Abstract
Metal contaminants cross the placenta, presenting a heightened risk of perturbing fetal development. Information on placental concentrations and transfer of multiple potentially toxic metals from low to moderate exposure is lacking. We measured concentrations of Cd, Pb, Hg, Mn, Se and Zn in 750 placentas collected from women enrolled in the New Hampshire Birth Cohort Study and examined the correlation between elements, and profiles of potentially toxic metals (Cd, Pb, Hg and Mn) stratified by nutrient concentrations (Zn and Se) using Principal Components Analyses (PCA). We further examined the indirect effects of maternal metal concentrations on infant metal concentrations through placenta metal concentrations using structural equation models. Placental metal concentrations were all correlated, particularly Zn and Mn, and Zn and Cd, and the principal component of metals differed by stratum of high versus low Zn and Se. Associations were observed between placenta and maternal toenail Se (β 63.49, P<0.0001) and Pb (β 0.90, P<0.0001) but not other metals. Structural equation models did not indicate any statistically significant indirect effects through placental metal concentrations. Placental metal concentrations may represent a distinct biomarker of metal exposure and adverse health impacts to the fetus, particularly those stemming from the placenta.
INTRODUCTION
Metal contaminants are ubiquitous in the environment; they cross the human placenta and pose a risk of adversely impacting the fetus during sensitive stages of development that may affect health throughout life (1–3). The human fetus is entirely composed of elements transferred from maternal to fetal blood via the placenta (4). The placenta is an organ of fetal origin that develops during pregnancy to control the transport of nutrients, respiratory gases and waste products between mother and fetus while hormonally regulating the progression of pregnancy. Cadmium (Cd), lead (Pb), and mercury (Hg) are developmental toxicants (5–8) with no known biological function that are transported across the placenta (9, 10) with varying degrees of efficiency. Manganese (Mn) is an essential nutrient that acts as a neurodevelopmental toxicant at supraoptimal concentrations (11). Selenium (Se) is an essential micronutrient in mammals that has a narrow window of sufficiency (12), and can be toxic in extreme excess (13). Zinc is also an essential nutrient, which plays key roles in embryogenesis, fetal growth and development and mammary gland function for milk synthesis and secretion (14). Short-term health effects from acute in utero exposure to individual metals include spontaneous abortion, stillbirth, low birth weight, pre-term birth, reduced fetal growth, impaired neurodevelopment and congenital malformation (15–20). Both arsenic and cadmium independently have been related to growth suppression among children (21), but there is evidence that metal mixtures may present risks such as impaired cognitive development (19, 20).
Essential metals play critical cellular roles, including structural components of biomolecules, signaling molecules, catalytic cofactors and regulators of protein expression. Their concentrations are tightly regulated via complex homeostatic networks (22), and altered metal homeostasis is characteristic of disease: including neurodegenerative disorders (23), pathogenic disease and cancer (24). Transport of non-essential metals across biological membranes is thought to be based on the similarity of their molecular size and charge to that of essential metals, a phenomenon known as molecular mimicry (25, 26). It has been shown in animal studies that placental nutrient transport systems can also recognize xenobiotics as targets (27). For example, Cd may directly interact with membrane transporters for iron (Fe) and zinc (Zn) (28), reducing the efficiency of transport, or it may indirectly influence Zn transport by increasing metallothionein production in the placenta (29–32), reducing the efficiency of Zn transfer to the fetus. Recent data suggest that Se can act antagonistically with Cd (33, 34) with one study finding Se supplementation was associated with lower Cd-induced oxidative stress and lower Cd concentrations. There is evidence that Se may also be protective against the effects of methylmercury by direct binding to Hg (35) although this has not been observed epidemiologically. Mercury in its most toxic form as methylmercury is a highly specific, irreversible inhibitor of Se-dependent enzymes, which prevent and reverse oxidative damage particularly in the brain and neuroendocrine tissues (36). Epidemiological studies to some extent have examined levels of Cd, Pb and Hg in the human placenta in relation to other biomarkers of maternal and fetal metal exposure, such as blood and cord blood (10, 37, 38), but to our knowledge not in relation to concentrations of essential elements such as Mn, Se or Zn. Given the role of the placenta in regulating the transport of all essential nutrients and toxicants that reach the fetus during pregnancy, we sought to determine the relationships between the concentration of multiple elements: Cd, Pb and Hg, Mn, Se and Zn measured in human placenta with those measured in established maternal and infant biomarkers of metal exposure in a large pregnancy cohort.
MATERIALS AND METHODS
The study protocols for the New Hampshire Birth Cohort Study (NHBCS) were approved by the Committee for the Protection of Human Subjects at Dartmouth College. All study participants provided written informed consent.
The New Hampshire Birth Cohort Study
We used data collected from all individuals currently enrolled in the ongoing NHBCS on whom we analyzed placental samples for multiple elements, including non-essential metals. The NHBCS recruited pregnant women whose primary residential water source is a private well and who obtain their prenatal care at clinics in New Hampshire, a state with detectable As concentrations in private well water, which exceeds the current maximum contaminant limit (10 µg/L) in over 10% of these wells. To be eligible for the study, women were: a) currently pregnant, b) 18 to 45 years old, c) receiving routine prenatal care at one of the study clinics, d) using a private well that serves <15 households or 25 individuals at their place of residence, e) residing in the same place since their last menstrual period and f) not planning to move prior to delivery.
Placental Collection Protocol
Placental biopsies were uniformly collected from the fetal side, at the base of the cord insertion avoiding vasculature, and measuring approximately 1 cm deep and 1–2 cm in diameter. The maternal decidua was removed to avoid inclusion of calcium (Ca) deposits and connective tissue. Placental biopsies were store in trace element-free tubes, which were labeled with a sample barcode ID and stored at −80°C until analysis.
Maternal and Infant Toenails
At two weeks post partum, participants received an information packet requesting maternal and infant toenail clippings within eight weeks of birth, which represent exposure during pregancy. Maternal toenails underwent an additional washing procedure that included manual removal of visible dirt and five washes in an ultrasonic bath using Triton X-100 (LabChem Inc., PA) and acetone followed by deionized water, and allowed to dry. All toenail samples were subject to low-pressure microwave digestion using the method above for placenta digestions and were analyzed via ICP-MS.
