Abstract
Objective:
Because leisure-time physical activity (LTPA) is protective against incident dementia, we hypothesized that LTPA is protective against decline in domain-specific cognitive performance.
Methods:
As part of the Northern Manhattan Study, LTPA was ascertained at enrollment using a validated in-person questionnaire. We assessed cognition in participants in the Northern Manhattan Study MRI substudy using a standard neuropsychological examination (NPE) (n = 1,228), and a repeat examination was performed 5 years later (n = 876). LTPA was summarized as the maximum intensity of any activity performed, classified as none to light intensity (physical inactivity) (90%) vs moderate to heavy intensity (10%). The NPE was subcategorized using standardized z scores over validated domains: processing speed, semantic memory, episodic memory, and executive function. We used multivariable linear regression models to examine the association of LTPA with initial and change in cognitive performance. Analyses were adjusted for sociodemographics, cardiovascular disease risk factors, and MRI findings (white matter hyperintensity volume, silent brain infarcts, cerebral volume).
Results:
No/low levels of LTPA were associated with worse executive function, semantic memory, and processing speed scores on the first NPE. The associations were slightly attenuated and no longer significant after adjusting for vascular risk factors. Cognitively unimpaired participants reporting no/low LTPA vs moderate/high levels declined more over time in processing speed (β = −0.231 ± 0.112, p = 0.040) and episodic memory (β = −0.223 ± 0.117, p = 0.057) adjusting for sociodemographic and vascular risk factors.
Conclusions:
A low level of LTPA is independently associated with greater decline in cognitive performance over time across domains.
With an expected increase in the mean age and proportion of the population older than 65 years, the public health burden of cognitive impairment and dementia will become substantial.1 There is substantial evidence for a contribution of subclinical cerebrovascular disease to dementia, both through direct vascular injury to the brain and/or modification of neurodegenerative processes. Increasing leisure-time physical activity (LTPA) is one target for dementia prevention because it is modifiable, does not require the use of medications, and provides substantial benefits for other diseases of aging.
Prior studies have shown associations between LTPA and cognitive performance,2–8 and a recent meta-analysis9 has demonstrated a dose-response association between LTPA and subsequent risk of dementia. These studies tend to show a protective effect on only vascular dementia or on Alzheimer disease alone.10,11 Not all groups have documented an association between physical activity and cognition in older individuals.5,12,13 Randomized clinical trials of LTPA programs have shown conflicting results,14–16 with results suggesting improvement in functional but not cognitive status. An NIH State of the Science Statement suggested that there may be a protective effect of LTPA on cognitive decline, based on “low-quality” data.17,18 A Cochrane database review concluded that there is insufficient evidence to support the effect of LTPA on cognitive decline in older people.19
Several gaps in knowledge remain in our understanding of the role LTPA may have on cognitive decline. Most studies used a single measure of cognitive performance obtained at the same time as (or after) the LTPA assessment, rather than a change over time in cognitive scores. These studies also focused on crude measures of cognitive performance, such as the Mini-Mental State Examination, rather than comprehensive measures of cognition obtained from neuropsychological examinations (NPEs).20 There are few studies on populations older than 65 years and on Spanish speakers residing in the United States. Furthermore, prior studies have not considered the role that cognitive reserve and baseline cognitive impairment may have on subsequent cognitive decline. The aim of this study was to characterize the independent association of LTPA with baseline and change in cognitive performance in a racially/ethnically diverse elderly population. We hypothesized that LTPA would be protective against cognitive decline across multiple domains and that the effect would be modified by baseline crystallized abilities (Gc), an assessment of lifetime intellectual achievement as measured through vocabulary and general knowledge.
METHODS
Recruitment of the cohort.
