Highlights
-
•
This is the first report of angiosarcoma occurring after radiation on a non-operated breast.
-
•
The patient underwent mastectomy, surviving disease free for 6 years, despite the generally poor prognosis of angiosarcoma.
-
•
The potential difficulties of diagnosing angiosarcoma against background fibrosis caused by radiation should be kept in mind.
-
•
Kaplan-Meier analysis of 60 Japanese breast angiosarcoma patients showed significantly better prognosis in patients with a tumor 2 cm or smaller.
Abbreviation: FNAC, fine needle aspiration cytology
Keywords: Angiosarcoma, Radiation therapy, Breast surgery, Breast cancer, Occult breast cancer
Abstract
Introduction
Angiosarcoma consists only 0.04% of all breast malignancies and has a poor prognosis. This is the first reported case of an angiosarcoma arising in the non-operated breast after primary irradiation for occult breast cancer. The patient underwent mastectomy, surviving disease free for 6 years.
Presentation of case
A 73-year-old woman with a past history of irradiation of the non-operated left breast complained of skin thickening and crust formation on the left nipple 8 years post-irradiation. Considering the clinical history and radiological studies, recurrent cancer was suspected and biopsy was performed. However, no proof of malignancy was obtained. As clinical symptoms continued to advance, informed consent was obtained and mastectomy was performed. Histological examination of the surgical specimen revealed angiosarcoma.
Discussion
In this case, angiosarcoma occurred after radiation on a non-operated breast. Preoperative diagnosis was not achieved even with two cytology specimen and one biopsy. Each showed only fibrosis and inflammatory changes. The background breast tissue inflammation should have been caused by radiation. Marked fibrosis and the rather small number of sarcoma cells in the breast tumor in this case may be why bioptic diagnosis was difficult. Kaplan-Meier analysis of 60 Japanese breast angiosarcoma patients showed significantly better prognosis in patients with a tumor 2 cm or smaller.
Conclusion
Angiosarcoma may occur in the non-operated breast, post irradiation. The potential difficulties of diagnosing angiosarcoma against background fibrosis should be kept in mind. Initial radical surgery currently represents the only effective treatment for improving survival in these patients.
1. Introduction
Angiosarcoma represents less than 1% of soft-tissue sarcomas [1]. Angiosarcoma of the breast, which represents 0.04% of breast malignancies, can be divided into primary and secondary angiosarcoma. Factors that predispose individuals toward secondary angiosarcoma include radiotherapy, Stewart-Treves syndrome and chronic lymphedema [2]. Radiation-induced angiosarcoma is being reported with increasing frequency. Most cases occur in the setting of breast-conserving therapy and subsequent radiotherapy [1]. However, there has been no report about angiosarcoma after irradiation on a non-operated breast. Also, despite the generally poor prognosis of angiosarcoma, our case is disease free 6 years post-operation.
2. Case report
A 73-year-old woman received fine needle aspiration cytology (FNAC) for a swelling left axillary lymph node in November 2001. FNAC showed metastatic carcinoma, and axillary lymph node dissection was performed. As FNAC revealed lymph node metastasis, sentinel lymph node biopsy was unnecessary. The diagnosis was comedo carcinoma, estrogen and progesterone receptor negative, and HER2/neu positive. As the primary disease could not be identified on radiological examination, occult breast cancer was suspected. Postoperatively, she received 3 courses of cyclophosphamide, epirubicin and 5-fluorouracil, with subsequent irradiation (50 Gy) to the left breast. We have also proposed the choice of mastectomy, which the patient refused.
In November 2007, ultrasonographic study showed a hypoechoic area in the left breast. Core needle biopsy revealed inflammatory pseudotumor.
