Abstract
Objective:
Using a semiautomated volumetric MRI assessment method, we aimed to identify determinants of white matter hyperintensity (WMH) burden in patients with Fabry disease (FD).
Methods:
Patients with confirmed FD and brain MRI available for this analysis were eligible for this protocol after written consent. Clinical characteristics were abstracted from medical records. T2 fluid-attenuated inversion recovery MRI were transferred in electronic format and analyzed for WMH volume (WMHV) using a validated, computer-assisted method. WMHV was normalized for head size (nWMHV) and natural log-transformed (lnWMHV) for univariate and multivariate linear regression analyses. Level of significance was set at p < 0.05 for all analyses.
Results:
Of 223 patients with FD and WMHV analyzed, 132 (59%) were female. Mean age at MRI was 39.2 ± 14.9 (range 9.6–72.7) years, and 136 (61%) patients received enzyme replacement therapy prior to enrollment. Median nWMHV was 2.7 cm3 (interquartile range 1.8–4.0). Age (β 0.02, p = 0.008) and history of stroke (β 1.13, p = 0.02) were independently associated with lnWMHV. However, WMH burden—as well as WMHV predictors—varied by decade of life in this cohort of patients with FD (p < 0.0001).
Conclusions:
In this largest-to-date cohort of patients with FD who had volumetric analysis of MRI, age and prior stroke independently predicted the burden of WMH. The 4th decade of life appears to be critical in progression of WMH burden, as novel predictors of WMHV emerged in patients aged 31–40 years. Future studies to elucidate the biology of WMH in FD and its role as potential MRI marker of disease progression are needed.
Cerebrovascular disease, including stroke, is a significant source of morbidity in patients with Fabry disease (FD), an X-linked lysosomal storage disorder characterized by reduced activity of the enzyme α-galactosidase A.1 Burden of MRI-detectable white matter hyperintensity (WMH) has been linked to risk of stroke,2 poor poststroke outcome,3 and functional disability in aging adults4; however, there are no systematic studies of WMH in patients with FD.
Previously, nonquantitative studies have documented WMH lesions in adults with FD,5,6 pediatric patients,7 and the Fabry Outcome Survey registry participants.8 A comparison of CT and MRI findings in patients with FD suggested the potential utility of a rating scale in this population.9 The recently published Stroke in Young Fabry Patients (SIFAP1) study10 showed detailed neuroimaging MRI characteristics including WMH assessed using a validated ordinal scale11,12; however, it failed to identify any FD-specific MRI characteristics.
Although the pathogenesis of cerebral vasculopathy in FD is poorly understood, white matter changes may at least in part reflect the disease biology in FD. Therefore, we hypothesized that the severity of WMH may serve as a marker of disease progression in FD, and that a reliable, quantitative assessment of WMH burden in FD may yield novel determinants of disease severity. In this analysis, we aimed to identify determinants of WMH burden in a large cohort of patients with FD, using a validated semiautomated volumetric MRI assessment method.
METHODS
Standard protocol approvals, registrations, and patient consent.
All participating participants or their surrogate provided informed consent to be enrolled as part of this ongoing prospective cohort study. All aspects were approved by participating institutional review boards.
Study design, setting, and patient population.
We retrospectively analyzed the multicenter, international cohort of patients with FD, recruited through the specialized care clinics in the United States, Germany, Argentina, France, and Brazil between 2009 and 2014, who consented to participate in this study of MRI-detectable WMH. Diagnosis of FD was confirmed using biochemical or genetic testing, and both male and female participants were approached to participate, without age limitations. All patients with FD with brain MRI available in electronic format were approached for consent at the local centers, where recruitment fliers were available to all potential participants. Indications for brain MRI varied between and within centers including routine screening scans, headache, transient neurologic symptoms, and acute stroke evaluations. When multiple brain MRI scans were available for same participants, the baseline scan corresponding to the time of clinical variable collection was used in this analysis. All enrolled participants had an alphanumeric code assigned, and all medical and digitized MRI information was labeled with this code. Medical history information relevant to FD was collected from the participant's medical record or through personal communication with the participant, or a surrogate, at the onset of participation.
Clinical variables.
Age was recorded at the time of study MRI scan. Adult age range was prespecified as ≥21 years. Hypertension, diabetes mellitus, cardiac arrhythmia, cardiomyopathy (CMP), renal failure, proteinuria, and migraine (including with aura) were recorded based on the prior diagnosis at each specialized care center participating in this study. History of Fabry-associated pain in hands and feet, decreased sweating, and gastrointestinal (GI) problems characteristic for FD were recorded for each participant. History of prior stroke or TIA, hemodialysis, renal or cardiac transplant, current use of tobacco or alcohol, as well as treatment with enzyme replacement therapy (ERT) were abstracted from medical records or obtained in direct interview with the patient or the surrogate.
