Abstract
Previous MRI studies confirmed abnormalities in the limbic-cortical-striatal-pallidal-thalamic (LCSPT) network or limbic-cortico-striatal-thalamic-cortical (LCSTC) circuits in patients with major depressive disorder (MDD), but few studies have investigated the subcortical structural abnormalities. Therefore, we sought to determine whether focal subcortical grey matter (GM) changes might be present in MDD at an early stage. We recruited 30 first episode, untreated patients with major depressive disorder (MDD) and 26 healthy control subjects. Voxel-based morphometry was used to evaluate cortical grey matter changes, and automated volumetric and shape analyses were used to assess volume and shape changes of the subcortical GM structures, respectively. In addition, probabilistic tractography methods were used to demonstrate the relationship between the subcortical and the cortical GM. Compared to healthy controls, MDD patients had significant volume reductions in the bilateral putamen and left thalamus (FWE-corrected, p < 0.05). Meanwhile, the vertex-based shape analysis showed regionally contracted areas on the dorsolateral and ventromedial aspects of the bilateral putamen, and on the dorsal and ventral aspects of left thalamus in MDD patients (FWE-corrected, p < 0.05). Additionally, a negative correlation was found between local atrophy in the dorsal aspects of the left thalamus and clinical variables representing severity. Furthermore, probabilistic tractography demonstrated that the area of shape deformation of the bilateral putamen and left thalamus have connections with the frontal and temporal lobes, which were found to be related to major depression. Our results suggested that structural abnormalities in the putamen and thalamus might be present in the early stages of MDD, which support the role of subcortical structure in the pathophysiology of MDD. Meanwhile, the present study showed that these subcortical structural abnormalities might be the potential trait markers of MDD.
Keywords: Major depressive disorder, Volumetric analysis, Shape analysis, Putamen, Thalamus
Highlights
-
•
Structural abnormalities in putamen and thalamus might be the potential trait marker of MDD at the early stage.
-
•
The abnormality of LCSTC circuits, or LCSPT circuit, may contribute to the pathophysiology of MDD.
-
•
The shape analysis is more sensitive to subtle structural changes than volumetric and VBM analysis.
1. Introduction
Major depressive disorder (MDD) is a highly prevalent complex neuropsychiatric condition characterised by a broad range of symptoms, with a yearly increase in morbidity and a high risk of mortality (Raes et al., 2006, Sullivan et al., 2000). Approximately 50–75% of MDD patients experience more than one clinically significant episode in their lifetimes (Association, A.P., 2000), and subsequent episodes reduce the effectiveness of antidepressant medication (Angst, 1999). Early studies suggested that psychological treatments earlier in life and at earlier stages of illness reduced the rate of recurrence of depressive episodes (Kaymaz et al., 2008). Therefore, understanding the pathophysiology of MDD at the time of the first episode may provide insights into prevention and early treatment for this debilitating illness.
Whereas the pathophysiology of MDD remains unknown, computational analyses of brain structural MRIs, especially voxel-based morphometry (VBM) and volumetric analyses, are beneficial for understanding the relationship between structural changes and pathologic processes in MDD. In previous VBM reports, volume reductions in the frontal regions, especially the anterior cingulated cortex, orbitofrontal and prefrontal cortices, and the hippocampus, have the most consistent results in MDD patients (Bora et al., 2012, Lai, 2013, Serra-Blasco et al., 2013, Zou et al., 2010). Especially, the hippocampal volume reductions were considered to be an early potential marker in first-episode patients with MDD (Frodl et al., 2002b). Besides, larger volumes of amygdala were also found in first-episode patients with MDD (Frodl et al., 2002a, Frodl et al., 2003), and correlated positively with the severity of depressive state (van Eijndhoven et al., 2009). These investigations indicated that the anatomical structure changes are in accordance with the hypothesis of abnormality in the cortic-limbic circuit (Malykhin et al., 2012, Mayberg, 1997), which may contribute to the pathophysiology of MDD. Although the hippocampus, a part of the subcortical grey matter (GM), had been particularly well studied in MDD patients, several studies recently demonstrated that the functional and structural abnormalities of other subcortical GM associated with affective processing, especially the striatum (Heller et al., 2009) and thalamus (Webb et al., 2014), may also be associated with MDD. For example, the caudate nucleus is one of the central loci for reward-based behavioural learning, and therefore is intricately involved in pleasure and motivation (Haruno et al., 2004). There were also a few studies demonstrating that MDD may be associated with a neuropathological process affecting neurocircuitry involving the connections between the frontal cortex, striatum, thalamus and the related parts of the limbic system within the limbic-cortico-striatal -thalamic-cortical (LCSTC) circuits (Yeh et al., 2010) or the limbic-cortical-striatal-pallidal-thalamic (LCSPT) network (Drevets et al., 2008, Sheline, 2000), suggesting the involvement of some subcortical structures in the pathology of MDD. However, only the structural abnormality of the hippocampus was found in most VBM studies, and the structural abnormalities of other subcortical nuclei, especially the striatum and thalamus, were not well studied.