ICP-MS analysis
Prior to analysis placental samples were transferred to a −20°C freezer and then to a fridge (4°C) for a maximum of 2 days, and then brought to room temperature. 1 ml of HNO3/HCl at 9:1 ratio (Optima™) was added to samples with of up to 500 mg mass (wet weight), and 2 ml of was added to samples greater than 500 mg. Samples were digested via microwave (CEM, Microwave Assisted Reaction System), ramping the temperature to 95°C in 15 minutes, and holding at this temperature for 45 minutes. 0.25–0.35 ml H2O2 was added to each tube and the microwave digestion sequence was repeated. This method is based on EPA method 3050B and is used at the Dartmouth Trace Element Analysis Core for digestion of low masses of biological samples. The method gives clear digestate solutions and good recoveries (80–120%) for biological SRMs and fortified blanks. Quality control procedures for the digestion included analysis of laboratory fortified blanks, digestion blanks and standard reference material (NIST 1566b, Oyster tissue). All samples were analyzed by ICP-MS (7700x Agilent, Santa Clara, CA) and analysis was conducted following the quality control procedures outlined in EPA 6020a. Analysis quality control included the use of an internal standard, initial and continuing calibration verification and blanks, analytical duplicates and spikes. Selenium was analyzed in reaction cell mode with hydrogen, and all other elements were analyzed using helium as a collision gas (7700x, Agilent, Santa Clara, CA). For the laboratory control sample we used a laboratory-prepared reference placental digest prepared from multiple samples of de-identified placental tissue pooled to create a 2L bulk placental digest solution. The pooled sample was mixed, analyzed and an aliquot was included with each batch of placental samples analyzed. A summary of the quality control data is given in Supplemental Table 1. Method detection limits were calculated based on the procedure outlined in US Code of Federal Regulations, actual mean values for the digestion blanks across all analytical batches are comparable of better than the MDL values. Recoveries for the standard reference material were excellent (90–110%) across the analytical batches and the reproducibility of the laboratory control solution, a bulk placenta digest, was also good with relative standard deviation of 17% for Mn and <10% for Cd, Pb, Zn; the concentration of Hg in the laboratory control sample was below detection limits.
Statistical Analysis
We examined associations between placenta metals and a variety of potential covariates, including maternal age upon enrollment, pre-pregnancy maternal body mass index (BMI), parity, maternal smoking status and infant sex. One-way analyses of variance analyses were conducted on log10-transformed placental metal concentration data, expressed as ng/g, and potential covariates. We examined correlations between elements in placental specimens using Spearman’s correlation statistical tests on untransformed placental metal concentration data. Using structural equation modeling (SEM) we tested the indirect effects of maternal metal concentrations on infant metal concentrations through placenta metal concentrations. Structural equation modeling (SEM) is a convenient statistical tool for modeling causal pathways and indirect effects (i.e., mediation) by components of the pathway (39). The indirect effects for all six metals were calculated and tested using the Sobel Z test.
To assess the effect of placental Zn and Se status on the relationship between placental metal concentrations, we stratified data by the median Zn (10.1 µg/g) and Se (272 ng/g) concentrations in both Spearman correlational and principal component analyses (PCA) to examine the structure of six placental metals and effects of Zn and Se levels on this structure. Use of PCA is consistent with recent literature on the placental metallome (40) and on analyzing multiple contaminants in feto-maternal tissues (41). In statistical analyses, placental metal concentrations that fell below instrument detection limits were recorded as missing data and excluded.
Results
Descriptive characteristics of the cohort
The study population was predominantly white, with an average age of 31.3 (25.1 – 37.8), a mean body mass index (BMI) of 25.3 (10th – 90th percentile range of 20 – 32.1), and who gave birth primarily to full term infants (>37 weeks) with an equal male/female distribution. Placental metal concentrations were related to demographic characteristics of the population (Table 1). Placental tissue collected from women over 30 years of age at enrollment had 28% higher concentrations of Cd compared to women under 30. Placental tissues from women with a BMI of 30 or more (obese) had 35% lower concentrations of Cd, 45% lower Pb, 40% lower Hg, 8% lower Zn and 17% lower Mn than women with a BMI between 18.5 – 24.9 (normal weight). Placental tissue from male births had 7% higher Zn concentrations than those from female births. Placental tissue from women reporting smoking cigarettes at any time during their pregnancy had 29% higher concentrations of Cd in placental tissue than those with no exposure to smoking.