Neuroimaging analysis.
Image collection.
All MRI available in electronic format were collected, securely stored, and analyzed centrally (Massachusetts General Hospital). T2 fluid-attenuated inversion recovery (FLAIR) axial MRI were converted to Analyze format using MRIcro (www.mricro.com) for computer-assisted determination of WMH volume (WMHV).13–15
WMH volumetric analysis.
The volumetric analysis method used to assess WMH severity in this cohort was published previously.16 All images in this study were analyzed by research staff (N.S.R., L.C., K.M.F., A.S.K.), extensively trained in the volumetric method with the intraclass correlation coefficient for WMHV equal to 0.97.
All WMHV were adjusted for differences in head size by using a validated method for calculating intracranial area (ICA) as a surrogate measure of intracranial volume.17 Normalized WMHV (nWMHV) is calculated by dividing the patient's WMHV by the ratio of the patient's ICA to the mean ICA of the study population.
The total WMHV was computed as a sum of WMHV from each hemisphere, unless WMHV assessment was impeded by a large hemispheric FLAIR hyperintensity artifact from an acute or chronic infarction, in which case the total WMHV was derived by doubling the WMHV from a hemisphere unaffected by stroke (n = 2, both with history of prior stroke).
Statistical analysis.
WMHV values were log-transformed (lnWMHV) for all analyses in this study. All variables were reported as either a mean value (±SD), a median value with an interquartile range [IQR] or a proportion/percentage of total. Univariate linear regression analysis was used to assess the association between WMHV and clinical variables. Those variables that reached a p value <0.10 in univariate analysis