Recently, many studies demonstrated that traditional VBM analyses of structural MRIs were not sensitive to subtle changes in the subcortical GM (Menke et al., 2014, Nemmi et al., 2015). A few studies began to use the vertex-based shape analysis for exploring the morphological changes of the subcortical GM in Parkinson and Alzheimer's disease (Menke et al., 2014, Štepán-Buksakowska et al., 2014), providing useful information about the location and pattern of morphological changes of the subcortical GM. Because shape analysis can precisely localise regional shape deformations in the subcortical GM and detect changes that are not found in VBM and volumetric analyses, they are now increasingly used to study subcortical GM in a variety of neurological and psychiatric disorders. In this context, shape analyses could provide a sensitive, quantitative biomarker for focal subcortical GM atrophy.
This present study aims to determine whether focal subcortical GM changes are present at the early stage of MDD or not and to elucidate their relationships with clinical severities. To minimise confounding factors that are known to possibly affect GM changes, we only recruited untreated, first episode MDD patients. Specifically, voxel-based morphometry (VBM), automated volumetric and vertex-based shape analyses will be used to assess focal, subtle changes of the subcortical GM. In addition, probabilistic tractography will be used to demonstrate the relationships between subcortical and cortical GM.
2. Materials and methods
2.1. Subjects
This was a retrospective study and the ethics committee of Kunming Medical University approved the study protocol. In this study, 35 first episode and drug-naive patients were recruited from the psychiatry department of the First Affiliated Hospital of Kunming Medical University, but five of the 35 patients were excluded due to obvious structural abnormalities detected by T2-weighted MRI. Ultimately, 30 first episode and drug-naive patients (15 women and 15 men, age range 18–52 years; 100% right handed; education years range = 12–23) were recruited. Two experienced psychiatrists independently made the diagnosis of MDD according to the diagnostic assessment using the Structured Clinical Interview for DSM-IV-Patient Edition (SCID-P). All of the MDD patients also had a score of 18 or greater (scores range: 18–34) on the 17-item Hamilton Depression Rating Scale (HDRS). Those patients that had other comorbid Axis I and Axis II psychiatric disorders, such as schizophrenia, bipolar affective disorder, and personality disorders, were excluded from this study according to the SCID-I and SCID-II assessments. The MDD patients included in the study never received anti-depressive medications before the MRI examinations. All patients involved in the study provided written informed consent.
A total of 30 healthy control subjects (HCs), matched for age, gender and number of years of education, were also recruited from Kunming, but four of the 30 HCs were also excluded due to obvious structural abnormalities detected by T2-weighted MRI. Ultimately, 26 healthy control subjects (HCs) were recruited. They were screened using a diagnostic interview, the Structured Clinical Interview for DSM-IV Nonpatient Edition (SCID-NP), to rule out current or past DSM-IV Axis I disorders. They were also interviewed to affirm that there was no history of psychiatric illness in their first-degree relatives. All subjects were right-handed and without severe or acute medical conditions physically based on clinical evaluations and medical records. All of the healthy control subjects involved in the study provided written informed consent.
2.2. Magnetic resonance imaging acquisition
All participants were scanned on a Philips 3T achieva TX scanner with an eight-channel head coil. A high-resolution 3D TFE sequence was acquired with the following parameters: TR = 7.7 ms, TE = 3.6 ms, matrix = 228 × 228, FOV = 250 mm × 250 mm, 230 axial slices, acquisition time = 6 min 53 s. A diffusion tensor image sequence was applied with the following parameters: TR = 7173 ms, TE = 78 ms, matrix = 115 × 115, FOV = 230 mm × 230 mm, 50 axial slices, slice thickness = 3 mm, diffusion directions = 32, acquisition time = 9 min 7 s. In addition, axial T2-weighted MR images were acquired with the parameters: TR = 2500 ms, TE = 80 ms, matrix = 332 × 225, FOV = 250 mm × 220 mm, slice thickness = 6 mm, 18 axial slices, acquisition time = 55 s. The anatomical MR images were re-evaluated for any structural abnormalities and were reported as normal in all subjects.
