes in environmental and health studies. 2013;49(2):163–179. doi: 10.1080/10256016.2013.739562. http://dx.doi.org/10.1080/10256016.2013.739562 . [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Tokonami S, Yoshinaga S, Narazaki Y. Airborne and waterborne radon concentrations in areas with use of groundwater supplies. Journal of radioanalytical and nuclear chemistry. 2005;267:85–88. http://dx.doi.org/10.1007/s10967-006-0012-1 . [Google Scholar]
- Ju Y.-J, Ryu Y.-H, Jang H.-C. A Study on Concentration Measurements of Radon-222 (Uranium Series) and Radon-220 (Thoron Series) Emitted to the Atmosphere from Tex (Cementitious), Red Brick, and Ecocarat among Construction Materials. Korean Physical Society. 2012;60:1177–1186. http://dx.doi.org/10.3938/jkps.60.1177 . [Google Scholar]
- Ju Y.-J, Ryu Y.-H, Jang H.-C, Dong K.-R, Chung W.-K, Cho J.-H, Lim C.-S. P.-S. A Study on Concentration Measurements of Radon-222 (Uranium Series) and Radon-220 (Thoron Series) Emitted to the Atmosphere from Tex (Cementitious), Red Brick, and Ecocarat among Construction Materials. Korean Physical Society. 2012;60:1177–1186. http://dx.doi.org/10.3938/jkps.60.1177 . [Google Scholar]
- Kralik C, Friedrich M, Vojir F. Natural radionuclides in bottled water in Austria. Journal of environmental radioactivity. 2003;65:233–241. doi: 10.1016/s0265-931x(02)00099-1. http://dx.doi.org/10.1016/S0265-931X(02)00099-1 . [DOI] [PubMed] [Google Scholar]
- Lee J.-M, Kim G. A simple and rapid method for analyzing radon in coastal and ground waters using a radon-in-air monitor. Journal of Environmental Radioactivity. 2006;89:219–228. doi: 10.1016/j.jenvrad.2006.05.006. http://dx.doi.org/10.1016/j.jenvrad.2006.05.006 . [DOI] [PubMed] [Google Scholar]
- Mehra R, Bala P. Estimation of annual effective dose due to Radon level in indoor air and soil gas in Hamirpur district of Himachal Pradesh. Journal of Geochemical Exploration. 2013 [Google Scholar]
- Motesaddi S, Fakhri Y, Alizadeh A, Mohseni S. M, Jafarzadeh S, Mahvi A. H. Effective dose of Radon222 and thoron220 in the indoor air of Genow hot springs of Bandar Abbas. Advances in Environmental Biology. 2014;8:453–459. [Google Scholar]
- Oner F, Yalim H, Akkurt A, Orbay M. The measurements of radon concentrations in drinking water and the Yeşilırmak River water in the area of Amasya in Turkey. Radiation Protection Dosimetry. 2009;133:223–226. doi: 10.1093/rpd/ncp049. http://dx.doi.org/10.1093/rpd/ncp049 . [DOI] [PubMed] [Google Scholar]
- Przylibski T. A, Mamont-Cies'la K, Kusyk M, Dorda J, Kozlowska B. Radon concentrationsin groundwaters of the Polish part of the Sudety Mountains (SW Poland) Journal of Environmental Radioactivity. 2004;75:193–209. doi: 10.1016/j.jenvrad.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Radiation U. N. S. C. o. t. E. o. A. UNSCEAR 2000. Sources and effects of ionizing radiation. 2000:2. [Google Scholar]
- Rezaei D, Jalili-Majareshin A. Concentration rate measurement of radon gas in hot springs of Sarein as a tourism city with RAD7 and investigation physical methods to reduce radon concentration in water. Department of Physics, University of Mohagheghe Ardabili; 2011. [Google Scholar]
- Rožmarić M, Rogi M, Benedik L, Štrok M. Natural radionuclides in bottled drinking waters produced in Croatia and their contribution to radiation dose. Science of the Total Environment. 2012;437:53–60. doi: 10.1016/j.scitotenv.2012.07.018. http://dx.doi.org/10.1016/j.scitotenv.2012.07.018 . [DOI] [PubMed] [Google Scholar]