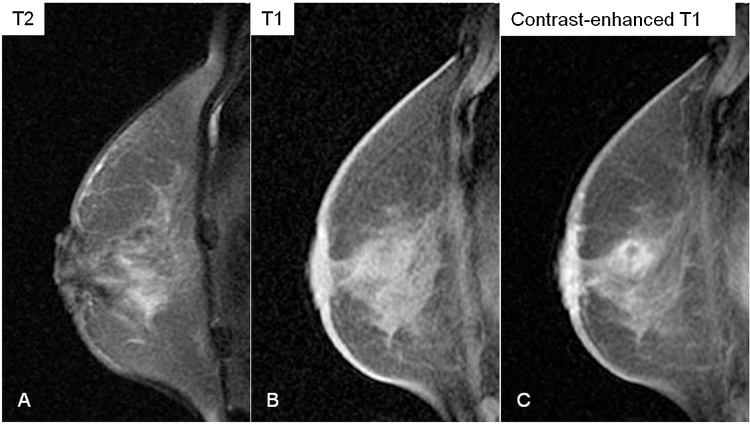
Then, in May 2009, she complained of skin-thickening and crust formation at the left areola. Mammography revealed skin-thickening and distortion around the left nipple (Fig. 1). Ultrasonography showed the hypoechoic area recognized in 2007, with thickening of the overlying skin on the left nipple. Magnetic resonance imaging showed a 3.5 cm mass with irregular margins. It was heterogeneous and hyperintense on T2-weighted imaging, hypointense on T1-weighted imaging, and gradually enhanced in a dynamic study (Fig. 2). Marked enhancement was suggestive of malignancy, but the gradual enhancement was inconsistent with common breast malignancies. Computed tomography showed skin thickening and an enhanced area with obscure borders beneath the left nipple.
Fig. 1.
Mammography.
Mediolateral oblique view revealing skin thickening and distortion around the left nipple (arrow). No tumor or calcification is apparent.
Fig. 2.
Magnetic resonance imaging.
A mass with irregular margins. The mass shows heterogeneous hyperintensity on T2-weighted imaging (A), and hypointensity on T1-weighted imaging (B). Contrast-enhanced T1-weighted imaging shows enhancement of the mass (C).
From radiological studies, primary breast cancer, recurrence or sarcoma was suspected. Given the clinical history, recurrence was strongly suspected. Even though FNAC, Vacuum-assisted biopsy (VACORA®; Bard Biopsy Systems, Tempe, AZ) and skin biopsy specimens were performed, diagnosis of malignant tumor was not obtained.
However, as skin thickening of the peri-nipple area continued to advance, clinically malignant tumor was suspected. After careful discussion with the patient, informed consent was obtained and simple mastectomy was performed in June 2009.
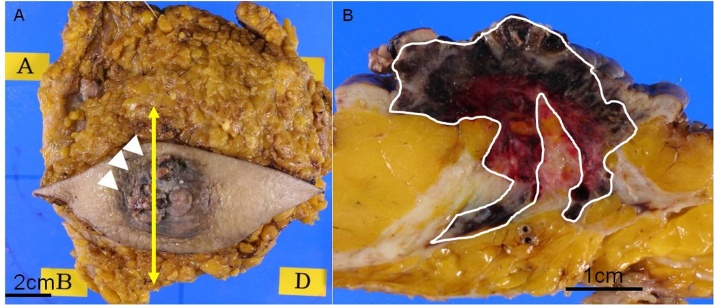
Macroscopically, an irregular shaped mass with crust formation and skin-thickening was observed in the areola region of the resected specimen (Fig. 3).
Fig. 3.
Pathology (gross).
(A) The resected specimen, showing an irregular mass accompanied by crust formation and skin thickening at the areola (arrows). (B) Cross-section of the specimen (vertical arrow in A). A brownish irregular mass leading into the skin thickening is seen beneath the nipple.
Histopathological examination revealed oval-shaped neoplastic stromal cells arranged in sinusoidal pattern, accompanying mitoses and background fibrosis (Fig. 4). The tumor was negative for estrogen, progesterone and HER2/neu receptors. Immunohistochemical staining yielded positive for CD34, vimentin and factor VIII, and negative for cytokeratin. The histological diagnosis was angiosarcoma of the breast.
Fig. 4.
Pathology (microscopy).
Microscopic examination of the surgically resected specimen, revealing oval-shaped cells arranged in the form of endovascular cells, accompanied by mitosis and background fibrosis.
Paclitaxel was administered as postoperative chemotherapy. Now, 6 years post-operation (February 2016), the patient is clinically free of disease.
3. Discussion
Breast angiosarcoma is rare, and primary angiosarcoma of the breast makes up only 0.00005% of all breast malignancies [2]. The frequency of angiosarcoma increases to 0.16% after radiotherapy. This represents an approximately 3200-fold increase in relative risk for patients who undergo breast-conserving surgery [3].