2.3. Voxel-based morphometry
T1-weighted 3D TFE data were analysed using FSL-VBM (Douaud et al., 2007), an optimised voxel-based morphometry protocol (Good et al., 2001) carried out using FSL tools (Smith et al., 2004). First, structural images were brain-extracted. Then, a tissue-type segmentation was carried out. The resulting grey matter partial volume images were then aligned to MNI152 standard space using the affine registration tool FLIRT (Jenkinson et al., 2002, Jenkinson and Smith, 2001), followed by nonlinear registration using FNIRT. Second, the registered images were averaged to create a study-specific template. Then, we registered all the grey matter images to the template, and smoothed these modulated segmented images using an isotropic Gaussian kernel with a sigma of 3 mm. Finally, the voxel-wise GLM was applied using an analysis of covariance (ANCOVA), and the effects of age and gender were regressed out. The statistical threshold was set at p < 0.05, corrected for multiple comparisons (family-wise error, FWE) using the cluster-wise approach. In addition, the associations between GM volume changes and clinical variables (Hamilton Depression Rating Scale (HDRS) score) were also explored (FWE-corrected, p < 0.05).
2.4. Subcortical grey matter volumetric analysis
We segmented the bilateral hippocampus, amygdala, accumbens nucleus, caudate nucleus, pallidum nucleus, putamen nucleus and thalamus, respectively, from each subject's T1 3D TFE image using FMRIB's Integrated Registration and Segmentation Tool (FIRST), part of FMRIB's Software Library (FSL 5.0.8, http://www.fmrib.ox.ac.uk/fsl).
For each subject, brain tissue volume, normalised for subject head size, was estimated with SIENAX, part of FMRIB's Software Library. SIENAX starts by extracting brain and skull images from the single whole-head input data. The brain image is then affine-registered to the MNI152 space (using the skull image to determine the registration scaling); this is primarily aimed at obtaining the volumetric scaling factor, to be used for normalisation of head size.
The results of each step of the image processing, most importantly the subcortical segmentation, were carefully examined to ensure accuracy of the results. Before conducting the statistical analyses, the volumes of each subcortical region-of-interest were adjusted for inter-individual head size differences via multiplication by the volumetric scaling factor derived from SIENAX (Smith, 2002). All statistical analyses were carried out using IBM SPSS Statistics (version 17.0; IBM, Armonk, New York). Statistical comparisons between MDD patients and healthy controls were carried out separately for the left and the right sides using an analysis of covariance (ANCOVA), adjusting for the effects of age and gender. The significance level was set at p < 0.05, corrected for multiple comparisons using Bonferroni correction.
Relationships between subcortical GM volumes and clinical factors were further explored. Specifically, correlations between GM volumes and the Hamilton Depression Rating Scale (HDRS) score were assessed using Pearson partial correlation analysis after controlling for the effects of age and gender (p < 0.05). Statistical analyses were conducted using SPSS.
2.5. Shape analysis of subcortical grey matter
A vertex analysis was performed using FIRST in a mode of operation that aims to assess group differences on a per-vertex basis by using a multi-variate General Linear Model. To normalise for inter-individual head size differences, the meshes were reconstructed in the MNI space. Group comparisons of vertices were carried out using F-statistics. The effects of age and gender were regressed out. p < 0.05 was considered statistically significant, corrected for multiple comparisons (family wise error, FWE) using the cluster-wise approach. The associations between regional shape changes of the subcortical GM and clinical variables (HDRS scores) were also explored (FWE-corrected, p < 0.05).
2.6. Probabilistic tractography
For each subject, we used all clusters that had a significant shape difference between two groups or correlations between the shape deformation and HDRS scores in MDD patients. These were seed masks for the probabilistic tractography. We linearly registered the individual fractional anisotropy maps to the relative T1 images using FLIRT (Jenkinson et al., 2002, Jenkinson and Smith, 2001). Then, we nonlinearly registered the T1 images to the MNI template using FNIRT. Finally, the affine matrix and warps calculated with FNIRT were combined to obtain a deformation field mapping of the B0 map of each subject to the MNI space. This final shape deformation field, which was calculated in the MNI space, was used to register the clusters of local atrophy to the diffusion space of each subject.