were used in the multivariate linear regression analysis of WMHV. Statistical significance was set at p < 0.05 in all analyses. Quantitative variables were treated as continuous variables. The statistical analysis was conducted using the SAS 9.1 statistical package (SAS Institute, Cary, NC).
RESULTS
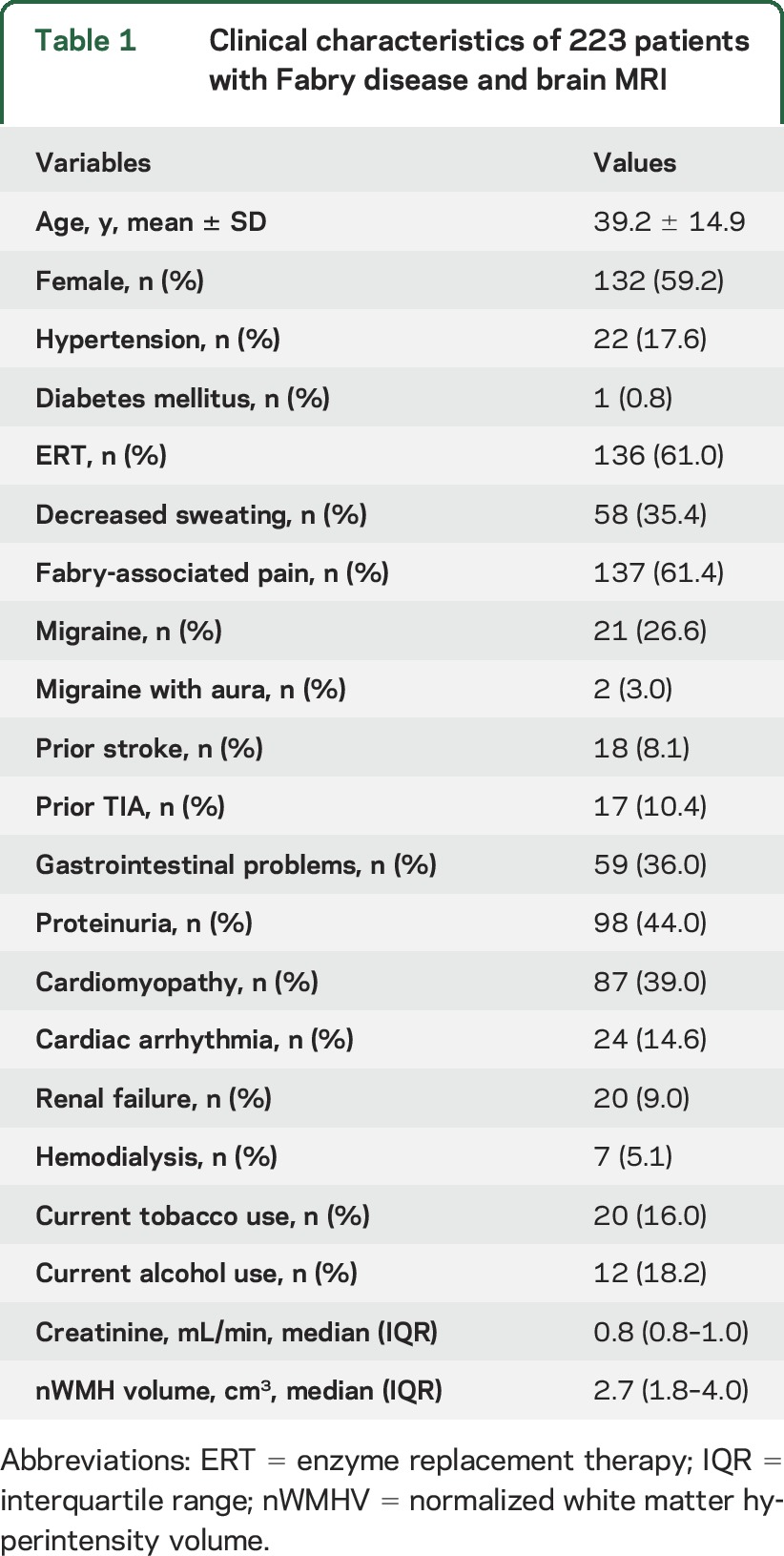
There were 223 patients with FD enrolled in this study: 85 (38.1%) from Germany, 59 (26.5%) from Argentina, 55 (24.7%) from the United States, 13 (5.8%) from France, and 11 (4.9%) from Brazil. Of these, 132 (59.2%) were women. Mean age was 39.2 (SD 14.9) years, ranging from 9 to 72.7 years; however, on average, men were younger (mean age 34.7 years) as compared to women (mean age 42.3 years). Median nWMHV was 2.7 cm3 (IQR 1.8–4.0), ranging from 0.3 to 61.2 cm3. Table 1 summarizes baseline patient characteristics.
Table 1.
Clinical characteristics of 223 patients with Fabry disease and brain MRI
Average WMH burden was similar between men (mean nWMHV = 4.7 cm3) and women (mean nWMHV = 4.9 cm3), as well as those exposed and nonexposed to ERT (p > 0.05). However, average WMHV differed between pediatric patients (age <21 years, mean nWMHV = 2.3 cm3) and adults (age ≥21 years, mean nWMHV = 5.3 cm3; p = 0.05).
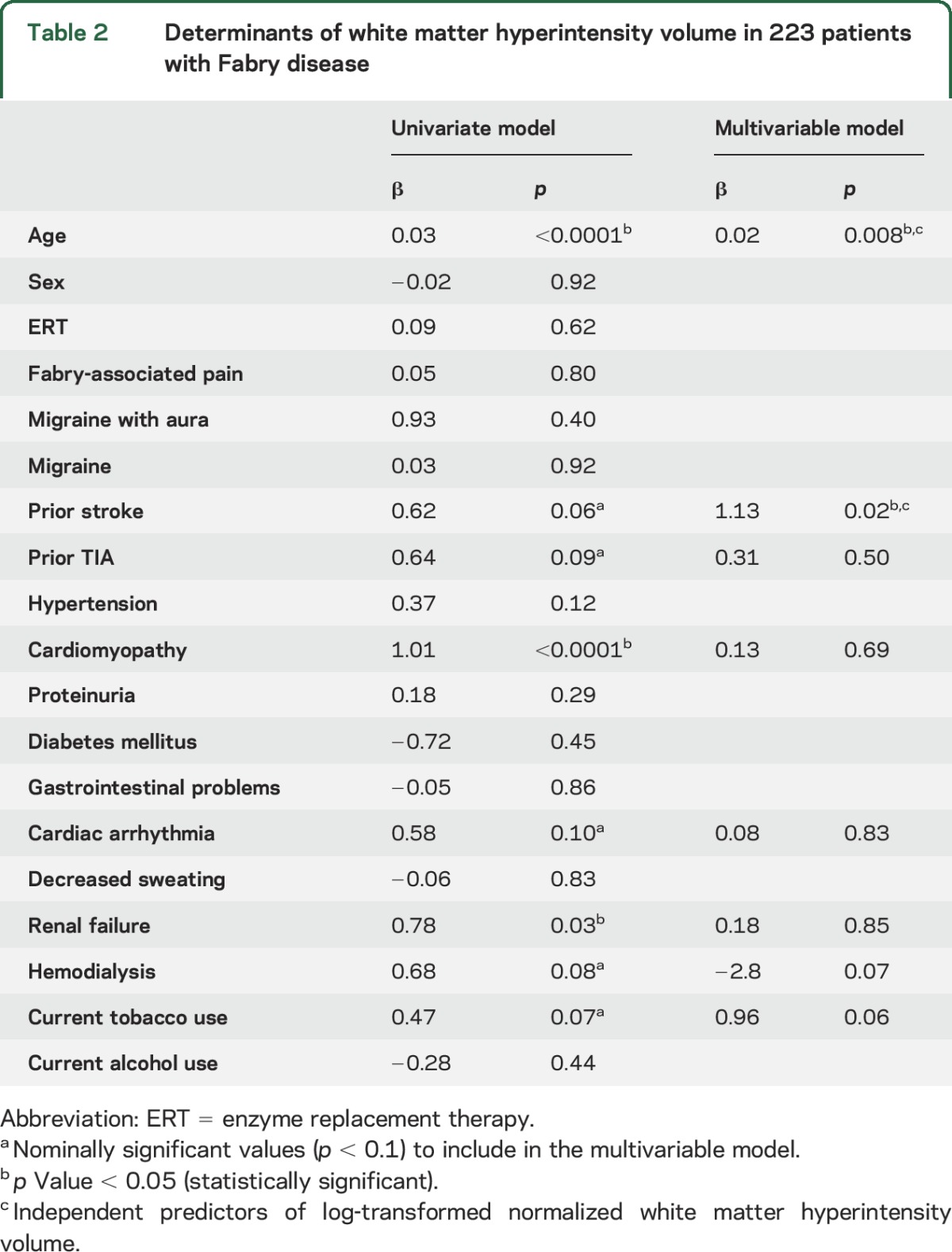
In univariate analysis, age at the time of MRI (β 0.03 per year, p < 0.0001), history of CMP (β 1.01, p < 0.0001), and renal failure (β 0.78, p = 0.03) were associated with lnWMHV (table 2). In the multivariable model including age, as well as history of CMP, renal failure, cardiac arrhythmia, hemodialysis, prior stroke, TIA, or current tobacco use (all p < 0.1 in univariate analysis), only age (β 0.02 per year, p = 0.008) and history of stroke (β 1.13, p = 0.02) were independent predictors of lnWMHV (table 2).