Table 1.
Cohort Characteristics. Arithmetic mean (± SD) of metal concentrations measured in placenta.
| Characteristics | Placenta Zn | Placenta Pb | Placenta Hg | Placenta Mn | Placenta Se | Placenta Zn | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | ng/g | N (%) | ng/g | N (%) | ng/g | N (%) | ng/g | N (%) | ng/g | N (%) | µg/g | ||
| Maternal: | |||||||||||||
| Age at enrollment (years): | *** | ||||||||||||
| < 30 years | 305 (41) | 3.0 ±1.9 | 307(40) | 2.3 ± 3.2 | 285 (40) | 2.7 ± 11.5 | 307 (40) | 76.6 ± 50.8 | 307 (40) | 283.4 ± 74.4 | 307 (40) | 10.7 ± 4.8 | |
| ≥ 30 years | 452 (59) | 3.9 ± 2.6 | 453 (60) | 2.4 ± 3.9 | 429 (60) | 2.1 ± 2.0 | 452 (60) | 72.4 ± 28.5 | 455 (60) | 278.5 ± 51.1 | 455 (60) | 10.5 ± 3.1 | |
| BMI at GW24 (kg/m2) | *** | ** | * | ** | * | ||||||||
| Normal (BMI <25) | 407 (54) | 3.8 ± 2.6 | 410 (54) | 2.7 4.6 | 384 (54) | 2.7 ± 10.0 | 409 (54) | 78.3 ± 47.7 | 411 (54) | 284.0 ± 70.2 | 411 (54) | 11.0 ± 4.6 | |
| Overweight (≥ 25 to < 30) | 134 (18) | 3.6 ± 2.2 | 134 (18) | 2.0 ± 1.9 | 128 (18) | 1.9 ± 2.1 | 134 (18) | 71.3 ± 24.3 | 134 (18) | 278.5 ± 46.0 | 134 (18) | 10.3 ± 2.7 | |
| Obese (≥30) | 157 (21) | 2.8 ± 1.6 | 156 (21) | 1.8 ± 1.6 | 147 (21) | 1.9 ± 2.1 | 156 (21) | 66.7 ± 24.9 | 157 (21) | 276.3 ± 52.5 | 157 (21) | 10.1 ± 2.8 | |
| Parity | ** | ** | |||||||||||
| First live birth | 310 (41) | 3.6 ± 2.2 | 310 (41) | 2.3 ± 2.0 | 294 (41) | 2.1 ± 2.1 | 310 (41) | 77.6 ± 47.4 | 310 (41) | 282.4 ± 66.4 | 310 (41) | 11.0 ± 4.1 | |
| 1 or more live birth | 453 (59) | 3.5 ± 2.5 | 448 (59) | 2.4 ± 4.4 | 418 (59) | 2.5 ± 9.6 | 447 (59) | 71.8 ± 32.0 | 450 (59) | 278.9 ± 58.0 | 450 (59) | 10.4 ± 3.7 | |
| Smoking status | ** | ||||||||||||
| Never | 665 (88) | 3.5 ± 2.3 | 667 (88) | 2.4 ± 3.8 | 627 (88) | 2.4 ± 7.9 | 666 (88) | 74.2 ± 40.7 | 669 (88) | 281.3 ± 63.1 | 669 (88) | 10.7 ± 4.0 | |
| Ever | 44 (6) | 4.5 ± 3.5 | 45 (6) | 2.3 ± 1.1 | 42 (6) | 2.0 ± 2.1 | 45 (6) | 76.5 ± 24.6 | 45 (6) | 277.5 ± 44.8 | 45 (6) | 10.4 ± 2.0 | |
| Infant: | |||||||||||||
| Sex | * | ||||||||||||
| Female | 375 (49) | 3.4 ± 2.1 | 376 (49) | 2.2 ± 2.8 | 352 (49) | 2.1 ± 2.2 | 375 (49) | 72.8 ± 30.4 | 377 (49) | 279.4 ± 57.6 | 377 (49) | 10.3 ± 3.4 | |
| Male | 382 (50) | 3.7 ± 2.7 | 383 (50) | 2.5 ± 4.3 | 361 (51) | 2.6 ± 10.2 | 383 (50) | 75.5 ± 46.0 | 384 (50) | 281.4 ± 65.3 | 384 (50) | 11.0 ± 4.3 | |
| Birth weight (g) | * | ||||||||||||
| Low (<2,500g) | 27 (4) | 3.2 ± 2.4 | 27 (4) | 2.1 ± 1.5 | 26 (4) | 1.9 ± 2.1 | 27 (4) | 82.6 ± 35.8 | 27 (4) | 296.0 ± 68.8 | 27 (4) | 11.8 ± 3.3 | |
| Normal (≥2,500g) | 721 (95) | 3.5 ± 2.4 | 723 (95) | 2.3 ± 3.7 | 678 (95) | 2.4 ± 7.6 | 722 (95) | 73.9 ± 39.4 | 725 (95) | 279.6 ± 61.4 | 725 (95) | 10.6 ± 3.9 | |
Results of one-way ANOVAs of log10-transformed variables: *P<0.05;
P<0.01;
P<0.001
Unknown/Missing: BMI at GW24 60 (*%); Parity 2 (0.3%); Smoking status 48 (6%); birthweight status 10 (1%)
Correlations between metals in the placenta
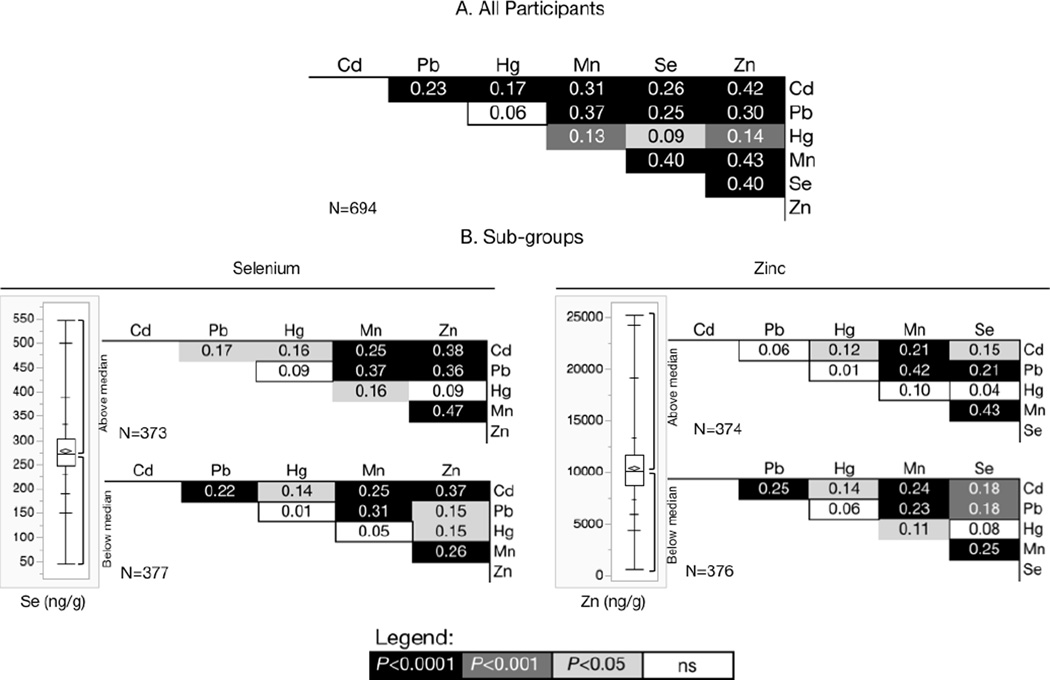
Placental metal concentrations were positively correlated (Figure 1A). The strongest correlations were between Zn and Mn, Zn and Cd, Zn and Se and between Se and Mn. Conversely, the weakest correlations were between non-essential metals Hg and Pb. Correlations with Hg were the weakest in placenta overall. We observed differences in placental metal correlations when we stratified placentas according to whether the concentration of essential nutrients Zn or Se were less than or more than the median value determined for the study population. In placentas with less than the median Zn concentration (10.1 µg/g) we found positive correlations between Pb and Cd (rS = 0.25, P < 0.0001) and between Mn and Hg (rS = 0.11, P < 0.001) that were not observed in placentas with above median Zn concentrations (Figure 1B). Further, in below-median Zn placentas, we observed a weaker correlation between Mn and Se than in higher Zn placentas (rS = 0.25 and 0.43 respectively). Likewise, correlations differed by Se concentrations, with a higher correlation between Mn and Hg in placentas with above median Se (rS = 0.05 ns, in below median Se and 0.15, P < 0.001 above). Correlations between Hg and Zn at below median Se (r = 0.15 P <0.001) were not observed in placental specimens with above median Se (rS = 0.09 ns). Zn and Pb were also more strongly correlated in above median Se placenta samples (rS = 0.36 P <0.0001), than in below median Se placentas (r = 0.15 P < 0.001).