Angiosarcoma should thus be considered in any post-radiation patient with skin thickening or mammographic density [4].
Angiosarcoma tend to present 5–6 years, after breast-conserving therapy and subsequent radiotherapy [5].
According to the recent literature, (1) axillary lymph node detection (ALND) + radiotherapy and (2) ALND + mastectomy are both the treatment of choice in occult breast carcinoma. (1) and (2) have the same survival outcomes [6], [7]. We would like to point out that one has to keep in mind the possibility of the development of angiosarcoma in the cases that received treatment choice (1).
The unique feature about this case is the cause of the angiosarcoma. In usual case reports, the patients receive both breast conservation surgery and radiotherapy. In the diagnostic imaging of angiosarcoma in these cases, it is hard to tell whether the back ground fibrosis was caused by surgery and radiotherapy, or the tumor itself. However, in our case, we had performed only radiotherapy, and it is reasonable to consider that angiosarcoma occurred from a radiation scar. This case is valuable in the respect that from its clinical course, rather intense fibrosis occurred in the breast caused by irradiation, and the development of angiosarcoma from the sclerosing breast was confirmed by the interpretation of the resected specimen.
In the reported cases, preoperative diagnosis of angiosarcoma is usually made by needle biopsy specimen [1], [8]. However, due to the variety of pathological features, some angiosarcoma cases are misdiagnosed as hemangioma [9].
In our case, preoperative diagnosis of angiosarcoma was not achieved even with two cytology specimen and one biopsy. Each showed only fibrosis and inflammatory changes.
Wijnmaalen et al. reported background breast tissue inflammation caused by radiation in 8 cases of angiosarcoma, also finding fibrosis in 7 (88%) [10].
When angiosarcoma occurs against such a background, correct diagnosis may be difficult. Marked fibrosis and the rather small number of sarcoma cells in the breast tumor in the present case may be why bioptic diagnosis was difficult. In this case, the tumor was considered to be high grade from its clinically aggressive nature, and as histology revealed abundant mitosis.
The potential difficulties of diagnosing angiosarcoma against background fibrosis should be kept in mind, particularly in tumors consisted of rather small number of angiosarcoma cells, and marked fibrosis caused by radiation.
Concerning treatment, initial radical surgery currently represents the only effective treatment for achieving long-term survival in angiosarcoma patients [8].
Other reports have indicated that hyperfractionated radiotherapy of secondary high-grade angiosarcoma has resulted in reduced cell repopulation [11].
Adjuvant chemotherapy, paclitaxel for example, may also reduce the local recurrence rate [1]. In the present case, paclitaxel was performed.
Angiosarcoma has a poor prognosis, and median overall survival is 22–37 months [3]. Vorburger et al. reported that tumors 5 cm or smaller in diameter have a better prognosis than those larger than 5 cm [12].
The clinicopathologic features of 60 reported Japanese angiosarcoma cases were reviewed. The relation between tumor diameter and prognosis was analyzed by Kaplan-Meier method (SPSS®; IBM, Armonk, NY).
The mean observation period was 22.7 months (2–84 months), the mean age was 41.5 years old (18–87). Mean tumor diameter was 7.8 cm (0.7–26 cm). The operations performed were mastectomy in 54 cases (90.0%), partial mastectomy in 6 cases (10.0%). Axillary dissection was performed in 28 cases (46.7%), but there were no lymph node metastasis. Distant metastasis was found in 32 (53.3%) cases (Table 1). Kaplan-Meier analysis showed significantly better prognosis in patients with a tumor 2 cm or smaller in diameter (p = 0.0457) [13].
Table 1.
