Abstract
Objective
Both electrical stimuli (endogenous and exogenous) and topographical cues are instructive to axonal extension. This report, for the first time, investigated the relative dominance of directional topographical guidance cues and directional electrical cues to enhance and/or direct primary neurite extension. We hypothesized the combination of electrical stimulation with electrospun fiber topography would induce longer neurite extension from DRG neurons than the presence of electrical stimulation or aligned topography alone.
Approach
To test the hypothesis, neurite outgrowth was examined on laminin-coated poly-L-lactide (PLLA) films or electrospun fibers (2 μm in diameter) in the presence or absence of electrical stimulation. Immunostained neurons were semi-automatically traced using Neurolucida software and morphology was evaluated.
Results
Neurite extension increased 74% on the aligned fibers compared to film controls. Stimulation alone increased outgrowth by 32% on films or fibers relative to unstimulated film controls. The co-presentation of topographical (fibers) with biophysical (electrical stimulation) cues resulted in a synergistic 126% increase in outgrowth relative to unstimulated film controls. Field polarity had no influence on the directionality of neurite, indicating topographical cues are responsible to guide neurite extension.
Significance
Both cues (electrical stimulation and fiber geometry) are modular in nature and can be synergistically applied in conjunction with other common methods in regenerative medicine such as controlled release of growth factors to further influence axonal growth in vivo. The combined application of electrical and aligned fiber topographical guidance cues described herein, if translated in vivo, could provide a more supportive environment for directed and robust axonal regeneration following peripheral nerve injury.
1.1 Introduction
Peripheral nervous system (PNS) injuries affect hundreds of thousands of people worldwide each year (Selecki, Ring et al. 1982; Noble, Munro et al. 1998; Robinson 2000; Burkhard Schlosshauer 2006; Portincasa 2007; Yegiyants, Dayicioglu et al. 2010). To restore function following injury, regenerating axons must navigate and bridge the injury site to re-connect with appropriate distal targets and peripheral glia (Schwann cells) must re-myelinate the regenerating axons. Following mild crush injuries, regenerating axons are often able to spontaneously regenerate without surgical intervention, however for severe large-gap nerve transections (>4 cm) nerve autografts are implanted to bridge the gap and create a more supportive environment for axonal extension (Reyes, Sosa et al. 2005; Portincasa 2007). Unfortunately, there is a limited supply of autologous graft material, and autografts may be unavailable following extensive trauma or in patients suffering from diseases impacting peripheral nerve quality (e.g. diabetes) (Kennedy and Zochodne 2005). The lack of available autologous donor tissue, associated co-morbidity, and limited functional recovery motivates the development of new treatment strategies such as engineered acellular nerve guidance channels.
Multiple types of nerve guidance channels have been developed and extensively studied within models of peripheral nerve injury, but to date, are only approved for use for small-gap injuries (<3 cm) as regeneration within large-gap injuries does not reach the functional recovery of gold-standard autographs (Chiu and Strauch 1990; Burkhard Schlosshauer 2006; Moore, Kasukurthi et al. 2009; Ray, Kasukurthi et al. 2010; Ray and Mackinnon 2010). The introduction of topographical features within guidance channels both direct and enhance neurite outgrowth (Goldner, Bruder et al. 2006; Hoffman-Kim, Mitchel et al. 2010; Tuft, Li et al. 2013). Electrospinning is one approach and by changing electrospinning parameters (such as polymer concentration within the electrospinning solvent, voltage drop between the needle tip and collection plate, humidity levels, or syringe pump flow rate), fiber surface structure and diameter can be altered (Wang, Mullins et al. 2010; Schaub, Britton et al. 2013). Furthermore, the use of a rotating collection mandrel can align the fibers, which is particularly useful for directing neurite outgrowth (Pham, Sharma et al. 2006). Using different electrospinning solvents allows for the fabrication of fibers of varying polymer type including synthetic polymers, natural polymers, or combinations thereof (Pham, Sharma et al. 2006; Kumbar, James et al. 2008; Sill and von Recum 2008). Careful attention to generating electrospun fiber samples consisting of highly aligned fibers is important, as crossed fibers can restrict directed axonal extension (Wang, Mullins, 2009). Fiber diameter can also influence neurite outgrowth. Small diameter PLLA fibers (293 nm) have been shown to support less extensive neurite outgrowth from chick DRG than medium (793 nm) or larger diameter fibers (1325 nm) (Wang, Mullins 2010). Therefore, both fiber alignment and diameter are important parameters to consider if electrospun fibers are incorporated within a guidance channel.
Like topographical features, other neurite stimulatory approaches, such as endogenous electric fields and/or exogenous electrical stimulation, are known to influence cell migration and neurite outgrowth (Hinkle, McCaig et al. 1981; McCaig and Rajnicek 1991; McCaig, Rajnicek et al. 2005). During development, natural current gradients created by epithelial layers act to direct cell migration, and perturbation of these endogenous signals disrupts proper limb bud formation demonstrating the importance of this naturally occurring cue (Stump and Robinson 1983; Hotary and Robinson 1990; Hotary and Robinson 1994). In vitro, neurons exhibit varying levels of responsiveness to exogenous electrical stimulation, exhibiting behaviors such as: directional outgrowth (Jaffe and Poo 1979; McCaig and Rajnicek 1991; Cork, McGinnis et al. 1994; Pan and Borgens 2012), increased outgrowth (Wood and Willits 2009), increased neurite branching (Hinkle, McCaig et al. 1981), or no response (Cormie and Robinson 2007). Many of these studies use isolated cells from fish or amphibians, model organisms known to exhibit more robust regenerative properties in comparison to mammalian models. Mammalian neurons may exhibit varied sensitivity to stimulation parameters (magnitude, duration, pulse duration, current, field) and/or inherent differences in responsiveness may be attributed to age, species, or specific cell differences (Patel and Poo 1982; McCaig 1990). We have previously reported significant increases in total neurite outgrowth and length for rat sensory neurons stimulated at 50 mV/mm (1 mA, 8 hour duration) relative to unstimulated neurons with no directional bias observed (no anode or cathodal bias, and/or no bias parallel or perpendicular to the field generated) (Koppes, Seggio et al. 2011). Since exogenous electrical stimulation promoted greater outgrowth in a non-directional manner, the incorporation of topographical cues to direct axonal outgrowth may be beneficial to promote and direct axons within a guidance channel.