Figure 1.
Spearman’s correlation coefficient (rS) matrix for (A) All participants: concentrations of metals measured in 694 placental specimens from the New Hampshire Birth Cohort Study, and (B) Subgroups: Spearman’s correlation coefficient (rS) matrix for concentrations of metals measured in placental specimens stratified into those with above or below the median concentration of selenium (272 ng/g) and zinc (10.11 µg/g). Shading indicates corresponding P value as shown, and ns = not significant.
Principal Components Analysis
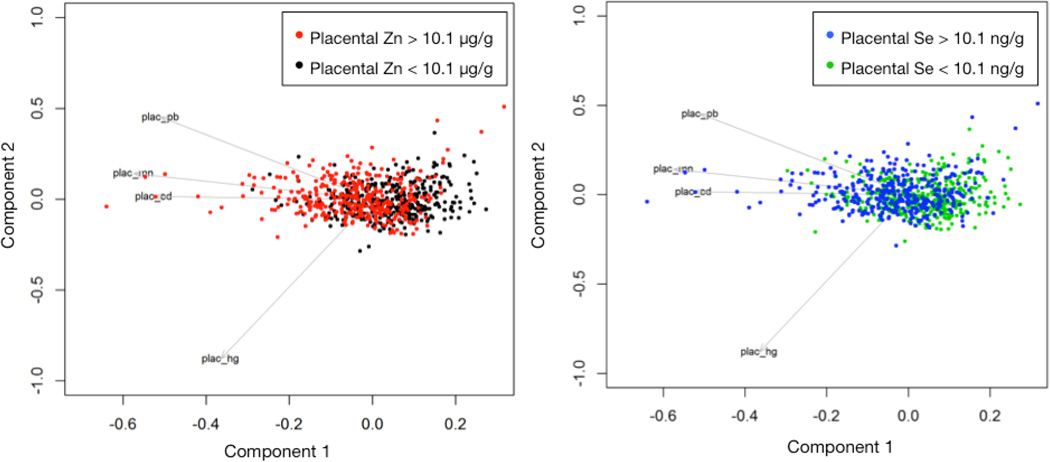
In our PCA analysis for all six metals, the first component explained 50% of the total variance and the second component 15%. Together, the first three components in this analysis explained 77% of the total variance. All variables loaded in the same direction on the first component and the loadings varied from 0.31 to 0.5 without a predominant metal, which was consistent with the positive correlations among placental metals. A principal component analysis using Cd, Hg, Pb and Mn to assess the effects of Zn and Se levels (dichotomized at the median values) on the structure showed that the first three components explained 48%, 22% and 16% of the total variance respectively. Loadings on the first component were in the same direction, and magnitudes varied from 0.42 to 0.56 with no indication of a predominant metal. The second component had a predominant loading from Cd (0.76) and a moderate loading from Pb (−0.56), whereas the third component had a predominant loading from Mn (−0.80) and a moderate loading from Cd (0.49) and Pb (0.33). The fourth component had a predominant loading from Hg (−0.78) and a moderate loading from Pb (0.58). All except the third component were statistically significantly different between high (above median) Zn group and low (below median) Zn group with P values 3.04 × 10−13, 1.49 × 10−7 and 5.21 × 10−6, for components 1,2 and 4 respectively. The same three components also differed between the high (above median) Se group and the low (below median) Se group with P values 2.94 × 10−7, 1.20 × 10−5, and 0.02. These results are additionally depicted in Figure 2 and align with results of the Spearman correlations.
Figure 2.
Principal components analysis of placental metal concentrations for 756 participants in the New Hampshire Birth Cohort Study, stratified by their median concentrations of Zn (left, stratified at 10.1 µg/g) and Se (right, at 272 ng/g).
Associations between biomarkers
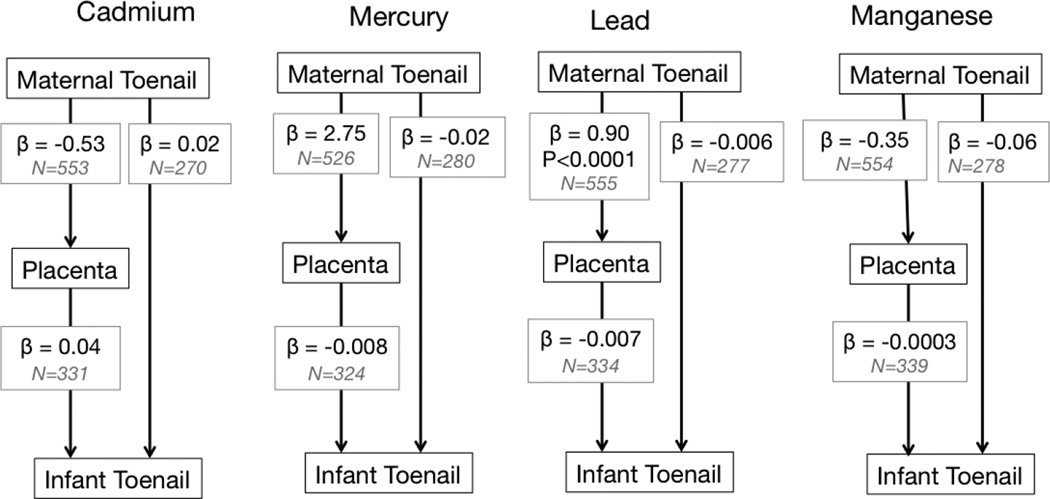
Results from the structural equation models are shown in Figures 3 and S1, with associations estimated simultaneously adjusting for covariance of the dependent variables. The indirect effects of maternal metals on infant metals through placenta metals were estimated as the product of the effects from mother to placenta and from placenta to infant and tested via the Sobel Z test. The six mediated effects were −0.02 (95% CI: −0.13, 0.08) for Cd, −0.02 (−0.31, 0.27) for Hg, −0.06 (−0.38, 0.26) for Mn, −0.01 (−0.28, 0.27) for Pb, −0.96 (−10.13, 8.20) for Se and −0.001 (−0.01, 0.008) for Zn. While all coefficients were in the negative direction, the confidence intervals around the effect estimates were large. A strong positive relationship between maternal toenail and placental Se levels, and a weak association between maternal toenail and placental Pb were both statistically significant. All other analyses between metal concentrations in maternal toenail, placenta and infant toenail indicated few statistically significant relationships.