The Northern Manhattan Study (NOMAS) is a population-based prospective cohort study designed to evaluate the effects of various risk factors on the incidence of stroke in a stroke-free racially/ethnically diverse community cohort.21 Eligible participants had never been diagnosed with a stroke, were older than 40 years, and resided in Northern Manhattan for ≥3 months, in a household with a telephone. Participants were identified by random-digit dialing and recruited from the telephone sample to have an in-person baseline interview and assessment (n = 3,298). Starting in 2003, stroke-free participants were invited to participate in an MRI substudy if they were older than 50 and had no contraindications to MRI (n = 2,636). Of these, 488 had strokes or died before enrollment and an additional 1,057 had contraindications (n = 133), refused (n = 578), or had other reasons for not participating (n = 347). To reach recruitment targets, 199 stroke-free participants who were household members, but not first-degree relatives, of existing NOMAS participants were enrolled for a total sample of 1,290.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review boards at Columbia University Medical Center and the University of Miami Miller School of Medicine. All participants provided written informed consent.
Cohort evaluation.
Data regarding baseline demographics and risk factors were collected through interviews of participants by trained bilingual research assistants. Race/ethnicity was determined by self-identification. Standardized questions were asked regarding cardiovascular disease risk factors, such as hypertension and diabetes, as previously described. Smoking was categorized as current (within the past year), former, or never smoker of cigarettes, cigars, or pipes. Moderate alcohol use was defined as current drinking of >1 drink per month and ≤2 drinks per day. Insurance status was defined as having no insurance or Medicaid vs having private insurance/Medicare.22 Educational achievement was self-reported as number of years in school and degree achieved.
An NPE was administered in a quiet room by trained bilingual research assistants in English or Spanish, on the same day as the MRI. An exploratory factor analysis as well as a review of findings reported in previous studies informed the selection of tests for each cognitive domain and Gc.14,15 The z scores for processing speed, executive function, semantic memory, episodic memory, and Gc were calculated by averaging z-transformed neuropsychological test scores. Specifically, episodic memory was assessed with 3 subscores on a 12-word 5-trial list-learning task: list learning total score, delayed recall score, and delayed recognition score. Executive function was assessed with 2 subscores: the difference in time to complete the Color Trails Test, Form 1 and Form 2, and the sum of the Odd-Man-Out subtests 2 and 4. Processing speed was assessed with the Grooved Pegboard task in the nondominant hand, the Color Trails Test, Form 1, and the Visual-Motor Integration test.16 Semantic memory was assessed with 3 tests: picture naming (modified Boston Naming Test), category fluency (Animal Naming), and phonemic fluency (C, F, L in English speakers and F, A, S in Spanish speakers). Baseline Gc was assessed with the Wide Range Achievement Test for English Speakers, the Word Accentuation Test for Spanish speakers, and English or Spanish version of the Peabody Picture Vocabulary Test, third edition revised.23–25 Cognitive impairment at the time of initial neuropsychological testing was defined as having a z score of less than −1.5 on any of the initial cognitive domain scores.
Five years after the initial MRI, the cohort was invited to participate in a second NPE. For the change from initial to second NPE, the primary outcome in this study, we regressed the individual test scores at the second assessment on the corresponding initial test scores adjusting for age, education, and time interval between the 2 assessments using linear regressions, and used the standardized residuals from the regression models as the standardized change NPE test scores.26 The changes in neuropsychological domain scores were then obtained by averaging the relevant individual standardized residuals.
Imaging for subclinical cerebrovascular disease was performed on a 1.5T MRI system (Philips Medical Systems, Best, the Netherlands) at the Hatch Research Center. The processing of MRI scans to quantify white matter hyperintensity and cerebral, lateral ventricular, and intracranial volumes, and to identify subclinical brain infarcts, has been described previously.27
Assessment of LTPA.