Clinicopathologic features of 60 Japanese angiosarcoma cases.
| Author | Year | Age of patient at diagnosis | Tumor diameter at diagnosis | Operative method | Site of recurrence | Observation period (Months) | Outcome |
|---|---|---|---|---|---|---|---|
| Sakaguchi | 1932 | 27 | 16 | Mastectomy | Surgical site, Contralateral breast, Bilateral ovary | 37 | Dead |
| Ishizuka/Watanabe | 1965/1974 | 52 | 12 | Mastectomy + Axillary dissection | Lung, Bone | 51 | Dead |
| Tanaka/Hamazaki | 1971/1978 | 22 | 13 | Mastectomy + Axillary dissection | Surgical site, Lung, Liver | 37 | Dead |
| Watanabe | 1973 | 51 | 3 | Mastectomy | Stomach, Lung, Pharynx, Uterine Cervix, Chest wall | 14 | Dead |
| Kunii | 1982 | 42 | 1 | Mastectomy + Axillary dissection | None | 11 | Alive |
| Koike | 1982 | 21 | 7.2 | Mastectomy + Axillary dissection | Surgical site, Chest wall | 24 | Dead |
| Shikama | 1986 | 28 | 18 | Mastectomy | Contralateral breast, Bone, Lung, Bilateral ovary | 15 | Dead |
| Sakuma | 1987 | 19 | 22 | Mastectomy | Surgical site, Contralateral breast, Chest wall | 30 | Alive |
| Yamashina | 1987 | 71 | 16 | Mastectomy + Axillary dissection | Lung | 6 | Alive |
| Tamura | 1987 | 24 | 9 | Mastectomy + Axillary dissection | None | 26 | Alive |
| Kawazu | 1988 | 42 | 7.3 | Mastectomy + Axillary dissection | Skin | 12 | Alive |
| Matoba/Shou | 1988 | 25 | 16 | Mastectomy + Axillary dissection | Surgical site, Skin, Bilateral ovary | 27 | Alive |
| Iseki | 1989 | 60 | 1.3 | Mastectomy + Axillary dissection | None | 8 | Alive |
| Yokoyama | 1990 | 33 | 9 | Mastectomy + Axillary dissection | Surgical site, Skin | 21 | Alive |
| Ishikawa | 1990 | 26 | 20 | Mastectomy + Axillary dissection | Lung, Skin | 5 | Dead |
| Nagahara | 1993 | 19 | 8 | Mastectomy + Axillary dissection | Lung, Bone, Liver, Skin | 10 | Dead |
| Okanobu | 1994 | 34 | 1.3 | Mastectomy + Axillary dissection | None | 18 | Alive |
| Hayashi | 1994 | 52 | 9 | Mastectomy + Axillary dissection | Lung | 2.5 | Dead |
| Hakata | 1995 | 43 | 1 | Mastectomy + Axillary dissection | Skin | 15 | Alive |
| Nakano | 1995 | 82 | 2 | Mastectomy | Lung, Bone, Liver, Skin | 3 | Dead |
| Ishizawa | 1995 | 38 | 4 | Lumpectomy | Residual Breast, Liver | 39 | Dead |
| Tachibana | 1996 | 24 | 10 | Mastectomy + Axillary dissection | None | 10 | Alive |
| Nagasawa | 1997 | 64 | 2 | Mastectomy | None | 10 | Alive |
| Murakawa | 1997 | 46 | 1.7 | Mastectomy | None | 60 | Alive |
| Michigami | 1997 | 24 | 10 | Lumpectomy | Surgical site, Skin, Contralateral breast | 12 | Alive |
| Kagawa | 1997 | 35 | 5.5 | Lumpectomy | Surgical site, Skin, Chest wall, Lung, Mediastinum, Digestive tract, Thyroid, Kidney, Adrenal gland, Ovary, Gall bladder, Spleen, Bone | 6 | Dead |
| Takanashi | 1997 | 32 | 10 | Mastectomy + Axillary dissection | Head skin, Ovary, Lung, Liver | 8 | Dead |
| Takanashi | 1997 | 25 | 15 | Mastectomy + Axillary dissection | Ovary, Bone, Liver, Pelvis | 34 | Dead |
| Shichinohe | 1998 | 34 | 0.7 | Partial mastectomy + Axillary dissectionn | None | 60 | Alive |
| Kiyono T | 1998 | 64 | 26 | Mastectomy | None | 84 | Alive |
| Yamamoto | 1998 | 24 | 6 | Mastectomy | Ipsilateral breast, Lung, Liver, Bone | 29 | Dead |