Table 2.
Determinants of white matter hyperintensity volume in 223 patients with Fabry disease
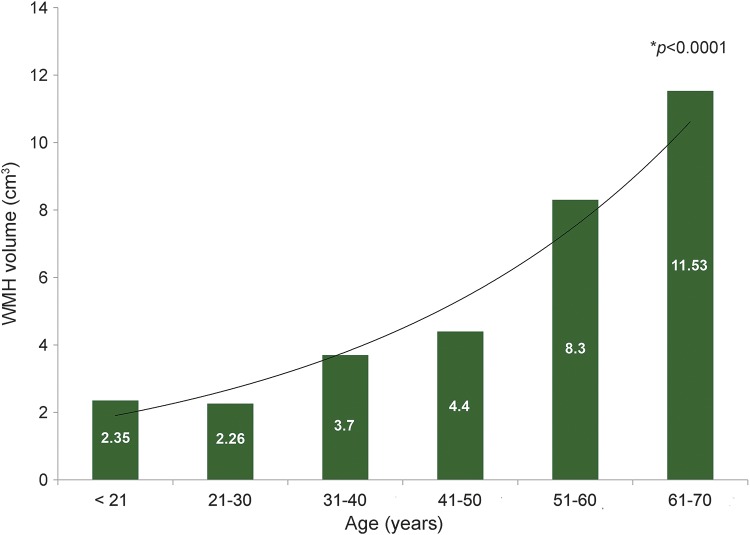
There was a difference in WMH burden, as measured by WMHV among the patients with FD based on the decade of age at MRI: <21 years (n = 28), mean nWMHV 2.35 cm3; 21–30 years (n = 41), mean nWMHV 2.26 cm3; 31–40 years (n = 43), mean nWMHV 3.7 cm3; 41–50 years (n = 51), 4.4 cm3; 51–60 years (n = 33), 8.3 cm3; 61–70 years (n = 19), 11.53 cm3 (p < 0.0001; figure).
Figure. White matter hyperintensity (WMH) burden in Fabry disease varies by decade of life.
WMH volumes (y axis) assessed on the brain MRI of 223 patients with Fabry disease vary by decade of life (x axis). The trend line and mean WMH volume values per each decade are shown. Wilcoxon rank-sum test p value < 0.0001.
The determinants of WMH burden varied by decade of age: <21 years, age (p = 0.0008); 21–30 years, none of the variables reached the nominal p value in univariate association with lnWMHV; 31–40 years, CMP (β 0.6, p = 0.01), prior stroke (β 0.8, p = 0.03), cardiac arrhythmia (β 0.6, p = 0.11), ERT (β −0.3, p = 0.14); 41–50 years, female sex (β −0.6, p = 0.02), prior stroke (β 0.9, p = 0.02), CMP (β 0.4, p = 0.09), cardiac arrhythmia (β 0.5, p = 0.09), Fabry-associated pain in hands and feet (β 0.4, p = 0.12), GI problems (β 0.3, p = 0.16); 51–60 years, proteinuria (β 0.7, p = 0.03), GI problems (β 0.8, p = 0.07), Fabry-associated pain in hands and feet (β 0.5, p = 0.09), female sex (β −0.5, p = 0.15); 61–70 years, CMP (β 1.5, p = 0.005), proteinuria (β 1.2, p = 0.02), TIA (β 1.4, p = 0.05), ERT (β 0.9, p = 0.09), age (β 0.1, p = 0.18) (table 3).