A single approach to nerve repair (e.g. controlled release of a soluble factor, substrate composition, topographical cues, mechanical cues, biophysical forces, or support cells) may not sufficiently overcome all the challenges required to restore nerve function following injury (Ceballos, Navarro et al. 1999; Fouad, Schnell et al. 2005). Miller and colleagues described the benefit of combining physical cues with biochemical cues to synergistically direct and enhance neurite outgrowth, with 95% of neurites extending preferentially within laminin coated polymeric microgrooves (Miller, Jeftinija et al. 2002). Similar benefits to neurite outgrowth were observed on laminin or fibronectin coated electrospun fibers, where protein presence supports longer neurite outgrowth in comparison untreated fibers (Rangappa, Romero et al. 2000; Mukhatyar, Salmeron-Sanchez et al. 2011). In this work, the combination of laminin coated, aligned electrospun fibers with simultaneous exogenous electrical stimulation were explored to examine the impact of biophysical and topographical cues on neurite outgrowth. Previously, we demonstrated that neurite outgrowth is independently enhanced by both aligned electrospun PLLA fibers (Hurtado, Cregg et al. 2011) and by exogenous electrical stimulation (50 mV/mm) (Koppes, Seggio et al. 2011). We hypothesize that the combination (topography with exogenous electrical stimulation) will synergistically enhance neurite outgrowth in a directional manner. Furthermore, this study will elucidate the dominance of either electrical or topographical cues by presenting aligned topography oriented parallel and perpendicular to the exogenous electric field. Characterizing the benefits of co-presenting electrical and topographical cues will provide insight into relative importance and sensitivity necessary to develop multi-cue guidance channels to support nerve repair.
1.2 Materials and Methods
1.2.1 Isolation and Culture of Dissociated Dorsal Root Ganglia Neurons
Dorsal root ganglia (DRG) were isolated from Sprague Dawley P2 neonatal rats as previously described (Taconic Farms, Inc.) (Seggio, Ellison et al. 2008; Seggio, Narayanaswamy et al. 2010; Koppes, Seggio et al. 2011). Whole DRG were first digested in a trypsin-collagenase A solution (0.1% trypsin (MediaTech, Inc.) and 1 mg/mL collagenase (Sigma Aldrich) diluted in 1x Hanks Balanced Salt Solution (HBSS; Fisher Scientific, Pittsburgh, PA), followed by a second 0.1% trypsin digestion and subsequent mechanical trituration in Dulbecco’s Modified Eagle Medium (DMEM; MediaTech, Inc.). The dissociated neurons were cryopreserved (1×106 cells/mL) for up to 12 months for use on-demand.
1.2.2 Electrospun Poly-L-Lactic Acid Fibers
Aligned, electrospun fiber scaffolds were fabricated as described previously (Wang, Mullins et al. 2009). In brief, fibers were produced by electrospinning an 8% (wt./wt.) polymer solution of poly-L-lactide (PLLA, Natureworks™; grade 6201D, Cargill Dow LLC, Minnetonka, MN) dissolved in a 1:1 mixture of dichloromethane and chloroform (Sigma-Aldrich, St. Louis, MO). The PLLA solution was loaded into a syringe and delivered at a rate of 2.0 mL/hour through a 22-gauge needle insulated with polymer tubing. Fibers were collected on acid-etched, 10 × 20 mm glass coverslips (Belco Glass, Vineland, New Jersey, USA) coated with a PLLA thin film. The film was produced by evaporation casting a 4% (wt./wt.) PLLA solution dissolved in 1:1 mixture of dichloromethane and chloroform (Figure 1A). The PLLA-coated coverslips were secured to a grounded aluminum collector disc with double-sided tape (3M, St. Paul, MN). The distance between the needle tip and the collector disc was set at 6 cm. A high voltage power supply connected to the needle was set at 10 kV, and the rotation speed of the collector wheel was set to 1000 rpm. Fiber samples were spun for 20 minutes. Parallel fibers were generated by aligning the long edge of the glass cover glass with the aluminum collector (Figure 1B). Perpendicular fibers were generated by rotating the long edge of glass cover glass 90° to the aluminum collector (Figure 1C). All fiber samples were sterilized using an ethylene oxide sterilization system (Andersen Sterilizers, Inc., Haw River, NC) for 12 hours prior to cell culture and degassed for a minimum of 24 hours prior to use. Samples were stored in desiccated ambient air for up to two weeks.
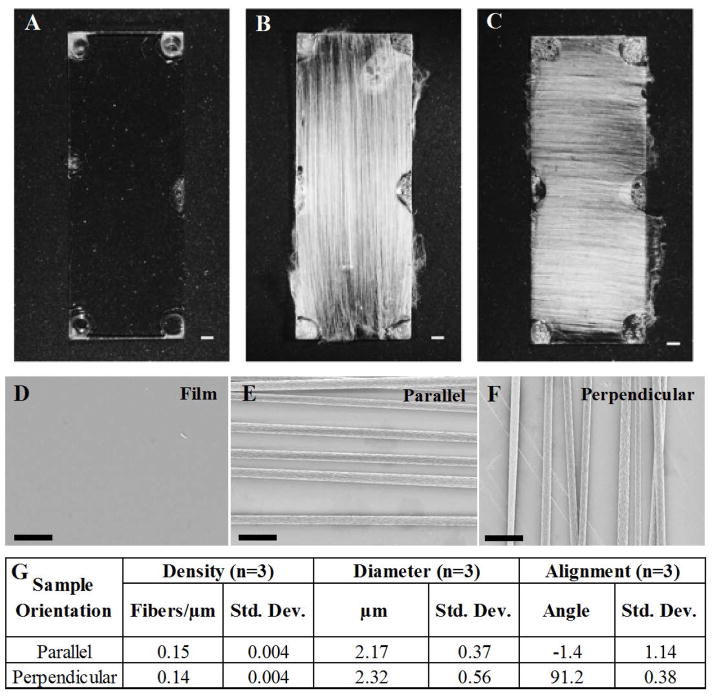
Figure 1.
Morphology of electrospun PLLA fibers. Brightfield microscopy of PLLA films (A) exhibit a uniform flat topography while aligned electrospun PLLA fibers are oriented either (B) parallel or (C) perpendicular to the long axis of the glass coverslip (bar = 1 mm). (D) PLLA films exhibit a uniform flat topography while electrospun PLLA fibers oriented either (E) parallel or (F) perpendicular to the long axis of the glass coverslip and applied electrical stimulus exhibit high degrees of alignment. (G) Images were captured using a Carl Zeiss Supra55 with Direct Write Attachment with a 5.00 mm working distance, 20 μm aperture diameter, and an accelerating voltage of 3kV. n=3, m=3, bar = 10 μm. The aligned fibers images were analyzed using ImageJ and the fibers exhibit similar density, diameter, and alignment regardless of fiber orientation (parallel (0°) or perpendicular (90°) to long axis of the glass coverslip).
1.2.3 Scanning Electron Microscopy (SEM) Imaging of Fiber Samples
SEM images were captured using a Carl Zeiss Supra55. The fiber samples were attached to glass slides using conductive copper tape (3M, St. Paul, MN), and the edges of the fibers were secured to the copper tape using Pelco® Colloidal Silver Liquid (Ted Pella Inc., Redding, CA). A Denton Desk IV Sputterer (Denton Vacuum, Moorestown, NJ) coated all the fiber samples with a 5 nm conductive coating of platinum prior to SEM analysis. The SEM was operated using a 5.00 mm working distance, 20 μm aperture diameter, and an accelerating voltage of 3kV. Images were captured using a 3:1 mixing ratio of an in-lens secondary electron detector and Robinson backscattered electron detector. Line integration scanning (n=50 per line) at a reduced scanning rate was used to enhance image resolution.