- Sahin L, Çetinkaya H, Saç M. M, Içhedef M. Determination of radon and radium concentrations in drinking water samples around the city of Kutahya. Radiation Protection Dosimetry. 2013;155:474–482. doi: 10.1093/rpd/nct019. http://dx.doi.org/10.1093/rpd/nct019 . [DOI] [PubMed] [Google Scholar]
- Somlai K, Tokonami S, Ishikaw T, Vancsur P, Gáspár M, Jobbágy V, Somlai J, Kovács T. 222Rn concentrations of water in the Balaton Highland and in the southern part of Hungary, and the assessment of the resulting dose. Radiation Measurements. 2007;42:491–495. [Google Scholar]
- Somlai K, Tokonami S, Ishikawa T, Vancsura P, Gáspár M, Jobbágy V, Kovács T. 222Rn concentrations of water in the Balaton Highland and in the southern part of Hungary, and the assessment of the resulting dose. Radiation Measurements. 2007;42:491–495. http://dx.doi.org/10.1016/j.radmeas.2006.11.005 . [Google Scholar]
- Todorovic N, Jovana Nikolov, Sofija Forkapic, Istvan Bikit, Dusan Mrdja, Miodrag Krmar, Veskovic M. Public exposure to radon in drinking water in SERBIA. Applied RadiationandIsotopes. 2012;70:543–549. doi: 10.1016/j.apradiso.2011.11.045. http://dx.doi.org/10.1016/j.apradiso.2011.11.045 . [DOI] [PubMed] [Google Scholar]
- Todorovic N, Nikolov J, Forkapic S, Bikit I, Mrdja D, Krmar M, Veskovic M. Public exposure to radon in drinking water in SERBIA. Applied Radiation and Isotopes. 2012;70:543–549. doi: 10.1016/j.apradiso.2011.11.045. [DOI] [PubMed] [Google Scholar]
- Torres-Durán M, Barros-Dios J. M, Fernández- A, Ruano-Ravina V. A. Residential radon and lung cancer in never smokers: A systematic review. Cancer Letters. 2014 doi: 10.1016/j.canlet.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Villalba L, Sujoa L. C, Cabrera M. E. M, Jime'neza A. C, Villalobos M. R. a, Mendoza C. J. D, Tenorio L. A. J, Rangeld I. D. v, Peraz E. F. H. Radon concentrations in ground and drinking water in the state of Chihuahua, Mexico. Journal of Environmental Radioactivity. 2005;80:139–151. doi: 10.1016/j.jenvrad.2004.08.005. http://dx.doi.org/10.1016/j.jenvrad.2004.08.005 . [DOI] [PubMed] [Google Scholar]
- WHO. Guidelines for drinking-water quality: Recommendations. World Health Organization; 2004. p. 1. [Google Scholar]
- WHO. Guidelines for drinking-water quality: First addendum to volume 1, Recommendations. World Health Organization; 2006. p. 1. [Google Scholar]
- Xinwei L. Analysis of radon concentration in drinking water in Baoji (China) and the associated health effects. Radiation Protection Dosimetry. 2006;121:452–455. doi: 10.1093/rpd/ncl048. http://dx.doi.org/10.1093/rpd/ncl048 . [DOI] [PubMed] [Google Scholar]
- Yalcin S, Gurler O, Akar U. T, Incirci F, Kaynak G, Gundogdu O. Measurements of radon concentration in drinking water samples from Kastamonu (Turkey) Isotopes in environmental and health studies. 2011;47:438–445. doi: 10.1080/10256016.2011.618270. http://dx.doi.org/10.1080/10256016.2011.618270 . [DOI] [PubMed] [Google Scholar]
- Yiğitoğlu I, Öner F, Yalim H, Akkurt A, Okur A, Özkan A. Radon concentrations in water in the region of Tokat city in Turkey. Radiation Protection Dosimetry. 2010;142:358–362. doi: 10.1093/rpd/ncq191. http://dx.doi.org/10.1093/rpd/ncq191 . [DOI] [PubMed] [Google Scholar]


