BACKGROUND AND SIGNIFICANCE
Intracerebral hemorrhage (ICH) remains one of the most catastrophic stroke subtypes, with high case-fatality rate and poor functional outcomes. In the Journal Club article “Time trends in incidence, case fatality, and mortality of intracerebral hemorrhage” by Jolink et al.,1 hospital and population registries were used to assess age- and sex-specific trends in incidence and case fatality of patients with ICH in the Netherlands. The study provides a significant epidemiologic contribution examining a large cohort of patients with ICH from 1980 to 2010. The authors report a decline in incidence, case-fatality, and mortality rates of ICH in men and women younger than 75 years but stable rates in patients 75 years and older in the Dutch population. Although these data do not have direct implications for clinical practice, and their generalizability to all ICH patient populations may be limited, they are very useful in consolidating epidemiologic knowledge about ICH in the last decades.
HYPOTHESIS AND DESIGN
The authors hypothesized that the overall stable incidence of ICH identified in a previous systematic review by this group2 may mask variations in time trends in incidence and in case fatality according to different subgroups of patients. The study utilized linked registries to retrospectively collect data from 41,068 patients aged 35 to 94 years with first-ever ICH.
METHODS
Linking the national hospital discharge registry by selecting patients with International Classification of Diseases, Ninth Revision (ICD-9) code 431 and the Dutch population registry, a nationwide cohort of all patients admitted with first-ever ICH between 1998 and 2010 was constructed. By definition, ICD-9 code 431 included all ICH types (spontaneous/primary and secondary), excluding only traumatic ICH. This cohort was linked with the cause of death registry of Statistics Netherlands to identify out-of-hospital deaths, stratified by year, sex, and age at time of death, and corresponding population estimates over the same time period.
The authors assessed the accuracy of ICH diagnosis coding, selecting a sample of all patients with a main diagnosis of ICH (ICD-9 code 431) from the hospital discharge registry of a university medical center and a large nonuniversity hospital (January 1995 to December 2010). A total of 1,052 patients' medical records were systematically screened and cross-checked against hospital discharge registry files for the correct diagnosis. ICH diagnosis was found to be correct in 91% in this subgroup.
ICH incidence per 100,000 persons was calculated by age and sex. The age categories were 35–54, 55–74, and 75–94 years. Although somewhat arbitrary, this grouping resulted in a large enough sample size per group for analysis. Mann-Kendall trend tests were performed by age–sex group to investigate whether incidence changed between 1998 and 2010. For 30-day and 1-year (31–365 days) case fatality, the survival time (i.e., time from initial admission date for ICH to the date of death from any cause) was calculated according to the actuarial life-table method and expressed as a percentage. The Mann-Kendall test was again used to explore whether 30-day case fatality and 1-year case fatality changed significantly between 1998 and 2010.
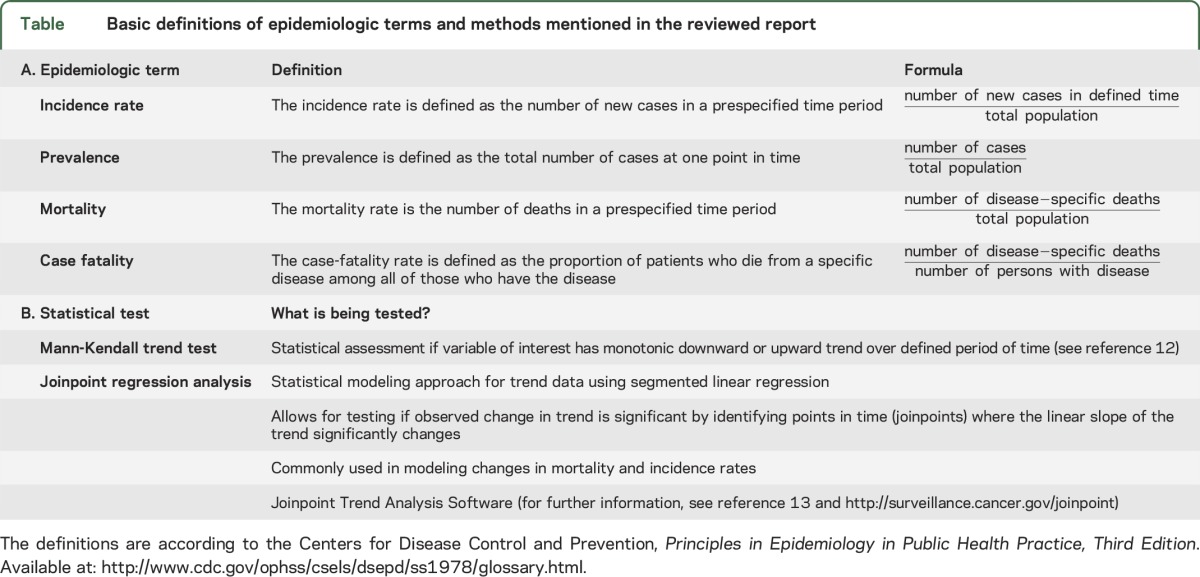
Age- and sex-specific trends in total ICH mortality rates between 1980 and 2010 were explored by using joinpoint regression analyses with software developed by the Surveillance Research Program for the US National Cancer Institute. This statistical approach is well validated and used in cancer research. Briefly, trend data (e.g., mortality rates) are fitted with the best and simplest joinpoint model, based on significant changes identified in the linear slope of the trend (using a log scale) over the study period. For a basic definition of the epidemiologic terms mentioned and the main methods used in the article, see the table.