1.2.4 Characterization of Electrospun Fiber Alignment and Diameter
Electrospun fiber characterization was performed as previously described (Wang, Mullins et al. 2009). Electrospun fiber samples were produced in three batches of fifteen samples per batch. One sample from each batch was chosen at random for fiber characterization using scanning electron microscopy (5kX magnification). Each sample was imaged at three randomly selected locations generating nine images per condition. Fiber alignment was quantified by measuring the angle between a reference line drawn parallel to the long axis of the rectangular glass co-verslip using ImageJ (NIH) with a minimum of twenty fibers per image transecting the reference line. Fiber samples were considered highly aligned if 95% of the fibers were ±10° from the average angle relative to the reference line. Fiber density was calculated by counting the number of fibers that cross a reference line of known length. Five lines were drawn over each image and the number of fibers per unit length was calculated. The diameters of twenty fibers per image were measured in ImageJ.
1.2.5 Electrical Stimulation Culture Chamber Design and Construction
Electrical stimulation (ES) culture chambers used in this study were previously described in detail, including a schematic of the chamber (Koppes, Seggio et al. 2011). In brief, PDMS was cured in rectangular polystyrene plates (Thermo Fisher Scientific, Waltham, MA). Two channels (7 mm × 30 mm × 1.5 mm) were excised from the cured PDMS (1 experimental, 1 control) and 2% agarose salt bridges were formed on plates to minimize cell exposure to potential pH changes (Koppes 2011). An additional external salt bridge was placed on the anodal side of the experimental chamber to both add resistance and further minimize electrical by-products. A bench-top DC power supply (Marlin P. Jones & Assoc., Inc., Lake Park, FL) was used to supply current through two platinum electrodes (1 anode, 1 cathode; 1 mm ID each, Biological Grade, Sigma Aldrich). Two platinum reference electrodes were placed at the ends of each chamber to measure the voltage drop across the channel during each experiment via SCC-FT01 feed through components and LabView (National Instruments, Austin, TX). The chamber provides a constant electric field and the reference electrodes provide real-time read out to confirm the stability of the field produced. DC stimulation was chosen to evaluate if directionally applied electric fields impact anodal or cathodal neurite extension.
1.2.6 Electrical Stimulation of Neurons on Electrospun Fibers
Ethylene oxide-sterilized PLLA fibers and films were plasma-treated using a Harrick Plasma Cleaner (Harrick Plasma, Ithaca, NY) for 60 seconds to enhance laminin adsorption. Plasma-treated samples were coated with 50 μg/mL of laminin (BD Biosciences, San Jose, CA) for 60 minutes and rinsed three times with HBSS (5 min each). Dissociated neurons were seeded at 7.1 × 103 cells/cm2 on the laminin-coated, electrospun fiber or film coated coverslips in growth medium (DMEM supplemented with 10% Fetal Bovine Serum (FBS; Hyclone, Logan, UT), 2 mM L-glutamine (L-glut, Hyclone), 50 U/ml penicillin/streptomycin (P/S, MediaTech, Inc.), 10 μg/mL Bovine Pituitary Extract (BPE; BD Biosciences, San Jose, CA), 6.6 mM Forskolin (Sigma Chemical) and supplemented with 50 ng/mL 2.5S nerve growth factor (NGF; Invitro-gen). This seeding density allows for the imaging and subsequent quantification of individual neuron morphology and limits neuron-neuron contact. Neurons were incubated (37° C, 5% CO2) for 4 hours for cell attachment with no visible neurite extension. Prior to electrical stimulation, samples were rinsed with warm PBS to remove non-adherent cells. Next, warm growth medium was gently added, and samples were exposed to a constant DC electrical stimulus for 8 hours at 50 mV/mm, DC (1mA). Paired controls were treated similarly, but remained unstimulated. 50 mV/mm was selected based on previous results where both total neurite outgrowth and neurite length was maximized within the range of stimuli tested (0–100 mV/mm) on laminin-coated cover glass (Koppes, Seggio et al. 2011). Following stimulation, the samples were transferred to 6 well dishes containing fresh medium and cultured for an additional 12 hours (0 mV/mm) prior to fixation.
1.2.7 Immunofluorescent Staining
The samples were fixed with a 4% paraformaldehyde, 4% sucrose (Sigma Aldrich) in 2xPHEM buffer (60mM PIPES, 25mM HEPES, 10mM EGTA, 2mM MgSO4, pH 7.0 with KOH in DI H2O; Sigma Aldrich) for 15 minutes at room temperature. Fixed samples were rinsed three times with warm HBSS (5 minutes each), permeabilized for 10 minutes with 0.1% Triton X-100 (Sigma Chemical) in PBS and blocked with 2.5% goat serum (GS) diluted in HBSS (Invitrogen) for 1 hour at room temperature. To visualize neurons, samples were incubated for 30 minutes at room temperature with mouse anti-β-III-tubulin primary antibody (1:500 in 2.5% GS, Invitro-gen), rinsed in HBSS (3x, 5 minutes each) and incubated for 30 minutes at room temperature with goat-anti-mouse Alexa Fluor 488 IgG2b secondary antibody (Invitrogen) (1:1000 v:v in 2.5% GS). Samples were rinsed in HBSS (3x, 5 minutes each) prior to mounting with Prolong Gold Anti-fade containing 4′,6-diamidino-2-phenylindol (DAPI; Invitrogen) to label all cell nuclei. The sealed samples were stored at −20°C prior to imaging.
1.2.8 Imaging and Analysis of Neuron Morphometrics
For each experimental condition and control (film, perpendicular fiber, parallel fiber +/− electrical stimulation), a minimum of 20 individual neurons (not in contact with adjacent neurons) were imaged using a 20x dry objective on an Olympus IX81 inverted microscope (Olympus, Center Valley, PA). Images were acquired with Metamorph image acquisition software (Molecular Devices, Downingtown, PA). A minimum of 10 neurons per experiment (n=3) were randomly selected from the pool of 20 neurons imaged for each condition (films, perpendicular fibers, parallel fibers) resulting in a minimum of 30 neurons per condition. Samples were blinded and randomly selected for analysis using a random number generator in Excel. Images were then semi-automatically traced using Neurolucida software (MBF Bioscience, Wilmington, VT) and quantitatively analyzed with respect to basic morphometric features such as: total neurite outgrowth (sum of all outgrowth from each individual neuron), longest neurite, number of primary neurites, branching, and orientation of neurite outgrowth as described previously (Seggio, Ellison et al. 2008; Koppes, Seggio et al. 2011). The entirety of each neuron was traced, and neurite extension was binned every 0.2 μm increments extending outward from the neuronal cell body and plotted as a polar histogram including all outgrowth extending 0–360° prior to exportation to Excel for statistical analysis. Biased neurite extension towards the anode or cathode was examined by binning the total outgrowth in the half the polar plot facing the cathode (centered at 0°, 270°–90°) or anode (centered at 180°, 90–270°). Neurite outgrowth parallel to the applied field was calculated as all outgrowth within +/− 10° from direction of field, centered at 0° and 180° for fibers parallel to the applied field and 90° and 270° for fibers perpendicular to the applied field.
