Reliable predictors of motor improvement in individual patients after stroke are scarce. Acute determination of upper limb Fugl-Meyer assessment (FMA) appears to have predictive value.1,2 This approach predicts that patients will improve approximately 70% of the difference between the maximum upper extremity FMA score and the score first tested for a given individual (recovery-typical). However, a significant subset of patients improves much less than predicted (recovery-atypical). Alternative models using other techniques like diffusion tensor imaging (DTI)3,4 also fail to predict recovery in some patients. Here, we show that a combination of FMA and DTI obtained in the first weeks after stroke accurately discriminate between recovery-typical and recovery-atypical patients. In addition, we identify an alternative set of model parameters required for predictions in the recovery-atypical subgroup.
We examined 25 patients (mean age 61 years, 11 female) with first ischemic stroke. Patients had a mean NIH Stroke Scale score of 11 (table e-1 on the Neurology® Web site at Neurology.org). FMA5 and DTI (appendix e-1) were obtained 2 weeks and 3 months after stroke onset. The FMA was performed by a trained occupational therapist blinded to the DTI results. Corticospinal tract (CST) asymmetry was calculated from the mean fractional anisotropy (FA) of the CST3:
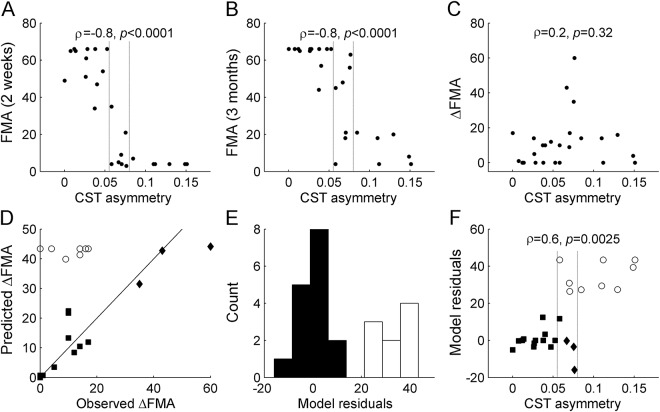
As with previous observations,3,4 patients with greater CST asymmetry at 2 weeks had proportionally more severe motor deficits, both 2 weeks and 3 months after stroke onset (Spearman ρ < −0.8, p < 0.0001, figure 1, A and B). However, CST asymmetry was not significantly related to FMA improvement from 2 weeks to 3 months (figure 1C). These findings were also confirmed when FAispilesional/FAcontralesional ratios were used as predictors rather than CST asymmetry.
Figure 1. Fugl-Meyer assessment (FMA) and diffusion tensor imaging (DTI) predictors of motor improvement.
Corticospinal tract (CST) asymmetry at 2 weeks correlated with the severity of motor deficits at (A) 2 weeks and (B) 3 months after stroke, but not with (C) the change between these 2 time points. (D) The model ΔFMA = 0.7*(66-FMAintial), represented by a continuous line, predicted future FMA improvement in most patients (filled symbols), but there was a significant recovery-atypical subset (n = 9; unfilled circles). (E) Recovery-atypical patients were defined as cases with model residuals >20 (unfilled bars). (F) CST asymmetry was linearly associated with the residuals of the FMA-based model, and accurately discriminated between recovery-typical and recovery-atypical patients. Dashed vertical lines indicate cutoffs for 100% positive and negative predictive values, respectively.
As expected, a majority of patients followed the FMA-based model predictions (figure 1D; filled symbols), while a subgroup of patients exhibited recovery-atypical profiles (figure 1D; unfilled circles). In order to further characterize the 2 patient subgroups, we computed the model residuals as the difference between the predicted FMA improvement and the observed FMA improvement. The residuals clearly differentiate recovery-typical (figure 1E; filled bars) from recovery-atypical patients (unfilled bars). All recovery-atypical patients (model residuals >20) had low initial FMA scores <25. However, this information alone was not capable of segregating the subgroups, as 3 of the 12 patients with initial scores <25 actually displayed typical recovery profiles (figure 1, D and F; filled diamonds). When recovery-atypical patients were excluded, a least-squares linear fit of the maximum possible improvement vs the observed improvement yielded a slope of 0.76, which approaches the values reported previously.1,2 Conversely, recovery-atypical patients only recovered up to 30% of the difference between maximum and initial FMA.