Table 3.
Determinants of white matter hyperintensity burden per decade of life in patients with Fabry disease
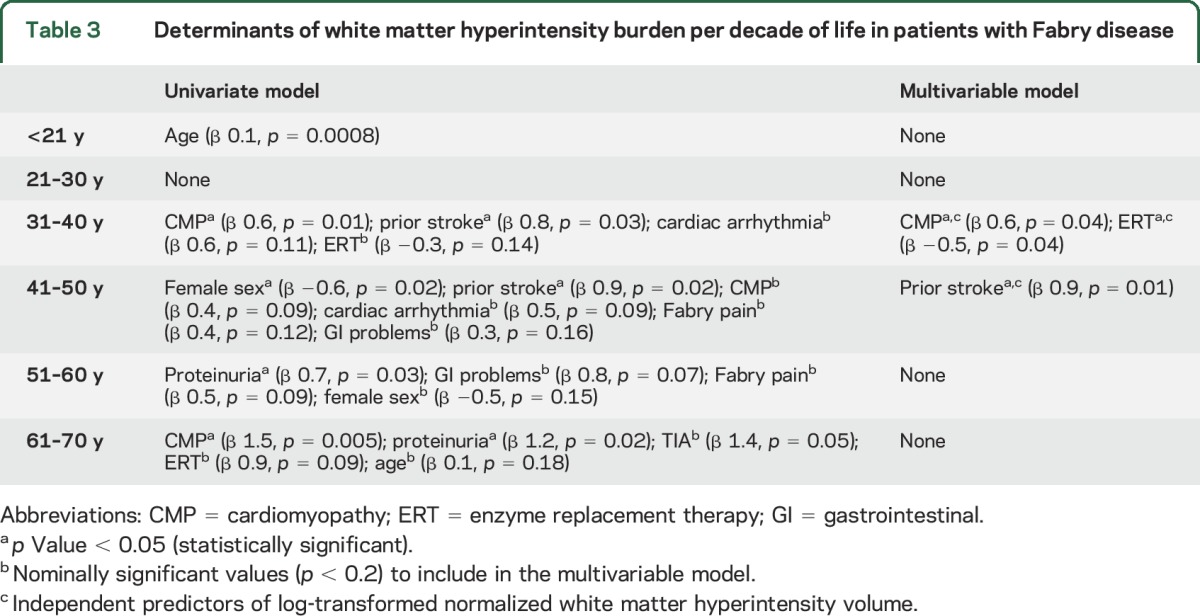
In a multivariable linear regression model including CMP, prior stroke, cardiac arrhythmia, and ERT, history of CMP was independently associated with increased lnWMHV (β 0.6, p = 0.04), whereas prior exposure to ERT was independently associated with decreased lnWMHV (β −0.5, p = 0.04) in patients with FD aged 31–40 years. In a multivariable linear regression model including sex, prior stroke, CMP, cardiac arrhythmia, Fabry-associated pain in hands and feet, and GI problems, only prior stroke was independently associated with increased lnWMHV (β 0.9, p = 0.01) in patients with FD aged 41–50 years (table 3).
DISCUSSION
In this largest-to-date, multicenter, international cohort of patients with confirmed FD and volumetric MRI analysis, we demonstrated that age and history of stroke were independent predictors of WMH burden. Furthermore, the effect of age on WMH burden, as well as predictors of WMHV, varied significantly by decade of life of patients with FD. In particular, novel markers of WMH burden have emerged for patients with FD in their 4th decade of life, when CMP appeared to be independently associated with increased WMHV, while exposure to ERT was independently associated with lesser WMH load.