Figure 3. Maternal fetal metal transfer models for Cd, Hg and Pb.
The model shows beta coefficients (β), sample size (N) and P values (if < 0.05) from structural equation modeling between maternal toenail, placental biopsies and infant toenail metal concentration data.
Discussion
The concentrations of potentially toxic metals (Cd, Pb and Hg) in placental tissue collected from women enrolled in the NHBCS fell at the lower end of the ranges reported worldwide. For placental Cd, arithmetic mean concentrations have been reported to be in the range of 1–53 ng/g (10) (all concentrations are reported as wet weight), and we report an arithmetic mean of 3.53 ng/g (±SD 2.35), which agrees with Cd measured in a cohort in North Carolina (42). In Western Europe and North America, placental Pb concentrations are below 50 ng/g since the removal of lead from gasoline (10), and our study reports arithmetic mean of 2.2 ng/g (±SD 2.72) (10). A review of worldwide placental Hg concentrations provides a range of 10–180 ng/g and in the NHBCS we measured a mean concentration of 2.05 ng/g (±SD 2.08), possibly due to low fish consumption in our population. Comparison of our data with a study by Al-Saleh (43), suggest similar median Cd levels (3.01 and 5.83 ng/g respectively using the dry to wet weight conversion described in Esteban-Vasallo et al (10)), lower median Hg (1.49 and 5.16 ng/g respectively) and much lower median Pb (1.55 and 75 ng/g respectively). To our knowledge, there are no reports of placental Mn or Se from large birth cohort studies.
We found strong positive correlations both between essential mineral nutrients in the placenta and between potentially toxic elements. Correlations with Hg, which were present at particularly low concentrations in this study, were the weakest overall. Correlation between mineral nutrients in placenta may be a characteristic of homeostatic control mechanisms for mineral nutrients within the placenta, or an indication of placental transport efficiency. Principal components analysis of placental metal concentrations stratified into participants with either above or below median concentrations of Zn or Se indicated differences by both elements. Together with Spearman’s correlations (Figure 1) this suggested that nutrient concentrations may influence concentrations of non-essential potentially toxic elements in the placenta, and vice versa. In particular, in placentae stratified by median Zn, correlations with- and between-contaminants were stronger in the low Zn group. For stratification by median Se, which literature suggests may play a protective role during metal exposure (35), inter-element correlations differed in the above and below median groups. The hypothesis that placentae with higher concentrations of essential nutrients may decrease contaminant metal accumulation in the placenta should be examined further in experimental studies. These preliminary findings are in agreement with a study by Laine et al (42) who measured placental Cd levels in women enrolled in a US cohort with preeclampsia (N=172), and found that the Cd-associated odds ratio for preeclampsia was lower with higher placental Se levels.
The finding that placental elemental concentrations of Cd, Hg, Pb, Zn and Mn fell with increasing maternal BMI is consistent with the literature. Reduced placental efficiency (defined as fetal to placental weight ratio (44)) was associated with higher BMI in a study of 55,105 pregnancies (45), suggesting that transplacental metal transport is suppressed. Inhibited mineral nutrient transport across the placenta has been implicated in diabetic pregnancies (46, 47). In maternal obesity, there is an increased flux of macronutrients such as fatty acids across the placenta, hypothesized to affect energy signaling and expression of genes involved in nutrient transport (48). Studies on glucose and fatty acid transporter expression in placenta from obese mothers have been inconclusive, with one study finding no changes in glucose transporter expression (49), and others showing increases or decreases in the expression or activity of various transporters (50–52). Wallace et al (45) found that maternal BMI was positively related to placental weight, suggesting that lower element concentrations could simply be related to an element/nutrient-dilution effect. We also observed differences in placental Zn concentrations between male and female placentas, (Table 1), although stratifying placental metal concentration data by infant sex did not reveal altered biomarker associations.
Studies that directly compare placental metal concentrations with those in other maternal and/or infant biomarkers are summarized in Supplemental Tables 2–4 (9, 29,63–70). Of the studies reviewed by Esteban-Vasallo, only 29 reported placenta Hg concentrations, and of those, only 4 studies included more than 100 women. Placental Hg concentrations correlated with concentrations measured in cord blood, umbilical tissue and hair from mother and child, but results for maternal blood were inconsistent.
Our study used maternal and infant toenails as biomarkers for metal exposure, in contrast to many comparative placental metal analysis studies, which have primarily used blood. Placental specimens contain varying amounts of both maternal and infant blood, whereas toenails provide an independent indicator of exposure. Quantification of elements in human finger- and toenails is an established biomarker for numerous essential and non-essential elements with and without known biological functions (53). Nails have been used previously as a biomarker for exposure to Hg (38, 54–57), Mn (58), Se (57) and Zn (59, 60). Fewer studies have used nails as biomarkers of Cd and Pb, and for Pb have observed correlations with blood Pb concentrations, and with exposure (38). The utility of toenails as biomarkers for metal status stems both from their ease of collection, and relative lack of extraneous contamination in comparison with hair. Moreover, toenail clippings provide a stable, longer time-integrated exposure history because of their slower growth rate in comparison with fingernails (61) with a growth rate of approximately 0.1 mm/day for fingernails and 0.03–0.04 mm/day for toenails. In our study, which collected toenails two weeks post-partum, the approximate exposure window provided by toenail samples would be the beginning of the second trimester. In infants, nail growth begins at about 10 weeks gestation. Total nail lengths of between 3.2 – 5.7 mm have been measured at birth (62), indicating a comparable growth rate of about 0.03 mm/day.
The strengths of this study include the large size of the cohort, the low-to-moderate metal exposure of participants, the range and novelty of metals analyzed (particularly Mn, Se and Zn), and the availability of supporting biomarker data for mothers and infants. This study is one of the few studies to focus on associations between placental metal concentrations and independent time-integrated biomarkers of metal exposure in both mother and infant. The main limitation of the study was the availability of infant toenail data, which was limited to about half of the population (363 participants), however, demographic characteristics (maternal age at enrollment, parity, maternal BMI and infant birthweight) of the subset of the population for which infant toenail data was available did not appreciably differ from those for whom infant toenail data was missing (data not shown). Other limitations include the windows of exposure represented by the maternal and infant toenail biomarkers (estimated to be the beginning of the second trimester and the middle of the third trimester respectively), which may not have corresponded exactly with environmental exposures represented by specimens collected from term placental specimens.