1.2.9 Statistical Analysis
Neuron morphometric data was collected from three separate experiments with two conditions: electrically stimulated (50 mV/mm) and non-stimulated paired controls (0 mV/mm). To characterize directional neurite extension on the films versus fibers or preferentially towards the anode or cathode, a minimum of 10 neurons from 3 separate experiments were plotted as a polar histogram encompassing 360° of neurite extension. Outgrowth parallel or perpendicular to the field on aligned fibers was calculated as total growth within +/− 10° from direction of field (350–10° and 170–190° in 360° plot for parallel fibers; 80–100° and 260–280° in 360° plot for perpendicular fibers). Anderson-Darling normality tests were completed in Minitab (Minitab Inc. State College, PA). Statistical significance was determined using the Excel 2010 Analysis ToolPak to perform a two-way ANOVA with replication. p-values < 0.05 were considered statistically significant. A minimum of 10 neurons per group was determined using a 1-sample t-test (Minitab Inc. State College, PA.) in order for each experiment to have a 99% power to detect (α=0.05) a maximum difference of 251 μm within total outgrowth of control (unstimulated) neuron populations, assuming an average variability of 143 μm. Statistical analysis of fiber diameter was performed using Minitab Student Version 14. A two-sample t-test was used to test for differences in density and diameter of the electrospun fibers for the parallel and perpendicular PLLA fibers with p<0.05 considered significant (n=3).
1.3 Results
1.3.1 Electrospinning creates highly aligned and directional fibers
We started our work with characterization of the poly-L-lactic (PLLA) scaffolds. PLLA evaporation-cast films (Figure 1A), aligned fibers oriented along the long-axis of the glass coverslip (Figure 1B), and aligned fibers oriented perpendicular to the long axis of the glass coverslip (Figure 1C) were imaged using scanning electron microscopy. PLLA films exhibit a smooth and uniform surface from phase microscopy (Figure 1A) and SEM (Figure 1D); in contrast to the highly aligned electrospun PLLA fibers are oriented either parallel (Figure 1B & E) or perpendicular (Figure 1C & F) to the long axis of the glass coverslips with a uniform diameter (Figure 1G). The fibers printed parallel (denoted “parallel”) to the long axis of the glass coverslip had a mean diameter of 2.17+/−0.37 μm with a density of 0.15 fibers/μm (Figure 1G) whereas fibers printed perpendicular (denoted “perpendicular”) to the long-axis of the glass coverslip has a fiber diameter of 2.32+/−0.56 μm with a density of 0.14 fibers/μm (Figure 1G). Based on previous work (Wen and Tresco 2006; Wang, Mullins et al. 2009; Wang, Mullins et al. 2010), a fiber diameter of ~2 μm was selected as smaller diameter fibers (<1.3 μm) did not promote greater and longer outgrowth. A specific density of ~0.15 fibers/μm was selected for this study to minimize fiber crossing and neurite crossings between adjacent fibers, which would likely impact the anisotropic growth and possibly branching (Wang, Mullins et al. 2009). Fibers were highly aligned with 95% of the fibers oriented ±10° parallel or perpendicular to the long axis (Supplemental Figure 1). Regardless of fiber orientation, there were no statistically significant differences for fiber diameter, density (p>0.05, Figure 1G) and alignment. The fact that there were no statistical differences between fiber alignment, density, or diameter between fiber samples electrospun parallel or perpendicular to the long axis of the glass coverslip means that any differences in neurite outgrowth on the fiber substrates are not attributable to variations in the physical properties of the electrospun fiber scaffolds.
1.3.2 Neurite outgrowth is enhanced by topographical features and electrical stimulation
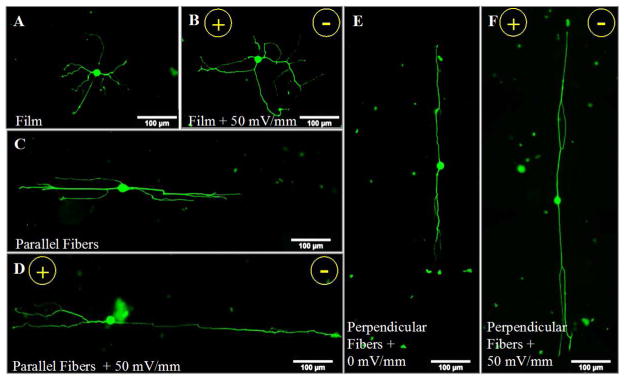
Next, to investigate neurite outgrowth in response to either aligned topographical cues from highly aligned electrospun fibers or exogenous electrical stimulation, dissociated DRG neurons were cultured on either PLLA films or aligned PLLA fibers with or without electrical stimulation. Representative immunofluorescent images show that electrical stimulation promoted greater outgrowth on the laminin coated PLLA films relative to the paired, unstimulated control (0 mV/mm) (Figure 2A & B). As expected, topographical cues presented by the aligned fibers supported longer processes, with more outgrowth extending along fibers (Figure 2C & E) compared to extension on planar films (Figure 2A). Co-presentation of electrical stimulation with the aligned fiber topography served to further enhance neurite outgrowth (Figure 2D & F). Regardless of fiber orientation (parallel or perpendicular to the electric field), electrically stimulated neurons extended greater neurite outgrowth on the aligned fibers in comparison to neurons presented with a single cue.
Figure 2.
Electrical stimulation and topographical cues from aligned fibers both serve to promote neurite outgrowth. Immunofluorescent images of neurons on laminin-coated PLLA films and electrospun fibers with or without electrical stimulation are shown. Neurite outgrowth on the unstimulated, control PLLA films (A) was shorter than outgrowth emanating from electrically stimulated neurons (50 mV/mm; 8 hr) (B). Neurite outgrowth was greater on the aligned fibers (C, E) in comparison to planar films (A). Combining electrospun fibers with electrical stimulation further enhanced neurite outgrowth (D, F), relative to either the aligned fibers (C, E) or electrical stimulation alone (B). No anodal/cathodal bias was observed following electri cal stimulation on films or fibers. Scale bar = 100 μm, green = β-III-tubulin neurons, direction of the electric field is shown (+/−).
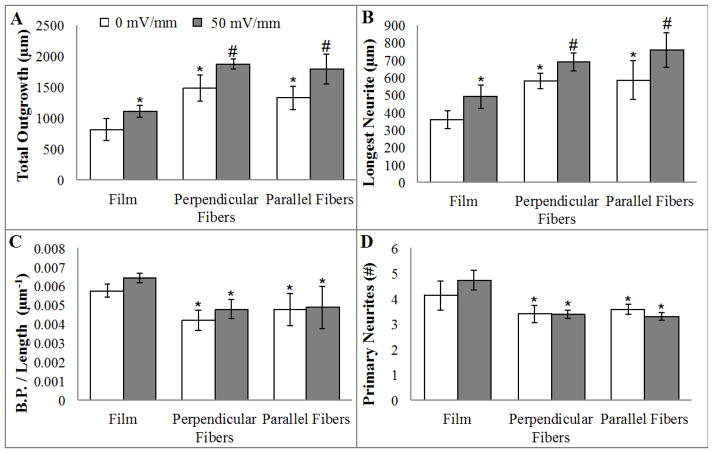
Quantification of neurite extension demonstrates that electrical stimulation results in an average 32% greater total neurite outgrowth on PLLA films compared to the paired unstimulated controls (Figure 3A, p<0.05). The fiber topography alone results in a 74% increase in total outgrowth on compared to neurons on unstimulated, paired film controls (p<0.05). Co-presenting both topographical features (fibers) with electrical stimulation significantly impacts total outgrowth, resulting in an average 126% increase in the total neurite outgrowth compared to outgrowth on unstimulated film controls (p<0.05). Differences observed in total neurite outgrowth paralleled changes measured in longest neurite (Figure 3B). The increase in outgrowth and longest neurite from electrical stimulation is not due to differences in neuronal morphology as no differences in branching and primary neurites were detected (Figure 3C & D). In contrast, neuron morphology is less complex on the aligned fibers exhibiting 19% less branching and 17% fewer primary neurites in comparison to growth on planar films (p<0.05).