| Tanaka | 1999 | 26 | 8 | Mastectomy + Axillary dissection | None | 12 | Alive |
| Yamada | 1999 | 42 | 6 | Mastectomy + Axillary dissection | Contralateral breast, Lung, Liver, Adrenal gland, Bone | 15 | Dead |
| Yoshida | 2000 | 41 | 1.5 | Partial mastectomy + Axillary dissectionn | None | 60 | Alive |
| Abe | 2000 | 79 | 2 | Mastectomy | Subcutaneous fat of ipsilateral breast | 6 | Alive |
| Ueki | 2000 | 19 | 1.5 | Mastectomy | None | 30 | Alive |
| Tsumagari K | 2000 | 52 | 6 | Mastectomy + Axillary dissection | None | 9 | Alive |
| Tsumagari K | 2000 | 26 | 11 | Mastectomy + Axillary dissection | None | 18 | Alive |
| Tsumagari K | 2000 | 19 | 9 | Mastectomy + Axillary dissection | Bone, Liver, Lung, Brain, Surgical site, Nasal cavity | 27 | Dead |
| Kitamura | 2001 | 46 | Hand fist size | Mastectomy | Skin, Liver, Lung | 24 | Dead |
| Fujisawa | 2003 | 65 | 5 | Mastectomy | Lung, Skin | 16 | Alive |
| Nishiyama | 2003 | 18 | 14 | Mastectomy | Surgical site, Lung, Liver | 9 | Dead |
| Nakayama | 2004 | 40 | 3 | Mastectomy | Contralateral breast | 51 | Alive |
| Nakata | 2004 | 51 | 8 | Mastectomy + Axillary dissection | None | 16 | Alive |
| Yoneyama | 2005 | 32 | 5.5 | Mastectomy + Axillary dissection | None | 72 | Alive |
| Sadashima | 2007 | 60 | 2 | Mastectomy | None | 12 | Alive |
| Ueda S | 2008 | 28 | 7 | Mastectomy | Bone | 6 | Alive |
| Yoshida C | 2008 | 38 | 9 | Mastectomy | None | 2 | Alive |
| Ueda S | 2008 | 44 | 4 | Mastectomy | Bilateral breast, Bilateral ovary, Bone, Lung, Liver | 41 | Dead |
| Sakamoto M | 2009 | 32 | 8 | Mastectomy | None | 4 | Alive |
| Takenaka M | 2009 | 87 | 11 | Mastectomy | Lung | 2 | Dead |
| Miyasaka M | 2010 | 59 | 12 | Mastectomy | None | 17 | Alive |
| Komatsu M | 2010 | 54 | 5.5 | Mastectomy | None | 2 | Alive |
| Oda T | 2011 | 27 | 5 | Mastectomy + Tissue expander | Ovary, Bone, Liver, Pelvis | 3 | Alive |
| Satou F | 2011 | 61 | 10 | Partial mastectomy + sentinel lymph node biopsy | Ovary, Bone, Liver, Pelvis | 14 | Alive |
| Hosono Y | 2011 | 47 | 3.2 | Mastectomy + Sentinel lymph node biopsy | Lung, Liver | 29 | Dead |
| Hoskimoto N | 2012 | 27 | 7 | Mastectomy | Skin | 40 | Alive |
| Okaminami Y | 2013 | 33 | 5 | Mastectomy | None | 42 | Alive |
| Mizumoto S | 2014 | 78 | 16 | Mastectomy + lymph node sampling | Rt knee, pleura | 13 | Dead |
| Our case | 2015 | 73 | 3.5 | Mastectomy | None | 60 | Alive |
Conflict of interest
None.
Funding
None.
Ethical approval
This study has been approved by the ethics committee (Ethics and Compliance Committee of Shizuoka Saiseikai General Hospital). Reference No. 27-17-01.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. I can provide this should the Editor ask to see it.
Author contribution
Takaaki Ito and Kenichiro Tanaka: study concept or design, writing the paper, operating surgeon.
Kiyoshi Suzumura: data analysis or interpretation, operating surgeon.
Shouji Hoshi: histological examination.
Yoshichika Okamoto, Koji Oda and Masaki Terasaki: study concept or design, critical revision.
Guarantor
Takaaki Ito, Kenichiro Tanaka.
Contributor Information
Takaaki Ito, Email: takaakiito@hotmail.com.
Kenichiro Tanaka, Email: ken.tanaka@acc-aichi.com.
Kiyoshi Suzumura, Email: k156574@siz.saiseikai.or.jp.