In summary, comparing metal levels in toenail biomarkers of mother-infant pairs with placental metal levels provided no evidence of an indirect effect through placenta metals. We found evidence that the correlation between non-essential metal concentrations differed by essential nutrient levels, Se and Zn. Thus, understanding the concentrations and distribution of metals in the placenta may help to elucidate the adverse impacts of these metals on fetal and lifelong health.
Supplementary Material
Acknowledgments
This work was supported in part by the following P20 GM104416 from the National Institute of General Medical Sciences, P01ES022832 from the National Institute of Environmental Health, and grant RD83544201 from the Environmental Protection Agency. The Trace Metal Analysis Facility is funded in part by P42 ES007373.
Footnotes
The authors declare they have no actual or potential competing financial interests.
Supplemental Information
Supplemental Table 1. Quality control information for ICP-MS analysis of placental biopsies reported in this study.
Supplemental Table 2. Studies reporting cadmium measurements in human placenta from 1976 to the present day.
Supplemental Table 3. Studies reporting lead measurements in human placenta from 1976 to the present day.
Supplemental Table 4
Studies reporting total mercury measurements in human placenta and other biomarkers from 1976 to the present day.
References
- 1.Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, Ji Y, Hong X, Caruso D, Bartell T, Gong Y, Strickland P, Navas-Acien A, Guallar E, Wang X. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J. Exp. Sci. Environ. Epi. 2014;24(5):537–544. doi: 10.1038/jes.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyer RA. Transplacental transport of lead. Environ. Health Perspect. 1990;89:101–105. doi: 10.1289/ehp.9089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rayman MP, Wijnen H, Vader H, Kooistra L, Pop V. Maternal selenium status during early gestation and risk for preterm birth. Can. Med. Assoc. J. 2011;183(5):549–555. doi: 10.1503/cmaj.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salafia CM, Misra DP, Yampolsky M, Charles AK, Miller RK. Allometric metabolic scaling and fetal and placental weight. Placenta. 2009;30(4):355–360. doi: 10.1016/j.placenta.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ATSDR. US Department of Health and Human Services; 1999. Toxicological Profile for Mercury. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=115&tid=24. [Google Scholar]
- 6.ATSDR. US Department of Health and Human Services; 2007. Toxicological Profile for Lead. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=96&tid=22. [Google Scholar]
- 7.ATSDR. US Department of Health and Human Services; 2012. Toxicological Profile for Cadmium. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15. [Google Scholar]
- 8.ATSDR. Toxicological Profile for Manganese. 2012 http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=102&tid=23. [PubMed]
- 9.Needham LL, Grandjean P, Heinzow B, Jorgensen PJ, Nielsen F, Patterson DG, Jr, Sjodin A, Turner WE, Weihe P. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ. Sci. Technol. 2011;45(3):1121–1126. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteban-Vasallo MD, Aragones N, Pollan M, Lopez-Abente G, Perez-Gomez B. Mercury, cadmium, and lead levels in human placenta: a systematic review. Environ. Health Perspect. 2012;120(10):1369–1377. doi: 10.1289/ehp.1204952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roels HA, Bowler RM, Kim Y, Claus Henn B, Mergler D, Hoet P, Gocheva VV, Bellinger DC, Wright RO, Harris MG, Chang Y, Bouchard MF, Riojas-Rodriguez H, Menezes-Filho JA, Tellez-Rojo MM. Manganese exposure and cognitive deficits: a growing concern for manganese neurotoxicity. Neurotoxicology. 2012;33(4):872–880. doi: 10.1016/j.neuro.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris JS, Crane SB. Selenium toxicity from a misformulated dietary supplement, adverse health effects, and the temporal response in the nail biologic monitor. Nutrients. 2013;5(4):1024–1057. doi: 10.3390/nu5041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurokawa S, Berry MJ. Selenium. Role of the essential metalloid in health. Metal Ions Life Sci. 2013;13:499–534. doi: 10.1007/978-94-007-7500-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donangelo CM, King JC. Maternal zinc intakes and homeostatic adjustments during pregnancy and lactation. Nutrients. 2012;4(7):782–798. doi: 10.3390/nu4070782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellinger DC. Teratogen update: Lead and pregnancy. Birth Defects Res. Part A. Clin. Mol. Teratol. 2005;73(6):409–420. doi: 10.1002/bdra.20127. [DOI] [PubMed] [Google Scholar]
- 16.Axelrad DA, Bellinger DC, Ryan LM, Woodruff TJ. Dose-response relationship of prenatal mercury exposure and IQ: an integrative analysis of epidemiologic data. Environ. Health Perspect. 2007;115(4):609–615. doi: 10.1289/ehp.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grazuleviciene R, Madiasauskiene R, Buinauskiene J, Grazulevicius T. Effects of elevated levels of manganese and iron in drinking water on birth outcomes. Pol. J. Environ. Stud. 2009;18(5):819–825. [Google Scholar]
- 18.Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, Robertson RT. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol. Teratol. 2007;29(2):181–187. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Ha EH, Park H, Ha M, Kim Y, Hong YC, Kim EJ, Kim BN. Prenatal lead and cadmium co-exposure and infant neurodevelopment at 6 months of age: the Mothers and Children's Environmental Health (MOCEH) study. Neurotoxicology. 2013;35:15–22. doi: 10.1016/j.neuro.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Yu XD, Yan CH, Shen XM, Tian Y, Cao LL, Yu XG, Zhao L, Liu JX. Prenatal exposure to multiple toxic heavy metals and neonatal neurobehavioral development in Shanghai, China. Neurotoxicol. Teratol. 2011;33(4):437–443. doi: 10.1016/j.ntt.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Gardner RM, Kippler M, Tofail F, Bottai M, Hamadani J, Grander M, Nermell B, Palm B, Rasmussen KM, Vahter M. Environmental exposure to metals and children's growth to age 5 years: a prospective cohort study. Am. J. Epidemiol. 2013;177(12):1356–1367. doi: 10.1093/aje/kws437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292(5526):2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 23.Bourassa MW, Miller LM. Metal imaging in neurodegenerative diseases. Metallomics. 2012;4(8):721–738. doi: 10.1039/c2mt20052j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mounicou S, Szpunar J, Lobinski R. Metallomics: the concept and methodology. Chem. Soc. Rev. 2009;38(4):1119–1138. doi: 10.1039/b713633c. [DOI] [PubMed] [Google Scholar]