LTPA was measured using an in-person questionnaire adapted from the National Health Interview Survey of the National Center for Health Statistics.28 The questionnaire captures the duration and frequency of LTPA performed for exercise for the 2 weeks before the interview. Participants were then asked whether they performed any LTPA in the preceding 2 weeks, and those who answered no were coded as inactive. For each activity, duration of activity and the number of times engaged in this same activity were obtained. If the duration of activity was less than 10 minutes, it was coded as “no activity.” This questionnaire has been previously reported as reliable and valid in this population,29 and correlates with body mass index and activities of daily living. Objective measures of physical fitness correlate well with LTPA questionnaires.30 Questionnaires were correlated with compendia of LTPA to summarize activity in metabolic equivalents (METs).31 Participant activity was defined as the maximum intensity (in METs) of any activity performed and as moderate–heavy activity (MET ≥6), light intensity (<6), and none. For analytical purposes, we combined light intensity and no activity as the referent groups because only moderate–heavy activity was protective against stroke in our cohort.29
Statistical analysis.
Baseline demographics by LTPA categories were compared using χ2 tests for categorical variables and Student t tests for continuous variables.
The primary exposure was LTPA summarized as a categorical variable, with moderate–heavy LTPA as the reference, and no/light LTPA as the main exposure of interest. A secondary analysis was conducted in which the no and light LTPA were examined separately, with no LTPA as the reference. This analysis (table e-1 on the Neurology® Web site at Neurology.org) showed similar cognitive performance and decline over time between these 2 groups, while the conclusions regarding cognition in the moderate–heavy LTPA group remained consistent, confirming the primary analysis that grouped no/light LTPA together as the referent category. A series of multivariable-adjusted linear regression models were constructed to examine the association between LTPA and the 4 cognitive domains (executive function, memory, language, processing speed) at the first NPE, and with the z scores for the change in the domains from first assessment to follow-up assessment. Model 1 was adjusted for age at MRI, sex, education, medical insurance status (Medicaid or no insurance vs Medicare or private insurance), Gc, and the time from baseline to first cognitive assessment. Model 2 was additionally adjusted for modifiable vascular risk factors (smoking, moderate alcohol consumption, body mass index, hypertension, diabetes). Model 3 was adjusted for variables in model 1 plus MRI markers (subclinical brain infarct, white matter hyperintensity volume, cerebral volume). The analyses of change in cognitive performance did not include age at MRI or education as covariates because these variables were included in the derivation of the z scores. We examined potential effect modification by Gc and the MRI markers by including interaction terms between these variables and LTPA in model 3. We ran sensitivity analyses excluding participants who were cognitively impaired at baseline based on initial domain z scores, which was defined as having a z score of less than −1.5 on any one or more of the cognitive domains (n = 247). All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Description of the cohort.
The study population included NOMAS MRI cohort members (n = 1,228) with LTPA measures and NPEs at baseline. Baseline characteristics of the cohort are presented in table 1. Our cohort is elderly (mean age = 71 years) with a high proportion of Hispanics and participants who have not completed high school. Participants with 1 vs 2 NPEs did not differ by the measure of LTPA. The prevalence of participants who reported moderate–heavy physical activity was 10%. The scores on the NPE declined between the first and second examination in all 4 domains, with a slightly greater decline in processing speed compared to other domains.
Table 1.