Figure 3.
Co-presentation of topography and electrical stimulation significantly increase neurite outgrowth. (A) Total neurite outgrowth is enhanced by electrical stimulation alone on PLLA films. Neurite outgrowth is greater on the aligned electrospun PLLA fibers (topographical cues), and the co-presentation of both cues (electrical stimulation and the fibers) serves to further support greater outgrowth. A similar trend is seen in longest neurite length (B). Topographical cues but not electrical stimulation decreases the complexity of neuronal morphology. Both number of branch points (C) and number of primary neurites (D) are significantly reduced on aligned fibers. *= p<0.05 to flat films; #= p<0.05 to flat film and respective controls, n=3.
1.3.3 Neurite outgrowth is directed by fiber alignment and not by the DC electric field orientation
After investigating the effects of electrical stimulation or aligned fiber topography to impact increased neurite extension, it remained unknown if guidance cues from aligned fibers or the electric field is dominant in directing neurite outgrowth. Directed neurite outgrowth would be beneficial in improving speed and accuracy of targeted regeneration across large-gap nerve injuries. Neurons grown on PLLA films with (50 mV/mm) or without (0 mV/mm) electrical stimulation (Figure 4; Film) do not exhibit directional bias (anodal/cathodal nor parallel/perpendicular to the electric field) (Figure 4; Supplemental Figure 2; p>0.05). In contrast to unbiased outgrowth on flat films, more than 95% of all neurite outgrowth on the aligned fibers extends within ± 45° of the mean fiber orientation, tracking along the aligned fibers (p<0.05). Fiber distribution (Figure 1G, Supplemental Figure 1 and 3) was slightly broader due to fabrication for the perpendicular samples, however no gross changes in neurite outgrowth (length or direction) were observed between parallel or perpendicular fibers (Figure 3).
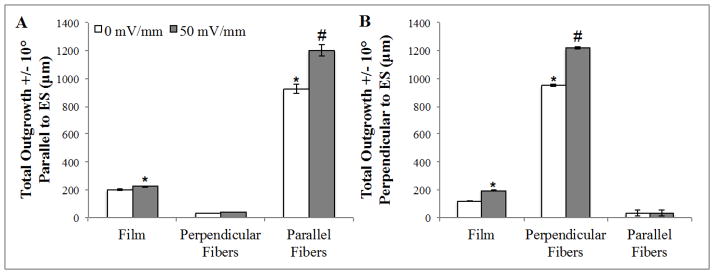
Figure 4.
Aligned topographical cues are dominant over directionality of an applied electrical stimulus. (A) Minimal outgrowth is observed on fibers aligned perpendicular to the direction of the electric field, indicating topographical cues are dominant in the observed increase in neurite outgrowth. Outgrowth was calculated as total growth within +/− 10° from direction of field (350–10°; 170–190° in 360° plot). (B) A similar trend is observed for neurite outgrowth on fibers aligned perpendicular to the electric field, with minimal growth observed parallel to the perpendicularly aligned fibers in the presence of absence of stimulation. Outgrowth was calculated +/− 10° perpendicular to the field (80–100°; 260–280° in 360° plot (Supplemental Figure 3))*= p<0.05 to respective controls; #= p<0.05 to all conditions, n=3.
Irrespective of culture surface (fiber or film), no cathodal/anodal bias in outgrowth was induced by DC electrical stimulation (Supplemental Figure 2; Figure 4). On the fibers, there is no difference in total outgrowth for fields that are parallel or perpendicular to fiber alignment (p=0.22). For example, when the field and fiber are aligned the majority (69.7%) of neurite outgrowth extends on the fibers (oriented +/−10° from the direction of the electric field) (Figure 4A, Supplemental Figure 3). In the case where the field is perpendicular to the fiber alignment, 64.8% of total neurite outgrowth extends on along the fibers (Figure 4B). To summarize, electrical stimulation and fiber topography both promoted greater outgrowth, but the directional guidance was solely due to fiber orientation.
1.4 Discussion
Enhancing neurite outgrowth via a single cue such as electrical stimulation or electrospun fibers is promising but has not translated to the clinic for large-gap nerve injuries. Due to the complex biological milieu, a multi-cue approach may better serve regenerating axons and efficiently support nerve repair (Ceballos, Navarro et al. 1999; Fouad, Schnell et al. 2005). Herein, we investigate the potential benefit of using topographical cues from electrospun fibers with exogenous electrical stimulation to enhance neurite outgrowth and determine the relative dominance of each individual cue. Implications of this investigation are threefold: 1) that co-presentation of electrical stimulation and aligned fibers serves to synergistically increase directed neurite outgrowth, 2) topographical guidance cues elicit longer neurites than electrical stimulation alone, and 3) the extent of neurite outgrowth does not depend on the whether the fibers are aligned parallel or perpendicular to the electrical field. Collectively, this experimental evidence strongly motivates the combined presentation of electrical stimulation with a guidance channel comprised of aligned fibers to treat peripheral nerve injury. This is an important extension of our previous work, which showed that neurite extension can be increased by either electrical stimulation on planar glass or on highly aligned electrospun fibers (Wang, Mullins et al. 2009; Wang, Mullins et al. 2010; Hurtado, Cregg et al. 2011; Koppes, Seggio et al. 2011; Koppes, Nordberg et al. 2013). This work is an important step towards understanding the relative importance of electrical and topographical guidance cues for neural engineering, and the combination thereof, which will aid effective clinical treatment strategies for peripheral nerve repair.
Towards improved functional recovery following large-gap injuries, the use of biomateri-als and/or electrical interventions requires several considerations including: optimized stimulation parameters (AC, DC, duration, strength), surface topography, incorporation of bioactive molecules (e.g. laminin), and determination of the impact of these cues on not only neurons but non-neuronal support cells that will also be present at the injury site. It is well established that topographical cues are able to significantly impact neurite extension, and current efforts aim to enhance the extent, speed, and accuracy of neurite outgrowth on fibers for improved nerve repair. For one example, the addition of aligned fibers with bioactive extra cellular matrix molecules (e.g. laminin) increases the rate of axonal regeneration, presumably due to improved cellular adhesion (Koh, Yong et al. 2008; Xie, MacEwan et al. 2009). As of yet, there is no definitive universal mechanism for axonal elongation in response to topographical guidance cues, however, contact dependence and axonal path finding mechanisms, such as adhesion-based focal adhesion kinase (FAK) signaling, may be activated in response to attachment on electro-spun fibers (Wang, Mullins et al. 2010; Suter and Miller 2011).