- 25.Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharmacol. 2005;204(3):274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allred BE, Rupert PB, Gauny SS, An DD, Ralston CY, Sturzbecher-Hoehne M, Strong RK, Abergel RJ. Siderocalin-mediated recognition, sensitization, and cellular uptake of actinides. Proc Natl Acad Sci U S A. 2015;112(33):10342–10347. doi: 10.1073/pnas.1508902112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leazer TM, Klaassen CD. The presence of xenobiotic transporters in rat placenta. Drug Metab. Disposition. 2003;31(2):153–167. doi: 10.1124/dmd.31.2.153. [DOI] [PubMed] [Google Scholar]
- 28.Zalups RK, Ahmad S. Molecular handling of cadmium in transporting epithelia. Toxicol. Appl. Pharmacol. 2003;186(3):163–188. doi: 10.1016/s0041-008x(02)00021-2. [DOI] [PubMed] [Google Scholar]
- 29.Tekin D, Kayaaltı Z, Aliyev V, Söylemezoğlu T. The effects of metallothionein 2A polymorphism on placental cadmium accumulation: is metallothionein a modifiying factor in transfer of micronutrients to the fetus? J. Appl. Toxicol. 2011;32:270–275. doi: 10.1002/jat.1661. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, Ohba K, Suzuki K, Ohta H. Health effects of low-level cadmium intake and the role of metallothionein on cadmium transport from mother rats to fetus. J Toxicol Sci. 2012;37(1):149–156. doi: 10.2131/jts.37.149. [DOI] [PubMed] [Google Scholar]
- 31.Benitez MA, Mendez-Armenta M, Montes S, Rembao D, Sanin LH, Rios C. Mother-fetus transference of lead and cadmium in rats: involvement of metallothionein. Histol. Histopathol. 2009;24(12):1523–1530. doi: 10.14670/HH-24.1523. [DOI] [PubMed] [Google Scholar]
- 32.Boadi WY, Yannai S, Urbach J, Brandes JM, Summer KH. Transfer and accumulation of cadmium, and the level of metallothionein in perfused human placentae. Arch. Toxicol. 1991;65:318–323. doi: 10.1007/BF01968966. [DOI] [PubMed] [Google Scholar]
- 33.Al-Saleh I, Al-Rouqi R, Obsum CA, Shinwari N, Mashhour A, Billedo G, Al-Sarraj Y, Rabbah A. Interaction between cadmium (Cd), selenium (Se) and oxidative stress biomarkers in healthy mothers and its impact on birth anthropometric measures. Int. J. Hyg. Environ. Health. 2015;218(1):66–90. doi: 10.1016/j.ijheh.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Kawakami T, Nishiyama K, Kadota Y, Sato M, Inoue M, Suzuki S. Cadmium modulates adipocyte functions in metallothionein-null mice. Toxicol. Appl. Pharmacol. 2013;272(3):625–636. doi: 10.1016/j.taap.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Chen C, Yu H, Zhao J, Li B, Qu L, Liu S, Zhang P, Chai Z. The roles of serum selenium and selenoproteins on mercury toxicity in environmental and occupational exposure. Environ. Health Perspect. 2006;114(2):297–301. doi: 10.1289/ehp.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ralston NV, Raymond LJ. Dietary selenium's protective effects against methylmercury toxicity. Toxicology. 2010;278(1):112–123. doi: 10.1016/j.tox.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues EG, Kile M, Dobson C, Amarasiriwardena C, Quamruzzaman Q, Rahman M, Golam M, Christiani DC. Maternal-infant biomarkers of prenatal exposure to arsenic and manganese. J. Exp. Sci. Environ. Epi. 2015;26(6):639–648. doi: 10.1038/jes.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders AP, Miller SK, Nguyen V, Kotch JB, Fry RC. Toxic metal levels in children residing in a smelting craft village in Vietnam: a pilot biomonitoring study. BMC Public Health. 2014;14:114. doi: 10.1186/1471-2458-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoyle RH. Handbook of Structural Equation Modeling. New York, NY: The Guildford Press; 2015. [Google Scholar]
- 40.Roverso M, Berte C, Marco VD, Lapolla A, Badocco D, Pastore P, Visentin S, Cosmi E. The metallome of the human placenta in gestational diabetes mellitus. Metallomics. 2015 doi: 10.1039/c5mt00050e. [DOI] [PubMed] [Google Scholar]
- 41.Kim JT, Son MH, Lee DH, Seong WJ, Han S, Chang YS. Partitioning Behavior of Heavy Metals and Persistent Organic Pollutants among Feto-Maternal Bloods and Tissues. Environ. Sci. Technol. 2015 doi: 10.1021/es5051309. [DOI] [PubMed] [Google Scholar]
- 42.Laine JE, Ray P, Bodnar W, Cable PH, Boggess K, Offenbacher S, Fry RC. Placental Cadmium Levels Are Associated with Increased Preeclampsia Risk. PloS one. 2015;10(9):e0139341. doi: 10.1371/journal.pone.0139341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Saleh I, Shinwari N, Mashhour A, Rabah A. Birth outcome measures and maternal exposure to heavy metals (lead, cadmium and mercury) in Saudi Arabian population. Int. J. Hyg. Environ. Health. 2014;217(2–3):205–218. doi: 10.1016/j.ijheh.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Fowden AL, Sferruzzi-Perri AN, Coan PM, Constancia M, Burton GJ. Placental efficiency and adaptation: endocrine regulation. J. Physiol. 2009;587(Pt 14):3459–3472. doi: 10.1113/jphysiol.2009.173013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallace JM, Horgan GW, Bhattacharya S. Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in women delivering singleton babies. Placenta. 2012;33(8):611–618. doi: 10.1016/j.placenta.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Brett KE, Ferraro ZM, Yockell-Lelievre J, Gruslin A, Adamo KB. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci. 2014;15(9):16153–16185. doi: 10.3390/ijms150916153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cetin I, Parisi F, Berti C, Mando C, Desoye G. Placental fatty acid transport in maternal obesity. J. Develop. Origins Health Dis. 2012;3(6):409–414. doi: 10.1017/S2040174412000414. [DOI] [PubMed] [Google Scholar]