Study cohort characteristics
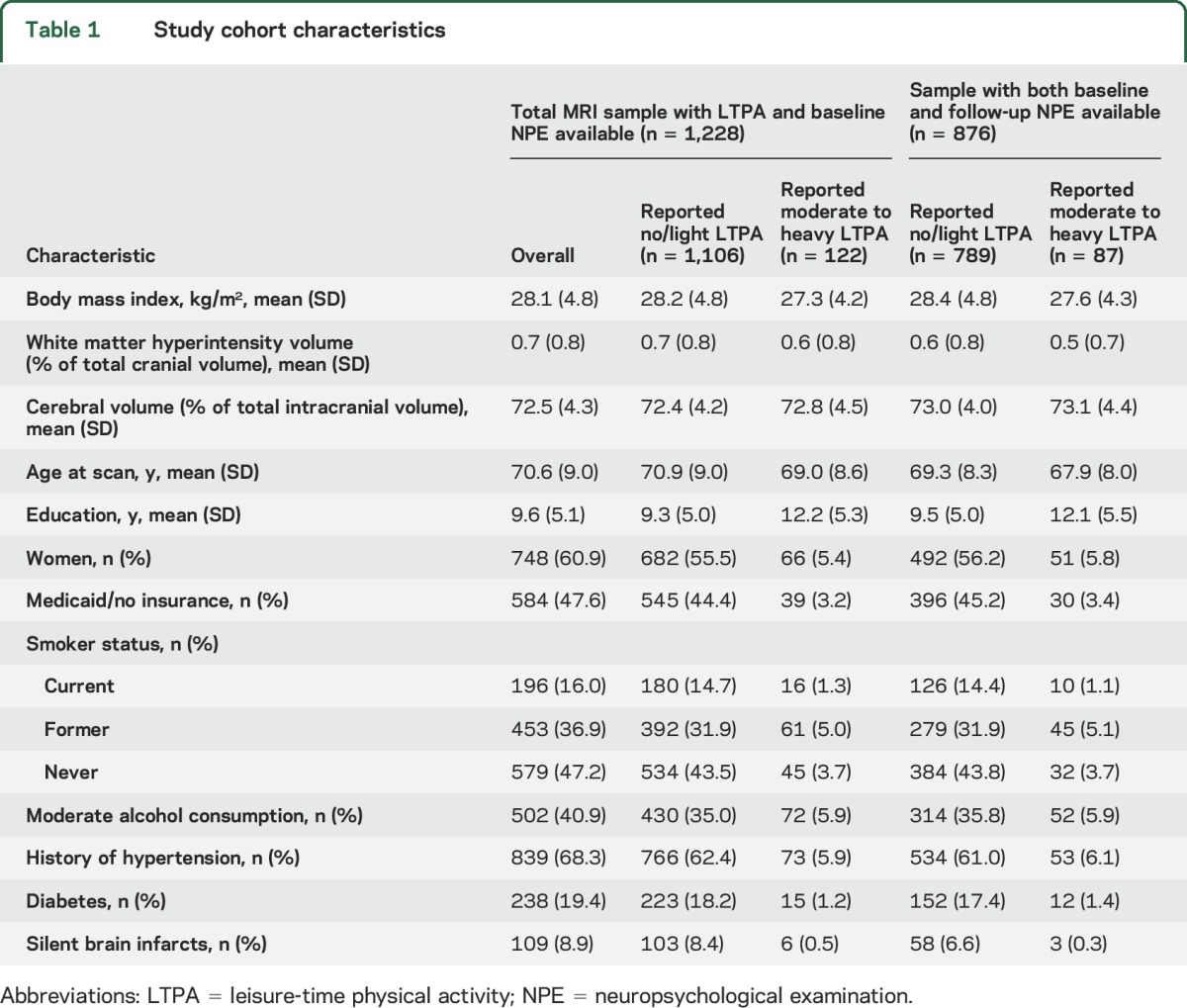
Association of LTPA and initial NPE.
In models adjusted for baseline demographics at the time of MRI (age, sex, education, insurance, Gc, and time from baseline to first cognitive assessment), no/light LTPA was associated with worse scores in all cognitive domains compared to moderate- to heavy-intensity LTPA, although the association was not statistically significant for episodic memory (table 2). After adjusting for modifiable stroke risk factors and MRI findings, the results attenuated slightly and were no longer significant. In a sensitivity analysis, we restricted the sample to those without evidence of cognitive impairment at baseline and found similar results, with worse scores on processing speed for those who were physically inactive. Similarly, this association was slightly attenuated and failed to reach statistical significance in models adjusting for vascular risk factors and MRI variables (table 2). We found no interactions between LTPA and Gc or MRI markers in relation to any of the cognitive domains (data not shown).
Table 2.