Due to the ability for topographical cues to enhance and direct neurite extension, we chose to utilize highly aligned electrospun fibers as one method to increase neurite extension from primary neurons. As expected, neurite outgrowth on aligned laminin coated PLLA fibers increased extension and longest neurite length significantly compared to growth on flat PLLA films (Figure 3–4). This enhancement in outgrowth is in agreement with those observed on other laminin coated electrospun fibers (poly(caprolactone) (PCL)) (Xie, MacEwan et al. 2009). Furthermore, Xie et al. reported both average neurite length and maximum neurite length increased nearly two-fold on aligned PCL fibers compared to randomly oriented fiber mat controls (Xie, MacEwan et al. 2009). In this study it is observed that on aligned fibers, neuronal morphology is less complex with a significant reduction in branching (19%) and primary neurites (17%) relative to neurons on PLLA films (Figure 3C & D). Based on this reduction in branching and sprouting, it is likely that part of the enhanced neurite extension on the fibers may be due to the neuron efficiently producing fewer processes and thus extension is localized and elongated (Liu, Chen et al. 2010). Since electrospun fibers alter the genetic expression of glial cells (Chew, Mi et al. 2008; Zuidema, Hyzinski-García et al. 2014), future work will focus on elucidating the molecular mechanisms by which electrospun fibers enhance neurite outgrowth (Zuidema, Hyzinski-García et al. 2014).
An alternative guidance cue, electrical stimulation, has gained interest in recent years due to the importance of endogenous currents in embryogenesis and wound healing as well as its clinical use in vivo (deep brain stimulation) for a variety of neurological disorders and neuro-degenerative diseases. Recently, exogenous bipolar electrical stimulation (1 hr, 20 Hz, 100 μS duration, variable amplitude) has been utilized in the less severe peripheral nerve crush injuries to treat human carpal tunnel syndrome following surgical release, with the stimulus applied alongside the decompressed median nerve resulting in enhanced axonal regeneration and conduction velocity (Gordon, Brushart et al. 2007; Gordon, Amirjani et al. 2010). However, over time the control unstimulated nerves catch up; implicating electrical stimulation may play a role in enhancing the rate, but not overall length or direction of neurite extension. Similarly, previous work in our lab has shown that voltage-gated calcium channels are activated on Schwann cells during DC electrical stimulation (50 mV/mm, 8 hours), resulting in an enhanced glial phenotype (e.g. enhanced neurotrophin release) that increases neurite extension on laminin coated glass in a uniform, non-directional manner (Koppes, Nordberg et al. 2013) (Koppes, Seggio et al. 2011). It is likely that electrical stimulation utilizes a different mechanism than adhesion based signaling on fibers, such as activation of voltage-gated ion channels known to influence cellular motility and neurotrophin release (Blackiston, McLaughlin et al. 2009; Huang, Ye et al. 2012; Koppes, Nordberg et al. 2013). Therefore, constant DC stimulation was used for this study to observe if anodal/cathodal biases exist on electrospun fibers (Does field polarity affect directional bias in growth?) or if differences in outgrowth due to field orientation exist (Is growth more robust along fibers aligned with or perpendicular to the applied field?). Results indicate that the increase in neurite extension due to electrical stimulation alone for neurons grown on either the aligned fibers or planar films was similar in magnitude (32%; Figure 3), and again neurons exhibited non-directional and uniform extension on planar films. Since electrical stimulation previously elicited enhanced, but non-directional neurite extension, we hypothesized the use of topographical cues would serve to efficiently guide the increased outgrowth toward a target of interest.
Due to the individual impact of either electrical stimulation or topographical cues from aligned electrospun fibers, this work aimed to utilize a combinatorial approach via presentation of chemical (laminin), topographical (aligned electrospun fibers), and biophysical (electrical stimulation) cues to drive enhanced rate and extent of axonal extension. To examine this phenomenon, dissociated rat DRG neurons were seeded on the laminin coated and aligned PLLA fibers oriented either parallel or perpendicular to the applied electrical stimulus. Following electrical stimulation of neurons on aligned fibers, both total neurite outgrowth and longest neurite length increased (126% and 102%) compared to outgrowth on unstimulated laminin coated PLLA film controls (Figure 3). Therefore, co-presentation is greater than each cue individually, suggesting that there is a synergistic increase neurite extension. These results are in agreement with previous work by Lee and colleagues (Lee, Lee et al. 2009) that found electrically stimulating (DC, 1 mV/mm, 2 hr, 250–670 nm diameter fibers) PC12 neuronal like cells on conductive moderately aligned electrospun fiber meshes increased outgrowth by approximately 50%. In this work, the use of highly aligned electrospun fibers and differential electrical stimulation parameters (DC, 50 mV/mm, 8 hr duration) are likely responsible for our observed increase in neurite extension up to 126% compared to planar films. Interestingly, culturing neurons on fibers oriented parallel or perpendicular while holding the field orientation constant resulted in increased total outgrowth, but no directional biaswas found as commonly noted in lower order models (fish, amphibian) with known regenerative capacities. For example, in vitro investigations of exogenous electrical stimulation of neurons has shown inconsistent sensitivity to the biophysical cue such as neurites turning towards the cathode (Jaffe and Poo 1979; McCaig 1990), turning towards the anode (Cork, McGinnis et al. 1994), increased branching (Hinkle, McCaig et al. 1981), increased outgrowth (Wood and Willits 2009), or no response (Cormie and Robinson 2007). Therefore the topographical features are the dominant cue and act by limiting branching and promoting longer neurites in the direction of the fibers, while electrical stimulation simultaneously serves to further enhance neurite length. No differences in morphology are noted due to electrical stimulation supporting the hypothesis that aligned fibers minimize aberrant growth leading to enhanced neurite extension.
These studies support further investigation of aligned, electrospun fiber scaffolds as tools to provide topographical cues to direct as well as promote more efficient axonal regeneration. Studies are underway to investigate the effects of electrical stimulation on non-neural support cells in 3D hydrogels and extending this work to co-culture with Schwann cells on electrospun fibers. Alternative stimulation parameters may also serve to increase the outgrowth on electro-spun fibers such as duration of exogenous stimulus, AC v. DC stimulation, or long-term stimulation (duty cycle), which have not been explored in depth to date. It is also important to remember that resident support cells (i.e. Schwann cells, endothelial cells, and fibroblasts) will be exposed to any exogenous electrical stimulation as well as interact with electrospun fibers prior to neuronal extension. It is known that these resident cells, along with macrophages, populate the injury site and thus a more comprehensive approach is needed to validate that stimulation and fibers are supportive of all key players in peripheral nerve repair (Schwan cell participation, revascularization, and prevention of disorganized fibroblast scarring) and studies are currently underway to examine how electrical stimulation and/or fibers impact non-neural cell phenotype. We know that electrically stimulated Schwann cells promote significant increases in neurite outgrowth relative to either neurons in co-culture with unstimulated Schwann cells or electrically stimulated neurons in the absence of neurons. More work is needed to understand and control cell migration and/or neurosupportive phenotypes of non-neural support cells through the presentation of external guidance cues that may provide an additive benefit leading to decreased healing time and improved functionality following peripheral nerve injury. This work provides a first step toward characterizing the mechanistic basis for a combinatorial intervention of electrical stimulation and highly aligned electrospun fiber topographical cues for improved repair of large-gap peripheral nerve injuries.