Yoshichika Okamoto, Email: y153575@siz.saiseikai.or.jp.
Koji Oda, Email: k-oda@acc-aichi.com.
Syouji Hoshi, Email: s100072@siz.saiseikai.or.jp.
Masaki Terasaki, Email: m129348@siz.saiseikai.or.jp.
References
- 1.Monroe A.T., Feigenberg S.J., Mendenhall N.P. Angiosarcoma after breast-conserving therapy. Cancer. 2003;97(8):1832–1840. doi: 10.1002/cncr.11277. [DOI] [PubMed] [Google Scholar]
- 2.Hunter T.B., Martin P.C., Dietzen C.D., Tyler L.T. Angiosarcoma of the breast. Two case of reports and a review of the literature. Cancer. 1985;56(8):2099–2106. doi: 10.1002/1097-0142(19851015)56:8<2099::aid-cncr2820560836>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 3.Strobbe L.A., Peterse H.L., van Tinteren H., Wijnmaalen A., Rutgers E.J. Angiosarcoma of the breast after conservation therapy for invasive cancer: the incidence and outcome. Breast Cancer Res. Treat. 1998;47(2):101–109. doi: 10.1023/a:1005997017102. [DOI] [PubMed] [Google Scholar]
- 4.Esler-Brauer L., Jaggernauth W., Zeitouni N.C. Angiosarcoma developing after conservative treatment for breast carcinoma: case report with review of the current literature. Dermatol. Surg. 2007;33(6):749–755. doi: 10.1111/j.1524-4725.2007.33156.x. [DOI] [PubMed] [Google Scholar]
- 5.Boyan W., Farr M., Georges R. High grade angiosarcoma fifteen years after breast conservation therapy with radiation therapy: a case report. Int. J. Surg. Case Rep. 2014;5(12):1176–1177. doi: 10.1016/j.ijscr.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francisco I.B., Joseph J.E., Jeff F. Optimal surgical management for occult breast carcinoma: a meta-analysis. Ann. Surg. Oncol. 2016;(February) doi: 10.1245/s10434-016-5104-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Natasha M.R., Dalliah M.B., Angela R.L. Breast conservation in the setting of contemporary multiimodality treatment provides excellent outcomes for patients with occult primary breast cancer. Surg. Oncol. 2015;22:90–95. doi: 10.1245/s10434-014-3991-0. [DOI] [PubMed] [Google Scholar]
- 8.Polgar C., Orosz Z., Szerdahelyi A., Fodor J., Major T., Magori A. Postirradiation angiosarcoma of the chest wall and breast: issues of radiogenic origin, diagnosis and treatment in two cases. Oncology. 2001;60(1):31–34. doi: 10.1159/000055293. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura R., Nagashima T., Sakakibara M., Nakano S., Tanabe N., Fujimoto H. Angiosarcoma arising in the breast following breast-conserving surgery with radiation for breast carcinoma. Breast Cancer. 2007;14(2):245–249. doi: 10.2325/jbcs.914. [DOI] [PubMed] [Google Scholar]
- 10.Wijnmaalen A., Ooijen B.V., Geel B.N., Henzen-Logmans S.C., Treurniet-Donker A.D. Angiosarcoma of the breast following lumpectomy, axillary lymph node dissection, and radiotherapy for primary breast cancer: three case reports and a review of the literature. Int. J. Radiat. Oncol. Biol. Phys. 1993;26(1):135–139. doi: 10.1016/0360-3016(93)90184-w. [DOI] [PubMed] [Google Scholar]
- 11.Glazebrook K., Magut M.J., Reynolds C. Angiosarcoma of the breast. Am. J. Roentgenol. 2008;190(2):533–538. doi: 10.2214/AJR.07.2909. [DOI] [PubMed] [Google Scholar]
- 12.Vorburger S.A., Xing Y., Hunt K.K. Angiosarcoma of the breast. Cancer. 2005;104:2682–2688. doi: 10.1002/cncr.21531. [DOI] [PubMed] [Google Scholar]
- 13.Ueda S., Tamaki Y., Okishiro M., Okabe T., Noguchi S. Primary angiosarcoma of the breast—a report of two cases. J. Jpn. Surg. Assoc. 2008;69:302–307. [Google Scholar]