Association of no/light leisure-time physical activity (vs moderate–heavy) and initial cognitive performance by domain
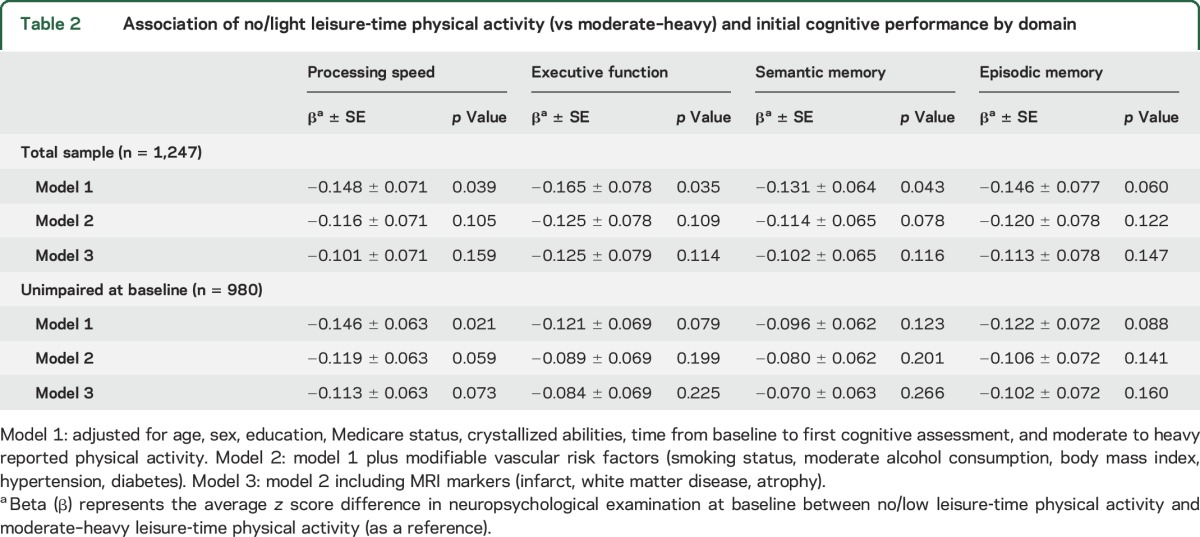
Association of LTPA with change in NPE scores.
When we examined change in cognitive performance over time, we found similar associations. Participants reporting no/light LTPA compared to moderate- to heavy-intensity LTPA showed greater decline over time in processing speed, adjusting for sociodemographic and vascular risk factors, but the difference no longer reached significance after adjusting for MRI markers (table 3). For episodic memory, the decline in scores was of a similar magnitude to that seen for processing speed for participants who were physically inactive compared to those reporting moderate–heavy LTPA but this did not reach significance adjusting for sociodemographic and vascular risk factors (p = 0.057, table 3). There was no association between LTPA and change in executive function or semantic memory. We also did not find any interactions between LTPA and Gc or MRI markers in relation to change in neuropsychological performance (data not shown). In additional analyses, we examined moderate- to heavy-intensity, light-intensity, and no-activity groups separately (tables e-1 and e-2). We found that those with moderate–heavy activity had higher baseline scores and slower decline in comparison to inactive patients, while those with light activity had no association with initial or change in NPE.
Table 3.