Table.
Basic definitions of epidemiologic terms and methods mentioned in the reviewed report
RESULTS
The overall ICH incidence, trends in 30-day and 1-year case fatality and mortality of ICH, as well as the trends in total ICH mortality were investigated. The overall ICH incidence remained stable in the oldest age stratum in both men and women but declined in both middle-aged men (from 53.3/100,000 to 37.2/100,000; p < 0.01) and women (from 36.1/100,000 to 26.4/100,000; p < 0.01) and in young women (from 6.3/100,000 to 5.1/100,000; p < 0.01). While the 30-day case fatality significantly declined in both sexes in the middle-aged cohorts as well as in young men (p < 0.01), it remained stable at approximately 40% in older patients (≥75 years). A similar observation was made for 1-year case fatality, which overall decreased in young and middle-aged men and middle-aged women (p < 0.01), but again remained stable throughout the oldest age stratum. A decrease in mortality rate was observed in young and middle-aged women alike, while the mortality rate did not significantly change in the oldest patients. In addition, time trends from 1980 to 2010 in age- and sex-specific total ICH mortality per 100,000 persons have been investigated. In the young patient group, ICH mortality per 100,000 declined in both sexes (in men by 43% and in women by 55%). A similar decrease was observed in the middle-aged group where the ICH mortality per 100,000 patients declined by 48% in men and by 46% in women. Again, the oldest age group did not show any change in mortality rate in the time span investigated.
INTERPRETATION
This work by Jolink et al. highlights important trends in ICH incidence and mortality over the past several decades.2 Their findings emphasize the importance of age and sex on these trends, and notably demonstrate the absence of any change in ICH incidence and mortality in patients older than 75 years. Key strengths of the study include (1) the large sample size, (2) the reliable registry-based methods with demonstrated high-quality linkage to define this cohort and capture mortality figures, (3) the nationwide coverage, almost approximating a population-based setting, (4) broad range of age groups across both sexes, and (5) the validation in a substudy of the correct coding of ICH in more than 90% of patients.
Potential limitations of the study—some of which are constraints inherent to registry-based studies—include the following:
The authors did not distinguish spontaneous (primary) ICH from bleeding secondary to neoplastic or vascular lesions. The latter category is more common in young patients3; as progress has been made in the last decades in diagnosis and endovascular treatment of cerebral vascular malformations, cerebral aneurysms, and other disorders, this may explain decreasing incidence and mortality. In addition, differentiating deep from lobar ICH is crucial, given their inherently different recurrence rates and risk associated with antithrombotic medications.4,5
The stable incidence of ICH has been frequently attributed to increased use of antithrombotic drugs in the elderly6 and the authors acknowledged the lack of this information as a major limitation of their study. Antithrombotic treatment in ICH survivors should also be discussed. Oral anticoagulant treatment resumption in patients with atrial fibrillation after ICH is associated with reduced mortality from all causes.7 However, restarting anticoagulants is less frequent in older patients with atrial fibrillation surviving ICH,8 and this is indeed an area without high-quality, evidence-based recommendations.
The age categories selected for the analysis are somewhat arbitrary. The risk factor profile of ICH changes significantly according to age. Hypertension is the main modifiable risk factor for ICH. However, its prevalence is less than 30% in young patients with ICH3 and, as discussed above, may point to other causes of ICH in this group. In addition, the age groups used in the analysis do not include adult patients aged between 18 and 34 years, which seems somewhat arbitrary.
ICH is still the most lethal type of stroke. However, it might not necessarily be the primary cause of long-term mortality, especially in persons older than 75 years with multiple comorbidities. Nonvascular diseases account for up to one-third of long-term mortality in stroke survivors.8 The exact causes of death are by definition hard to assess in registry-based studies. Large population-based studies with very close follow-up, or autopsy data are needed.9 Given the significant social and economic impact of ICH, functional outcome should also be assessed in future studies.
Early withdrawal of care was proven to be an independent predictor of mortality in patients with ICH10 and the authors were not able to analyze for this variable. Patients older than 75 years may be more likely to have care withdrawn because of their perceived lower chances to survive with a good functional outcome.