These novel findings may have implications for future studies of disease biology in FD and for clinical guidance in FD diagnosis and management. FD has long been regarded as a prototype monogenic disorder with distinct cerebrovascular pathology including risk of stroke.18,19 While etiology of stroke in FD is relatively well-defined and is thought to include both extrinsic (i.e., cardioembolism due to atrial fibrillation) and intrinsic (cerebral large-vessel atherosclerosis and small-vessel arteriopathy) mechanisms of cerebral ischemia,18,20 the pathophysiology of diffuse white matter abnormalities detected on T2 FLAIR MRI as WMH remains poorly understood. A small, nonquantitative analysis of 8 patients, studied before and after ERT, suggested deep white matter lesions (WMLs) may precede neurologic events,21 whereas a case report noted amelioration of WMLs in a single patient with FD in the context of ERT.22 Furthermore, a longitudinal MRI analysis based on manual measurement of the WML diameters in 41 patients with FD suggested that these WMLs were more likely to remain stable in patients on ERT.23 These data point to potential FD-specific mechanisms of WMH, which may in fact be affected by ERT, and are possibly explained by changes of the CNS resulting from lysosomal storage.20
Our data indicate that the processes affecting WMH burden might be significantly more complex. First, age emerged as a strong independent predictor of WMHV in the cohort of 223 patients with FD, whose age ranged from 9 to 72 years. Age is the most powerful and consistent predictor of WMH severity across all and any populations studied systematically since its evolvement as a marker and manifestation of diffuse cerebrovascular disease and a risk factor for stroke, cognitive impairment, and functional disability.2,24,4 This indicator of effect consistency by age on WMHV is especially significant considering the lack of direct comparison studies of WMH burden between general populations and patients with cerebrovascular disease. An average WMHV reported for stroke-free participants of the Framingham Offspring Study (mean age 58 ± 10 years) was 0.05 cm3;25 however, virtually no data exist with regard to WMHV in the young with exception of patients with early-onset stroke (mean age 46.1 ± 7.5 years) with average WMHV reported at 0.9 cm3.26 While no direct comparison could be made between these very different populations, mean WMHV of the relatively young FD cohort (mean age 39.2 ± 14.9 years) appears to be significantly greater at 4.7 cm3 in this analysis, on average.
Second, history of prior stroke was significantly associated with WMHV in this FD cohort. While no systematic data on prior stroke subtypes in these patients were available, WMH severity is known to be strongly linked to small-vessel (lacunar) stroke subtype in ischemic stroke patients without FD.16 One mechanism by which small-vessel infarcts may contribute to the overall burden of WMHV is by the long-term evolution of white matter lesion imaging appearances on T2 MRI.27 Thus, our findings may potentially point to the etiology of prior strokes among the patients with FD in this analysis; however, future dedicated analyses of stroke subtypes in patients with FD will be needed to fully assess this question.
Third, variability in WMH burden and its determinants emerged in the analysis of WMHV by decade of life of the patients with FD. It appears that at different disease stages, unique FD comorbidities may be associated with WMH accumulation reflecting the underlying mechanisms of pathobiology of progression. Children and adolescents under age 21 had the lowest WMHV, as expected; however, with the rise in prevalence of FD-specific comorbidities over time, WMH burden appeared to increase as well. In fact, the first 3 decades of life may represent the vulnerable period, during which the FD-related injury to the brain might accumulate along with injury to other organs such as heart, but ultimately manifest in patients with FD aged 31–40 years, when cardiac pathology (CMP) is independently linked to WMH severity. In the same age group, ERT exposure appears to decrease WMHV. Given that diffusion tensor imaging–detected microstructural damage to white matter in FD is known to exist,28 one might hypothesize that patients exposed to ERT prior to the 4th decade of life might exhibit lesser burden of WMH. Similarly, the effect of other interventions in FD might delay WML progression during this period; however, dedicated studies are needed to further test this hypothesis.
Beyond the 4th and 5th decade of life in patients with FD, several other Fabry comorbidities emerge in association with WMHV, possibly reflective of the shared disease pathology contributing to multiorgan damage: heart (CMP, cardiac arrhythmia), kidney (proteinuria), GI problems, Fabry-associated pain (small fiber neuropathy), and symptomatic cerebral ischemia (prior stroke and TIA). Interestingly, female sex was associated with lower WMHV in patients with FD aged 41–60, albeit not independently, possibly pointing toward a protective property of a heterozygous status. However, female participants in this study were not tested for X chromosome inactivation and prior studies have shown that female patients who seek medical attention are more severely affected by FD than their counterparts.29 Otherwise, with exception of history of stroke being an independent predictor of WMHV in the 5th decade of life, the profile of WMH burden determinants appears to reflect the spectrum of accumulating comorbidities typical in the lifetime of FD. Overall, caution should be exercised in interpreting the results of subset analyses, which were exploratory in nature and based on a relatively small number of patients included in per stratum analysis, limiting the power to discover significant associations with the outcome.
There are several limitations of this study. Patients were recruited from multiple sites; thus, the specifics of each individual site operations including the diagnosis and management of FD might have potentially influenced the recruitment and enrollment process. An effort to create a minimum dataset of relevant clinical FD variables resulted in the informative database for this analysis. However, a number of important characteristics were not systematically captured across different sites, including, for example, plasma cholesterol levels, prior stroke subtypes, timing of prior strokes, and indication for brain MRI. This variability in data collection is a typical, albeit significant, limitation of the retrospective study design, and does pose a risk of confounding in our analysis. If this were the case, the variability would have effectively modified the results of association in favor of a null hypothesis.