Association of no/light physical activity at baseline (vs moderate–heavy) and change in cognitive performance by domain
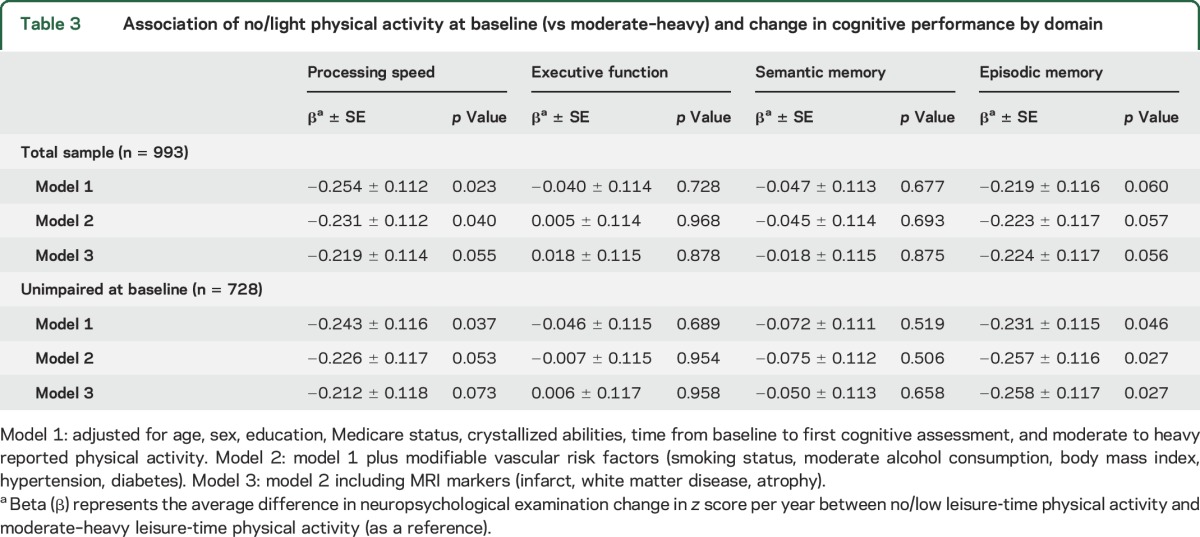
Association of LTPA with change in NPE scores excluding those with cognitive impairment at initial NPE.
When we excluded participants with evidence of cognitive impairment at initial NPE assessment, participants reporting no/light LTPA declined significantly more in processing speed and episodic memory compared to participants reporting moderate–heavy LTPA adjusting for sociodemographic and vascular risk factors (table 3). After further adjusting for structural MRI findings, the association remained significant for episodic memory (p = 0.027) but attenuated slightly for processing speed and no longer reached significance (p = 0.073). The magnitude of the decrease in episodic memory between the 2 NPEs was equivalent to 10 years of aging in our multivariable models (p < 0.001).
DISCUSSION
In this racially/ethnically diverse cohort of older adults, we found that, compared to moderate–heavy LTPA, no or low leisure-time physical activity was associated with a greater decline in processing speed among all participants, and episodic memory among those unimpaired at baseline. The degree of decline was equivalent to the expected decline associated with approximately 10 years of cognitive aging. Our study is unique in demonstrating that the different effect on decline in processing speed over time between 2 LTPA groups may be more appreciable among those without initial cognitive impairment. This observation is consistent with the paradigm that interventions aimed at preventing cognitive decline need to occur before symptoms are manifest. Similar to prior studies in non-Hispanic white populations, our results provide evidence that physical activity may thus delay the onset of cognitive decline, and that effect could be more prominent among those who are cognitively intact at baseline and add to the growing data establishing modifiable risk factors for dementia.32–34 Prior studies have limitations for understanding whether or not all individuals will have a protective effect against cognitive decline from LTPA. Our findings inform the role of LTPA in slowing the rate of cognitive decline in the presymptomatic state before pathology may become irreversible.9
The hypothesized mechanisms of action for the protective role of LTPA on cognition have been related to 2 broad categories: vascular and nonvascular. LTPA reduces the incidence of hypertension and diabetes and ameliorates end-organ damage, including subclinical cerebrovascular disease among those who already have it.35 In our study, this is unlikely to be the sole explanation given the continued association after adjusting for these factors, although residual confounding cannot be entirely excluded. LTPA is also associated with a lower risk of silent brain infarction, which is associated with cognition, and adjusting for subclinical MRI evidence of brain disease partially attenuated our results.22 There are extensive animal and human data to suggest that LTPA may influence several biological processes involved in cognition independent of cerebrovascular injury, including angiogenesis, neurogenesis, and neuronal connectivity.36,37
Our findings of a lack of a protective effect when we included those who were cognitively impaired are in keeping with results from randomized clinical trials in Alzheimer disease in which LTPA did not affect measures of memory.38 Episodic memory may be influenced by connectivity between the hippocampus and higher cortical structures, and although these pathways appear to be affected by ischemic damage, they are also dependent on the degree of β-amyloid deposition. Recent data suggest that LTPA may attenuate some of the deleterious effects of amyloid deposition in the preclinical stage,39 which we were not able to examine in our cohort.