Related to points 4 and 5 above, of note, the most pronounced changes in ICH incidence/outcome were shown for the middle-aged group of 55 to 74 years. The causes for this cannot be investigated within the current study but might include lifestyle factors or better prevention and vascular risk factor management in this population.
The role of surgery in supratentorial parenchymal hemorrhage is still controversial. However, some evidence suggests that young patients with ICH might benefit from early hematoma evacuation.11
The authors note that from 2005, the number of hospitals using the national hospital discharge registry was declining, resulting in an increasing number of missing records (from 1.1% in 2004, to 3.3%–14% in 2005–2010).1 Hence, the possibility that, at least partly, the missing data could have influenced the calculated incidence of ICH cannot be excluded.
Finally, despite the robust methodology and the large number of participants included, the results might be difficult to generalize beyond the Netherlands—a Northern European country with advanced stroke unit organization, highly active ICH research teams, overall close proximity to hospitals, and significant stroke awareness.
Beyond these limitations incurred by study design (many of them thoroughly discussed in the article), this important work is hypothesis-generating and will certainly inform future epidemiologic studies. This work will be a critical piece in our understanding of ICH and its consequences, most importantly in aging populations who often have many comorbid conditions (cardiovascular disease, diabetes, dementia, and cerebrovascular disease).
AUTHOR CONTRIBUTIONS
All coauthors actively participated in a structured discussion and critical appraisal of this paper at the Hemorrhagic Stroke Research Group Fellows Journal Club meeting. Andreas Charidimou: Fellows Journal Club organization, write-up, critical revisions. Andrea Morotti: write-up, critical revisions. Raffaella Valenti: write-up, critical revisions. Anne-Katrin Giese: write-up, critical revisions. Gregoire Boulouis: critical revisions. Marco Pasi: critical revisions. Duangnapa Roongpiboonsopit: critical revisions. Arne Lauer: critical revisions. Li Xiong: critical revisions. Thijs Wijnzen Van Harten: critical revisions. Hasan Karadeli: critical revisions. Panagiotis Fotiadis: critical revisions. Michael James Jessel: critical revisions. Anand Viswanathan: write-up, critical revisions, supervision of the journal club and the report.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Jolink WM, Klijn CJ, Brouwers PJ, Kappelle LJ, Vaartjes I. Time trends in incidence, case fatality, and mortality of intracerebral hemorrhage. Neurology 2015;85:1318–1324. [DOI] [PubMed] [Google Scholar]
- 2.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–176. [DOI] [PubMed] [Google Scholar]
- 3.Koivunen RJ, Satopaa J, Meretoja A, et al. Incidence, risk factors, etiology, severity and short-term outcome of non-traumatic intracerebral hemorrhage in young adults. Eur J Neurol 2015;22:123–132. [DOI] [PubMed] [Google Scholar]
- 4.Pezzini A, Grassi M, Paciaroni M, et al. Antithrombotic medications and the etiology of intracerebral hemorrhage: MUCH-Italy. Neurology 2014;82:529–535. [DOI] [PubMed] [Google Scholar]
- 5.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry 2012;83:124–137. [DOI] [PubMed] [Google Scholar]
- 6.Schols AM, Schreuder FH, van Raak EP, et al. Incidence of oral anticoagulant-associated intracerebral hemorrhage in the Netherlands. Stroke 2014;45:268–270. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen PB, Larsen TB, Skjoth F, Gorst-Rasmussen A, Rasmussen LH, Lip GY. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: a nationwide cohort study. Circulation 2015;132:517–525. [DOI] [PubMed] [Google Scholar]
- 8.Pennlert J, Asplund K, Carlberg B, et al. Antithrombotic treatment following intracerebral hemorrhage in patients with and without atrial fibrillation. Stroke 2015;46:2094–2099. [DOI] [PubMed] [Google Scholar]
- 9.van Beijnum J, Lovelock CE, Cordonnier C, et al. Outcome after spontaneous and arteriovenous malformation-related intracerebral haemorrhage: population-based studies. Brain 2009;132:537–543. [DOI] [PubMed] [Google Scholar]
- 10.Zahuranec DB, Brown DL, Lisabeth LD, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology 2007;68:1651–1657. [DOI] [PubMed] [Google Scholar]
- 11.Koivunen RJ, Satopaa J, Haapaniemi E, et al. Predictors of early mortality in young adults after intracerebral hemorrhage. Stroke 2014;45:2454–2456. [DOI] [PubMed] [Google Scholar]
- 12.Mann HB. Nonparametric tests against trend. Econometrica 1945;13:245–259. [Google Scholar]
- 13.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–351. Correction: 2001;20:655. [DOI] [PubMed] [Google Scholar]