1.5 Conclusion
In conclusion, this study investigated the combination of biophysical (electrical stimulation) and topographical (laminin coated electrospun fibers) cues and found that both serve to increase neurite outgrowth independently, but the aligned fibers are solely responsible for neurite guidance. This report, for the first time, investigated the relative dominance of directional topographical guidance cues compared to directional electrical cues to both enhance and direct primary neurite extension. Results indicate that topographical cues provided by the fibers are more dominant in supporting the extent and directionality of neurite outgrowth compared to electrical stimulation alone. Both cues are modular in nature and can be synergistically applied in conjunction with other common cues such as controlled release of growth factors to further influence axonal growth in vivo. Characterization of neurite outgrowth using multiple guidance cues individually and in combination will allow for the rational development of combinatorial therapeutic approaches towards improved interventions for the repair of large-gap and complex peripheral nervous injuries. The combined application of electrical and aligned fiber topographical guidance cues described herein, if translated in vivo, could provide a more supportive environment for directed and enhanced axonal regeneration following peripheral nerve injury.
Supplementary Material
Acknowledgments
The authors acknowledge financial support from NIH RO1 # 1R01EB013281 (DMT), NSF CAREER BMAT# 1150125 (RJG), and the NIH NINDS # R21NS62392 (RJG). The authors also thank members of the Thompson lab and Gilbert lab for their support and insight into this project.
Footnotes
The authors disclose no potential conflicts of interest in this work.
Contributor Information
A. N. Koppes, Email: eldria@rpi.edu.
N. W. Zaccor, Email: zaccon@rpi.edu.
C. J. Rivet, Email: rivetc2@rpi.edu.
L. A. Williams, Email: willilj12@rpi.edu.
J. M. Piselli, Email: piselj@rpi.edu.
R. J. Gilbert, Email: gilber2@rpi.edu.
D. M. Thompson, Email: thompd4@rpi.edu.
1.8 Literature Cited
- Blackiston DJ, McLaughlin KA, et al. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009;8(21):3527–3536. doi: 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard Schlosshauer LD, Schaller Hans-Eberhard, Sinis Nektarios. Synthetic Nerve Guide Implants in Humans: a Comprehensive Survey. Neurosurgery. 2006;59:740–748. doi: 10.1227/01.NEU.0000235197.36789.42. [DOI] [PubMed] [Google Scholar]
- Ceballos D, Navarro X, et al. Magnetically aligned collagen gel filling a collagen nerve guide improves peripheral nerve regeneration. Exp Neurol. 1999;158(2):290–300. doi: 10.1006/exnr.1999.7111. [DOI] [PubMed] [Google Scholar]
- Chew SY, Mi R, et al. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials. 2008;29(6):653–661. doi: 10.1016/j.biomaterials.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu DT, Strauch B. A prospective clinical evaluation of autogenous vein grafts used as a nerve conduit for distal sensory nerve defects of 3 cm or less. Plast Reconstr Surg. 1990;86(5):928–934. doi: 10.1097/00006534-199011000-00015. [DOI] [PubMed] [Google Scholar]
- Cork RJ, McGinnis ME, et al. The growth of PC12 neurites is biased towards the anode of an applied electrical field. J Neurobiol. 1994;25(12):1509–1516. doi: 10.1002/neu.480251204. [DOI] [PubMed] [Google Scholar]
- Cormie P, Robinson KR. Embryonic zebrafish neuronal growth is not affected by an applied electric field in vitro. Neurosci Lett. 2007;411(2):128–132. doi: 10.1016/j.neulet.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Schnell L, et al. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25(5):1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldner JS, Bruder JM, et al. Neurite bridging across micropatterned grooves. Biomaterials. 2006;27(3):460–472. doi: 10.1016/j.biomaterials.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Gordon T, Amirjani N, et al. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp Neurol. 2010;223(1):192–202. doi: 10.1016/j.expneurol.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Gordon T, Brushart TM, et al. The potential of electrical stimulation to promote functional recovery after peripheral nerve injury--comparisons between rats and humans. Acta Neurochir Suppl. 2007;100:3–11. doi: 10.1007/978-3-211-72958-8_1. [DOI] [PubMed] [Google Scholar]
- Hinkle L, McCaig CD, et al. The direction of growth of differentiating neurones and myoblasts from frog embryos in an applied electric field. J Physiol. 1981;314:121–135. doi: 10.1113/jphysiol.1981.sp013695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman-Kim D, Mitchel JA, et al. Topography, cell response, and nerve regeneration. Annu Rev Biomed Eng. 2010;12:203–231. doi: 10.1146/annurev-bioeng-070909-105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary KB, Robinson KR. Endogenous electrical currents and the resultant voltage gradients in the chick embryo. Dev Biol. 1990;140(1):149–160. doi: 10.1016/0012-1606(90)90062-n. [DOI] [PubMed] [Google Scholar]
- Hotary KB, Robinson KR. Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption. Dev Biol. 1994;166(2):789–800. doi: 10.1006/dbio.1994.1357. [DOI] [PubMed] [Google Scholar]
- Huang J, Ye Z, et al. Electrical stimulation induces calcium-dependent release of NGF from cultured Schwann cells. Glia. 2012;58(5):622–631. doi: 10.1002/glia.20951. [DOI] [PubMed] [Google Scholar]
- Hurtado A, Cregg JM, et al. Robust CNS regeneration after complete spinal cord transection using aligned poly-L-lactic acid microfibers. Biomaterials. 2011;32(26):6068–6079. doi: 10.1016/j.biomaterials.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LF, Poo MM. Neurites grow faster towards the cathode than the anode in a steady field. J Exp Zool. 1979;209(1):115–128. doi: 10.1002/jez.1402090114. [DOI] [PubMed] [Google Scholar]
- Kennedy JM, Zochodne DW. Impaired peripheral nerve regeneration in diabetes mellitus. J Peripher Nerv Syst. 2005;10(2):144–157. doi: 10.1111/j.1085-9489.2005.0010205.x. [DOI] [PubMed] [Google Scholar]
- Koh HS, Yong T, et al. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials. 2008;29(26):3574–3582. doi: 10.1016/j.biomaterials.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Koppes AN, Nordberg AL, et al. Electrical Stimulation of Schwann Cells Promotes Sustained Increases in Neurite Outgrowth. Tissue Engineering Part A not available. 2013 doi: 10.1089/ten.tea.2013.0012. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes AN, Seggio AM, et al. Neurite outgrowth is significantly increased by the simultaneous presentation of Schwann cells and moderate exogenous electric fields. Journal of Neural Engineering. 2011;8(4):046023. doi: 10.1088/1741-2560/8/4/046023. [DOI] [PubMed] [Google Scholar]