Our study has some important limitations. Close to one-quarter of the sample did not have a repeat NPE, limiting power to detect associations with change in cognitive performance and introducing possible selection bias. However, in our analyses, we found that participants with and without repeat NPE did not differ by LTPA level. Although this suggests that selection bias did not confound the association between LTPA and decline on NPE, it remains a limitation. We did not collect information on lifetime patterns of LTPA, such as midlife LTPA,5 which others have shown can have a significant effect on multiple outcomes associated with aging, including cardiovascular mortality and stroke. The NOMAS MRI study did not collect objective measures of physical fitness, such as actigraphy, but previous studies have shown moderate correlation of LTPA questionnaires with actigraphy. Information on occupational physical activity was also not captured, although the protective effect of occupational activities on cardiovascular disease has been inconsistent and this elderly population was less likely to be working. We did not correct for multiple testing across domains in our data analyses, although the domains were highly correlated in the sequential models and across domains.
Important strengths of our study include an understudied population with a high burden of cardiovascular disease risk factors, and use of more than one measure of cognitive function derived from a sensitive neuropsychological test battery. Of note, LTPA has protective effects against multiple other conditions associated with aging, with little associated risk,21 and our observational findings support the hypothesis that even the elderly benefit from LTPA.
We found that LTPA was associated with a protective effect against cognitive decline, independent of structural brain injury as captured by MRI. LTPA is a likely therapeutic target for cognitive decline with aging. Further studies are also required to understand the mechanism of action. Physical activity poses an attractive target for lessening the public health burden of cognitive impairment because of its low cost, lack of interaction with medications, and other health benefits.
Supplementary Material
GLOSSARY
- Gc
crystallized abilities
- LTPA
leisure-time physical activity
- MET
metabolic equivalent
- NOMAS
Northern Manhattan Study
- NPE
neuropsychological examination
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Study concept and design: Drs. Willey, Gardener, Elkind, Sacco, Wright. Acquisition of data: Drs. Willey, Sacco, Elkind, Wright. Analysis and interpretation: Dr. Gardener, Dr. Dong, Ms. Moon, Dr. Cheung. Critical revision of the manuscript for important intellectual content: all authors. Study supervision: Drs. Elkind, Sacco, Wright.
STUDY FUNDING
Dr. Willey is supported by the National Institute of Neurological Disorders and Stroke (NIH/NINDS K23 NS 073104). Dr. Wright is supported by a related grant from the National Heart, Lung, and Blood Institute (NIH/NHLBI R01 HL 108623) and by the Evelyn F. McKnight Brain Institute. Funding for this project was provided by NIH/NINDS R37 NS 29993. The sponsor had no role in the design, methods, participant recruitment, data collections, analysis, and preparation of the manuscript.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol 2009;66:1210–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etgen T, Sander D, Huntgeburth U, Poppert H, Forstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the INVADE Study. Arch Intern Med 2010;170:186–193. [DOI] [PubMed] [Google Scholar]
- 3.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006;144:73–81. [DOI] [PubMed] [Google Scholar]
- 4.Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med 2011;171:1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol 2005;4:705–711. [DOI] [PubMed] [Google Scholar]
- 6.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009;302:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vercambre MN, Grodstein F, Manson JE, Stampfer MJ, Kang JH. Physical activity and cognition in women with vascular conditions. Arch Intern Med 2011;171:1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003;348:2508–2516. [DOI] [PubMed] [Google Scholar]