Another limitation of this study is the lack of systematic data with regard to the native enzyme activity among the patients with FD included in this analysis. As a result, we are unable to identify a proportion of potential FD variants within this cohort, and therefore, not able to further evaluate the effect of classic vs variant FD subtype on the overall burden of WMH measured on brain MRI. Future analyses of larger FD cohorts, with detailed biochemical characterization, will be needed to assess this specific question.
Furthermore, we were limited by the lack of detailed information on the duration and consistency of ERT in this cohort, including potential ERT interruption due to shortages in the United States from September 2010 to March 2012. In the absence of these data, as well as a very limited longitudinal set of brain MRI, no meaningful cohort conclusions could be made regarding white matter disease progression and the potential modifying effects of ERT.
Strengths of this study include the following: (1) it is the largest-to-date cohort of patients with FD with brain MRIs; (2) the study was coordinated centrally, including the standardization of the clinical variable data abstraction and MRI analysis; and (3) the MRI scans were analyzed using a validated, semiautomated volumetric method, which has been adapted to the analysis of clinical scans and conducted by highly trained neuroimaging research analysts.
Current markers of CNS disease burden in FD are limited to event histories (such as TIA or stroke), and although clinically significant and not infrequent, these events represent an inadequate measure of disease burden and ongoing disease progression or improvement. Using a validated, quantitative MRI-based method of WMH analysis, we have been able to demonstrate that age and prior stroke are independent predictors of WMHV in patients with FD. Furthermore, we discovered that burden of WMH, as well as its determinants, varies by decade of life in this unique cohort of patients. These data highlight the lifelong CNS effects of FD-specific processes, and the potential timeline for intervention with disease-modifying treatments. Quantitative WMH analysis offers an opportunity for an accurate MRI-based biomarker such as WMHV to be used in natural history assessment, hypothesis-driven testing of associated FD comorbidities, and in the future, as a potential marker of efficacy of proposed therapies.
GLOSSARY
- CMP
cardiomyopathy
- ERT
enzyme replacement therapy
- FD
Fabry disease
- FLAIR
fluid-attenuated inversion recovery
- GI
gastrointestinal
- ICA
intracranial area
- IQR
interquartile range
- nWMHV
normalized white matter hyperintensity volume
- SIFAP1
Stroke in Young Fabry Patients
- WMH
white matter hyperintensity
- WMHV
white matter hyperintensity volume
- WML
white matter lesion
AUTHOR CONTRIBUTIONS
Natalia S. Rost: study concept/design, statistical analysis/interpretation of data, drafting/revising the manuscript for intellectual content. Lisa Cloonan: data acquisition, revising the manuscript for intellectual content. Allison S. Kanakis: data acquisition, revising the manuscript for intellectual content. Kaitlin Fitrzpatrick: data acquisition, revising the manuscript for intellectual content. Danielle R. Azzariti: data acquisition, revising the manuscript for intellectual content. Virginia Clark: data acquisition, revising the manuscript for intellectual content. Charles M. Lourenco: data acquisition, revising the manuscript for intellectual content. Dominique P. Germain: data acquisition, revising the manuscript for intellectual content. Juan M. Politei: data acquisition, revising the manuscript for intellectual content. György A. Homola: data acquisition, revising the manuscript for intellectual content. Nurcan Üçeyler: data acquisition, revising the manuscript for intellectual content. Claudia Sommer: data acquisition, revising the manuscript for intellectual content. Katherine B. Sims: study concept/design, data acquisition, interpretation of data, drafting/revising the manuscript for intellectual content.
STUDY FUNDING
Supported by NIH-NINDS K23NS064052 (N.S.R.) and an investigator sponsored grant from Genzyme (K.B.S, PI).