- Kumbar SG, James R, et al. Electrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater. 2008;3(3):034002. doi: 10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- Lee JY, Lee JW, et al. Neuroactive conducting scaffolds: nerve growth factor conjugation on active ester-functionalized polypyrrole. J R Soc Interface. 2009;6(38):801–810. doi: 10.1098/rsif.2008.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen J, et al. Guidance of neurite outgrowth on aligned electrospun polypyrrole/poly(styrene-beta-isobutylene-beta-styrene) fiber platforms. J Biomed Mater Res A. 2010;94(4):1004–1011. doi: 10.1002/jbm.a.32675. [DOI] [PubMed] [Google Scholar]
- McCaig CD. Nerve branching is induced and oriented by a small applied electric field. J Cell Sci. 1990;95(Pt 4):605–615. doi: 10.1242/jcs.95.4.605. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM. Electrical fields, nerve growth and nerve regeneration. Exp Physiol. 1991;76(4):473–494. doi: 10.1113/expphysiol.1991.sp003514. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, et al. Controlling cell behavior electrically: current views and future potential. Physiol Rev. 2005;85(3):943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- Miller C, Jeftinija S, et al. Synergistic effects of physical and chemical guidance cues on neurite alignment and outgrowth on biodegradable polymer substrates. Tissue Eng. 2002;8(3):367–378. doi: 10.1089/107632702760184646. [DOI] [PubMed] [Google Scholar]
- Moore AM, Kasukurthi R, et al. Limitations of conduits in peripheral nerve repairs. Hand (N Y) 2009;4(2):180–186. doi: 10.1007/s11552-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhatyar VJ, Salmeron-Sanchez M, et al. Role of fibronectin in topographical guidance of neurite extension on electrospun fibers. Biomaterials. 2011;32(16):3958–3968. doi: 10.1016/j.biomaterials.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble J, Munro CA, et al. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45(1):116–122. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- Pan L, Borgens RB. Strict perpendicular orientation of neural crest-derived neurons in vitro is dependent on an extracellular gradient of voltage. J Neurosci Res. 2012;90 (7):1335–1346. doi: 10.1002/jnr.22809. [DOI] [PubMed] [Google Scholar]
- Patel N, Poo MM. Orientation of neurite growth by extracellular electric fields. J Neurosci. 1982;2(4):483–496. doi: 10.1523/JNEUROSCI.02-04-00483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham QP, Sharma U, et al. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12(5):1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- Portincasa A, Gozzo G, Parisi D, Annacontini L, Campanale A, Basso G, Maiorella A. Microsurgical Treatment of Injury to Peripheral Nerves and Lower Limbs: A Critical Review of the Last 8 years. Microsurgery. 2007;27:455–462. doi: 10.1002/micr.20382. [DOI] [PubMed] [Google Scholar]
- Rangappa N, Romero A, et al. Laminin-coated poly(L-lactide) filaments induce robust neurite growth while providing directional orientation. J Biomed Mater Res. 2000;51(4):625–634. doi: 10.1002/1097-4636(20000915)51:4<625::aid-jbm10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Ray WZ, Kasukurthi R, et al. Functional recovery following an end to side neurorrhaphy of the accessory nerve to the suprascapular nerve: case report. Hand (N Y) 2010;5(3):313–317. doi: 10.1007/s11552-009-9242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray WZ, Mackinnon SE. Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol. 2010;223(1):77–85. doi: 10.1016/j.expneurol.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes O, Sosa I, et al. Promoting neurological recovery following a traumatic peripheral nerve injury. P R Health Sci J. 2005;24(3):215–223. [PubMed] [Google Scholar]
- Robinson LR. Traumatic injury to peripheral nerves. Muscle Nerve. 2000;23(6):863–873. doi: 10.1002/(sici)1097-4598(200006)23:6<863::aid-mus4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Schaub NJ, Britton T, et al. Engineered Nanotopography on Electrospun PLLA Microfibers Modifies RAW 264.7 Cell Response. ACS applied materials & interfaces. 2013;5(20):10173–10184. doi: 10.1021/am402827g. [DOI] [PubMed] [Google Scholar]
- Seggio A, Narayanaswamy A, et al. Local Orientation of Schwann cells directs Neurite outgrowth. Journal of Neural Engineering. 2010;7:046001. doi: 10.1088/1741-2560/7/4/046001. [DOI] [PubMed] [Google Scholar]
- Seggio AM, Ellison KS, et al. Cryopreservation of transfected primary dorsal root ganglia neurons. J Neurosci Methods. 2008;173(1):67–73. doi: 10.1016/j.jneumeth.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Selecki BR, I, Ring T, et al. Trauma to the central and peripheral nervous systems. Part II: A statistical profile of surgical treatment New South Wales 1977. Aust N Z J Surg. 1982;52(2):111–116. doi: 10.1111/j.1445-2197.1982.tb06081.x. [DOI] [PubMed] [Google Scholar]
- Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Stump RF, Robinson KR. Xenopus neural crest cell migration in an applied electrical field. J Cell Biol. 1983;97(4):1226–1233. doi: 10.1083/jcb.97.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter DM, Miller KE. The emerging role of forces in axonal elongation. Prog Neurobiol. 2011;94(2):91–101. doi: 10.1016/j.pneurobio.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuft BW, Li S, et al. Photopolymerized microfeatures for directed spiral ganglion neurite and Schwann cell growth. Biomaterials. 2013;34(1):42–54. doi: 10.1016/j.biomaterials.2012.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Mullins ME, et al. Creation of highly aligned electrospun poly-L-lactic acid fibers for nerve regeneration applications. J Neural Eng. 2009;6(1):016001. doi: 10.1088/1741-2560/6/1/016001. [DOI] [PubMed] [Google Scholar]
- Wang HB, Mullins ME, et al. Varying the diameter of aligned electrospun fibers alters neurite outgrowth and Schwann cell migration. Acta Biomater. 2010;6(8):2970–2978. doi: 10.1016/j.actbio.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Wen X, Tresco PA. Effect of filament diameter and extracellular matrix molecule precoating on neurite outgrowth and Schwann cell behavior on multifilament entubulation bridging device in vitro. J Biomed Mater Res A. 2006;76(3):626–637. doi: 10.1002/jbm.a.30520. [DOI] [PubMed] [Google Scholar]
- Wood MD, Willits RK. Applied electric field enhances DRG neurite growth: influence of stimulation media, surface coating and growth supplements. J Neural Eng. 2009;6(4):046003. doi: 10.1088/1741-2560/6/4/046003. [DOI] [PubMed] [Google Scholar]
- Xie J, MacEwan MR, et al. Neurite outgrowth on nanofiber scaffolds with different orders, structures, and surface properties. ACS Nano. 2009;3(5):1151–1159. doi: 10.1021/nn900070z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegiyants S, Dayicioglu D, et al. Traumatic peripheral nerve injury: a wartime review. J Craniofac Surg. 2010;21(4):998–1001. doi: 10.1097/SCS.0b013e3181e17aef. [DOI] [PubMed] [Google Scholar]
- Zuidema JM, Hyzinski-García MC, et al. Enhanced GLT-1 mediated glutamate uptake and migration of primary astrocytes directed by fibronectin-coated electrospun poly-l-lactic acid fibers. Biomaterials. 2014;35(5):1439–1449. doi: 10.1016/j.biomaterials.2013.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.