- 48.Brett KE, Ferraro ZM, Holcik M, Adamo KB. Placenta nutrient transport-related gene expression: the impact of maternal obesity and excessive gestational weight gain. The Journal of Maternal-Fetal and Neonatal Medicine. 2015:1–7. doi: 10.3109/14767058.2015.1049522. [DOI] [PubMed] [Google Scholar]
- 49.Colomiere M, Permezel M, Riley C, Desoye G, Lappas M. Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur. J. Endocrinol. 2009;160(4):567–578. doi: 10.1530/EJE-09-0031. [DOI] [PubMed] [Google Scholar]
- 50.Brass E, Hanson E, O'Tierney-Ginn PF. Placental oleic acid uptake is lower in male offspring of obese women. Placenta. 2013;34(6):503–509. doi: 10.1016/j.placenta.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farley DM, Choi J, Dudley DJ, Li C, Jenkins SL, Myatt L, Nathanielsz PW. Placental amino acid transport and placental leptin resistance in pregnancies complicated by maternal obesity. Placenta. 2010;31(8):718–724. doi: 10.1016/j.placenta.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, Jansson T, Powell TL. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J. Clin. Endocrinol. Metab. 2013;98(1):105–113. doi: 10.1210/jc.2012-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He K. Trace elements in nails as biomarkers in clinical research. Eur. J. Clin. Invest. 2011;41(1):98–102. doi: 10.1111/j.1365-2362.2010.02373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinners T, Tsuchiya A, Stern AH, Burbacher TM, Faustman EM, Marien K. Chronologically matched toenail-Hg to hair-Hg ratio: temporal analysis within the Japanese community (U.S.) Environ. Health Perspect. 2012;11:81. doi: 10.1186/1476-069X-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xun P, Liu K, Morris JS, Jordan JM, He K. Distributions and determinants of mercury concentrations in toenails among American young adults: the CARDIA Trace Element Study. Environ. Sci. Pollut. Res. Int. 2013;20(3):1423–1430. doi: 10.1007/s11356-012-1126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mozaffarian D, Shi P, Morris JS, Spiegelman D, Grandjean P, Siscovick DS, Willett WC, Rimm EB. Mercury exposure and risk of cardiovascular disease in two U.S. cohorts. New Engl. J. Med. 2011;364(12):1116–1125. doi: 10.1056/NEJMoa1006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xun P, Hou N, Daviglus M, Liu K, Morris JS, Shikany JM, Sidney S, Jacobs DR, He K. Fish oil, selenium and mercury in relation to incidence of hypertension: a 20-year follow-up study. J. Intern. Med. 2011;270(2):175–186. doi: 10.1111/j.1365-2796.2010.02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Christiani DC, Weisskopf MG. Toenail, blood, and urine as biomarkers of manganese exposure. J. Occup. Environ. Med. 2011;53(5):506–510. doi: 10.1097/JOM.0b013e31821854da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzalez A, Peters U, Lampe JW, Satia JA, White E. Correlates of toenail zinc in a free-living U.S. population. Ann. Epidemiol. 2008;18(1):74–77. doi: 10.1016/j.annepidem.2007.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Rorke MA, Cantwell MM, Abnet CC, Brockman AJ, Murray LJ, Group FS. Toenail trace element status and risk of Barrett's oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. Int. J. Cancer. 2012;131(8):1882–1891. doi: 10.1002/ijc.27434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yaemsiri S, Hou N, Slining MM, He K. Growth rate of human fingernails and toenails in healthy American young adults. J. Eur. Acad. Dermatol. Veneareol. 2010;24(4):420–423. doi: 10.1111/j.1468-3083.2009.03426.x. [DOI] [PubMed] [Google Scholar]
- 62.Seaborg B, Bodurtha J. Nail size in normal infants. Establishing standards for healthy term infants. Clin. Pedi. 1989;28(3):142–145. doi: 10.1177/000992288902800309. [DOI] [PubMed] [Google Scholar]
- 63.Roels HA, Hubermont G, Buchet JP, Lauwerys R. Placental transfer of lead, mercury, cadmium and carbon monoxide in women: III. Factors influencing the accumulation of heavy metals in the placenta and the relationship between metal concentration in the placenta and in maternal and cord blood. Environ. Res. 1977;16(1–3):236–247. doi: 10.1016/0013-9351(78)90159-7. [DOI] [PubMed] [Google Scholar]
- 64.Zadorozhnaja TD, Little RE, RK M, Mendel NA, Taylor RJ, Presley BJ, Gladen BC. Concentrations of arsenic, cadmium, copper, lead, mercuy and zinc in human placentas from two cities in Ukraine. J. Toxicol. Environ. Health, A. 2000;61(4):255–263. doi: 10.1080/00984100050136571. [DOI] [PubMed] [Google Scholar]
- 65.Al-Saleh I, Shinwari N, Mashhour A, Mohamed Gel D, Rabah A. Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int. J. Hyg. Environ. Health. 2011;214(2):79–101. doi: 10.1016/j.ijheh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Kayaaltı Z, Tekin D, Aliyev V, Yalcin S, Kurtay G, Soylemezoglu T. Effects of the interleukin-6 (IL-6) polymorphism on toxic metal and trace element levels in placental tissues. Sci. Total Environ. 2011;409(23):4929–4933. doi: 10.1016/j.scitotenv.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 67.Soria ML, Sanz P, Martinez D, Lopez-Artiguez M, Garrido R, Grilo A, Repetto M. Total mercury and methylmercury in hair, maternal and umbilical blood, and placenta from women in the Seville area. Bull. Environ. Contam. Toxicol. 1992;48(4):494–501. doi: 10.1007/BF00199063. [DOI] [PubMed] [Google Scholar]
- 68.Tsuchiya H, Mitani K, Kodama K, Nakata T. Placental transfer of heavy metals in normal pregnant Japanese women. Arch. Environ. Health. 1984;39(1):11–17. doi: 10.1080/00039896.1984.10545827. [DOI] [PubMed] [Google Scholar]
- 69.Marques RC, Garrofe Dorea J, Rodrigues Bastos W, de Freitas Rebelo M, de Freitas Fonseca M, Malm O. Maternal mercury exposure and neuro-motor development in breastfed infants from Porto Velho (Amazon), Brazil. Int. J. Hyg. Environ. Health. 2007;210(1):51–60. doi: 10.1016/j.ijheh.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Gundacker C, Frohlich S, Graf-Rohrmeister K, Eibenberger B, Jessenig V, Gicic D, Prinz S, Wittmann KJ, Zeisler H, Vallant B, Pollak A, Husslein P. Perinatal lead and mercury exposure in Austria. Sci. Tot. Environ. 2010;408(23):5744–5749. doi: 10.1016/j.scitotenv.2010.07.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.