Finally, we combined clinical and DTI assessments and observed that patients with greater CST asymmetry had proportionally greater FMA model residuals (figure 1F; ρ = 0.6, p = 0.0025). A logistic regression with CST asymmetry as a predictor successfully discriminated between recovery-typical and recovery-atypical patients. Leave-one-out cross-validation confirmed a positive predictive value of 0.75, a negative predictive value of 0.82, and an accuracy of 0.80 (p < 0.0008, permutation test). All patients with a CST asymmetry >0.080 showed atypical recovery, while all patients with CST asymmetry <0.058 improved as predicted by the model (vertical lines in figure 1). Lesion size (ρ = −0.2, p = 0.5), lesion extent in the primary motor cortex (ρ = 0.0, p = 0.8), initial NIH Stroke Scale score (ρ = 0.3, p = 0.14), and patient age (ρ = 0.1, p = 0.7) were not associated with model residuals (see also the lesion maps in figure e-1). A partial correlation including these factors as confounding covariates remained significant (ρ = 0.6, p = 0.0026). This confirms that our observation is specific to CST integrity. The change of CST asymmetry from 2 weeks to 3 months after stroke was also significantly associated with model residuals (n = 18, ρ = 0.57, p = 0.013) and the correlation at 3 months was even greater than at 2 weeks (ρ = 0.7, p = 0.0014) (figure e-2).
These findings demonstrate that a combination of FMA and DTI obtained in the first weeks after stroke successfully predicts impairment improvements after stroke. This is in agreement with a recent study6 and extends previous algorithms for prediction of the severity of impairment at the chronic stage7 to prediction of improvement gains. Patients with mild degeneration observed in the CST will recover about 70% of impaired upper limb function, while expected recovery in patients with severe CST degeneration drops to approximately <30%. Recovery of patients with moderate CST degeneration remains relatively unpredictable despite DTI and clinical assessments. Additional variables, such as functional and structural neuroimaging parameters, may further stratify these patients in the future.
Supplementary Material
Acknowledgments
Acknowledgment: The authors thank Jean-Michel Pignat for help with MRI data acquisition.
Footnotes
Supplemental data at Neurology.org
Author contributions: E.R.B. performed the DTI analyses and wrote the paper; S.R. and P.N. performed the DTI analyses; L.G.C. supervised the study and revised the paper; A.S. supervised the study and revised the paper; A.G.G. designed the study, performed the statistical analyses, and wrote the paper.
Study funding: A.G.G. was supported by the Swiss National Science Foundation (grants 320030-129679 and 320030-146639), S.R. by the Fonds Jean Falk-Vairant, and E.R.B. and L.G.C. by the intramural program of the NINDS, NIH.
Disclosure: The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
References
- 1.Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair 2008;22:64–71. [DOI] [PubMed] [Google Scholar]
- 2.Winters C, van Wegen EE, Daffertshofer A, Kwakkel G. Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabil Neural Repair 2015;29:614–622. [DOI] [PubMed] [Google Scholar]
- 3.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007;130:170–180. [DOI] [PubMed] [Google Scholar]
- 4.Kim KH, Kim YH, Kim MS, Park CH, Lee A, Chang WH. Prediction of motor recovery using diffusion tensor tractography in supratentorial stroke patients with severe motor involvement. Ann Rehabil Med 2015;39:570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair 2002;16:232–240. [DOI] [PubMed] [Google Scholar]
- 6.Byblow WD, Stinear CM, Barber PA, Petoe MA, Ackerley SJ. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol 2015;78:848–859. [DOI] [PubMed] [Google Scholar]
- 7.Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain 2012;135:2527–2535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.