- 9.Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med 2011;269:107–117. [DOI] [PubMed] [Google Scholar]
- 10.Ravaglia G, Forti P, Lucicesare A, et al. Physical activity and dementia risk in the elderly: findings from a prospective Italian study. Neurology 2008;70:1786–1794. [DOI] [PubMed] [Google Scholar]
- 11.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA 2004;292:1447–1453. [DOI] [PubMed] [Google Scholar]
- 12.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol 2005;161:639–651. [DOI] [PubMed] [Google Scholar]
- 13.Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology 2008;71:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol 2010;67:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness and neurocognitive function. Nature 1999;400:418–419. [DOI] [PubMed] [Google Scholar]
- 16.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008;300:1027–1037. [DOI] [PubMed] [Google Scholar]
- 17.Plassman BL, Williams JW, Jr, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med 2010;153:182–193. [DOI] [PubMed] [Google Scholar]
- 18.Daviglus ML, Bell CC, Berrettini W, et al. NIH state-of-the-science conference statement: preventing Alzheimer's disease and cognitive decline. NIH Consens State Sci Statements 2010;27:1–30. [PubMed] [Google Scholar]
- 19.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev 2008:CD005381. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 21.Willey JZ, Moon YP, Paik MC, Boden-Albala B, Sacco RL, Elkind MS. Physical activity and risk of ischemic stroke in the Northern Manhattan Study. Neurology 2009;73:1774–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willey JZ, Moon YP, Paik MC, et al. Lower prevalence of silent brain infarcts in the physically active: the Northern Manhattan Study. Neurology 2011;76:2112–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn L, Dunn L. Peabody Picture Vocabulary Test. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 24.Stone M, Jastak S, Wilkinson G. Wide range achievement test. Rasch Meas Trans 1995;8:403. [Google Scholar]
- 25.Del Ser T, Gonzalez-Montalvo JI, Martinez-Espinosa S, Delgado-Villapalos C, Bermejo F. Estimation of premorbid intelligence in Spanish people with the Word Accentuation Test and its application to the diagnosis of dementia. Brain Cogn 1997;33:343–356. [DOI] [PubMed] [Google Scholar]
- 26.Duff K. Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol 2012;27:248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willey JZ, Gardener H, Moon YP, et al. Lipid profile components and subclinical cerebrovascular disease in the Northern Manhattan Study. Cerebrovasc Dis 2014;37:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss AJ, Parsons VL. Current estimates from the National Health Interview Survey. United States, 1985. Vital Health Stat 1986;(160):i–iv, 1–182. [PubMed] [Google Scholar]
- 29.Sacco RL, Gan R, Boden-Albala B, et al. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke 1998;29:380–387. [DOI] [PubMed] [Google Scholar]
- 30.Siconolfi SF, Lasater TM, Snow RC, Carleton RA. Self-reported physical activity compared with maximal oxygen uptake. Am J Epidemiol 1985;122:101–105. [DOI] [PubMed] [Google Scholar]
- 31.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498–S504. [DOI] [PubMed] [Google Scholar]
- 32.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012;78:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES project. Alzheimer Dis Assoc Disord 2004;18:57–64. [DOI] [PubMed] [Google Scholar]
- 34.Daviglus ML, Plassman BL, Pirzada A, et al. Risk factors and preventive interventions for Alzheimer disease: state of the science. Arch Neurol 2011;68:1185–1190. [DOI] [PubMed] [Google Scholar]
- 35.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 2003;107:3109–3116. [DOI] [PubMed] [Google Scholar]
- 36.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011;108:3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 2007;30:464–472. [DOI] [PubMed] [Google Scholar]
- 38.Pitkala KH, Poysti MM, Laakkonen ML, et al. Effects of the Finnish Alzheimer disease exercise trial (FINALEX): a randomized controlled trial. JAMA Intern Med 2013;173:894–901. [DOI] [PubMed] [Google Scholar]
- 39.Okonkwo OC, Schultz SA, Oh JM, et al. Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology 2014;83:1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