DISCLOSURE
N. Rost received honoraria for serving as consultant to Genzyme. L. Cloonan, A. Kanakis, K. Fitzpatrick, D. Azzariti, and V. Clarke report no disclosures relevant to the manuscript. C. Lourenco received honoraria for speakers' fees from Actelion, Genzyme, and Shire HGT. All fees are donated to the CML Medical Foundation for Research and Diagnosis Support Research BV for research support. D. Germain received research grants from Genzyme and Shire. J. Politei and G. Homola report no disclosures relevant to the manuscript. C. Sommer received honoraria and research support from Genzyme Corp. N. Üçeyler received honoraria, travel grants, and research support from Genzyme Corp. and Shire Corp. K. Sims received investigator-sponsored grant from Genzyme and served on Genzyme sponsored Fabry Registry Advisory Board. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Germain DP. Fabry disease. Orphanet J Rare Dis 2010;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuller LH, Longstreth WT, Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ, Jr; Cardiovascular Health Study Collaborative Research Group. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke 2004;35:1821–1825. [DOI] [PubMed] [Google Scholar]
- 3.Arsava EM, Rahman R, Rosand J, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology 2009;72:1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfson L, Wei X, Hall CB, et al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci 2005;232:23–27. [DOI] [PubMed] [Google Scholar]
- 5.Crutchfield KE, Patronas NJ, Dambrosia JM, et al. Quantitative analysis of cerebral vasculopathy in patients with Fabry disease. Neurology 2007;50:1746–1749. [DOI] [PubMed] [Google Scholar]
- 6.Fellgiebel A, Müller MJ, Mazanek M, et al. White matter lesion severity in male and female patients with Fabry disease. Neurology 2005;65:600–602. [DOI] [PubMed] [Google Scholar]
- 7.Polieti JM, Capizzano AA. Magnetic resonance image findings in 5 young patients with Fabry disease. Neurologist 2006;12:103–105. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg L. Nervous system manifestations of Fabry disease: data from FOS: the Fabry Outcome Survey. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives From 5 Years of FOS. Oxford: Oxford PharamGenesis; 2006. [PubMed] [Google Scholar]
- 9.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001;32:1318–1322. [DOI] [PubMed] [Google Scholar]
- 10.Rolfs A, Fazekas F, Grittner U, et al. Acute cerebrovascular disease in the young: the stroke in young Fabry patients study. Stroke 2014;44:340–349. [DOI] [PubMed] [Google Scholar]
- 11.Fazekas F, Enzinger C, Schmidt R, et al. MRI in acute cerebral ischemia of the young: the Stroke in Young Fabry Patients (SIFAP1) study. Neurology 2013;81:1914–1921. [DOI] [PubMed] [Google Scholar]
- 12.Fazekas F, Enzinger C, Schmidt R, et al. Brain magnetic resonance imaging findings fail suspect Fabry disease in young patients with an acute cerebrovascular event. Stroke 2015;46:1548–1553. [DOI] [PubMed] [Google Scholar]
- 13.Chen YW, Gurol ME, Rosand J, et al. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology 2006;67:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology 2006;66:23–29. [DOI] [PubMed] [Google Scholar]
- 15.Nandigam RN, Chen YW, Gurol ME, Rosand J, Greenberg SM, Smith EE. Validation of intracranial area as a surrogate measure of intracranial volume when using clinical MRI. J Neuroimaging 2007;17:74–77. [DOI] [PubMed] [Google Scholar]
- 16.Rost NS, Rahman RM, Biffi A, et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology 2010;75:1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson KJ, Wardlaw JM, Edmond CL, Deary IJ, MacLullich AM. Intracranial area: a validated method for estimating intracranial volume. J Neuroimaging 2005;15:76–78. [DOI] [PubMed] [Google Scholar]
- 18.Meschia JF, Worral BB, Rich SS. Genetic susceptibility to ischemic stroke. Nat Rev Neurol 2011;7:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi JC. Genetics of cerebral small vessel disease. J Stroke 2015;17:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolodny E, Fellgiebel A, Hilz MJ, et al. Cerebrovascular involvement in Fabry disease. Stroke 2015;46:302–313. [DOI] [PubMed] [Google Scholar]
- 21.Jardim L, Vedolin L, Schwartz IV, et al. CNS involvement in Fabry disease: clinical and imaging studies before and after 12 months of enzyme replacement therapy. J Inherit Metab Dis 2004;27:229–240. [DOI] [PubMed] [Google Scholar]
- 22.Yamadera M, Yokoe M, Beck G, et al. Amelioration of white-matter lesions in a patient with Fabry disease. J Neurol Sci 2009;279:118–120. [DOI] [PubMed] [Google Scholar]
- 23.Fellgiebel A, Gartenschläger M, Wildberger K, et al. Enzyme replacement therapy stabilized white matter lesion progression in Fabry disease. Cerebrovasc Dis 2014;38:448–456. [DOI] [PubMed] [Google Scholar]
- 24.de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology 2001;56:1539–1545. [DOI] [PubMed] [Google Scholar]
- 25.Pikula A, Beiser AS, DeCarli C, et al. Multiple biomarkers and risk of clinical and subclinical vascular brain injury: the Framingham Offspring Study. Circulation 2012;125:2100–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C, Cloonan L, Fitzpatrick KM, et al. Determinants of white matter hyperintensity burden differ at the extremes of ages of ischemic stroke onset. J Stroke Cerebrovasc Dis 2015;24:649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wardlaw JM, Smith EE, Biessels GJ, et al. ; STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paavilainen T, Lepomäki V, Saunavaara J, et al. Diffusion tensor imaging and brain volumetry in Fabry disease patients. Neuroradiology 2013;55:551–558. [DOI] [PubMed] [Google Scholar]
- 29.Echevarria L, Benistan K, Toussaint A, et al. X-chromosome inactivation in female patients with Fabry disease. Clin Genet 2016;89:44–54. [DOI] [PubMed] [Google Scholar]