Probabilistic tractography was performed for every voxel in the chosen seed mask, generating 5000 samples from each seed voxel to build up the connectivity distribution. In addition, we discarded all pathways that entered any of the non-significant voxels by using the non-significant voxels as an exclusion mask. For each subject and seed mask, individual tractography maps were normalised by dividing the number of streamlined samples present in each voxel by the ‘waytotal’, which corresponds to the total number of streamlined samples. Then, the mean streamlined samples were calculated voxelwise for all subjects and for each cluster. These resulting maps were then thresholded to a value equal to 1% of the maximum voxel intensity value in the map and were binarised as the mask of these tracts(Menke et al., 2014, Nemmi et al., 2015).
Next, we registered the binarised tracts mask to each subject's FA, MD, and radial diffusivity (RD) maps using the previously described transformations from the MNI 1 mm space to the diffusion space. Then, we extracted the mean FA, MD, and RD values from the binarised tracts mask and compared those values between the MDD and the control groups using nonparametric two independent samples t-tests. The significance level was set at p < 0.05 (one-tailed and uncorrected)(Menke et al., 2014).
3. Results
3.1. Demographics and clinical information
Clinical data for 30 right-handed major depressive disorder (MDD) patients and 26 matched health controls (HCs) are presented in Table 1. The two groups did not differ in age (p = 0.276, gender (p = 1.00), or number of years of education (p = 0.119). The mean Hamilton Depression Rating Scale (HDRS) score for the MDD patients was 23.73 ± 4.8 (range = 18–34). Data are expressed as mean ± standard deviation. An unpaired t-test for the continuous variables and a Pearson chi-square test for gender were performed using the Statistical Package for Social Sciences (SPSS) (version 17.0; IBM, Armonk, New York).
Table 1.
Clinical data of MDD patients and control subjects.
| MDD patients (n = 30) | HCs (n = 26) | p | |
|---|---|---|---|
| Age(years) | 34 ± 10.75 | 31.42 ± 6.5 | p = 0.276 > 0.05 |
| Gender (female/male) | 15/15 | 13/13 | p = 1.00 > 0.05 |
| Hand(left/right) | 0/30 | 0/26 | |
| Education years | 15.06 ± 3.62 | 16.69 ± 4.05 | p = 0.119 > 0.05 |
| HDRS scores | 23.73 ± 4.8 |
Data are expressed as mean ± standard deviation.
Unpaired t-test for the continuous variables and Pearson chi-square test for the gender were performed using the SPSS.
MDD = Major Depressive Disorder, HCs = Healthy Control subjects, HDRS = Hamilton Depression Rating Scale.
3.2. Voxel-based morphometry analysis
VBM showed no significant regions of either reductions or increases in cortical and subcortical GM volume in MDD patients compared to controls at the threshold of FWE-corrected p < 0.05. In addition, there was no region with significant correlations of GM volume changes with disease severity (HDRS score) in the patients.
3.3. Volumetric analysis of subcortical grey matter
The volume of each subcortical GM was obtained respectively from each subject's T1 3D TFE image, normalised for inter-individual head size differences via multiplication by the volumetric scaling factor derived from SIENAX. Statistical comparisons between the MDD patients and healthy controls were carried out separately for the left and the right sides using ANCOVA, and the effects of age and gender were adjusted. Table 2 shows the statistical results for normalised volumes of the 14 subcortical GM structures. Compared to controls, MDD patients had a significant volume reduction in the left putamen nucleus (p = 0.033, Bonferroni corrected, p = 0.046), right putamen nucleus (p = 0.025, Bonferroni corrected, p = 0.038), and the left thalamus (p = 0.024, Bonferroni corrected, p = 0.016). But there was no difference in volumes of other subcortical GM between the MDD patients and controls (see Supplementary Table 1).
Table 2.
Normalised volumes of subcortical GM (mm3).
| MDD patients (n = 30) |
HCs (n = 26) |
ANCOVA p-value | Bonferroni-corrected p-value | |
|---|---|---|---|---|
| Left putamen | 7792 ± 1596 | 8571 ± 794 | 0.033 | 0.046 |
| Left thalamus | 11,471 ± 1089 | 12,173 ± 849 | 0.024 | 0.016 |
| Right putamen | 7834 ± 796 | 8334 ± 798 | 0.025 | 0.038 |
Volumes (mean ± standard deviation) are given in mm3. ANCOVA = analysis of covariance; MDD = Major Depressive Disorder, HCs = Healthy Control subjects.
Correlations between subcortical GM volumes and HDRS scores were assessed using the Pearson partial correlation analysis after controlling for the effects of age and gender. However, there were no correlations between the subcortical GM volumes changes and HDRS scores (see Supplementary Table 2).
3.4. Shape analysis of subcortical grey matter
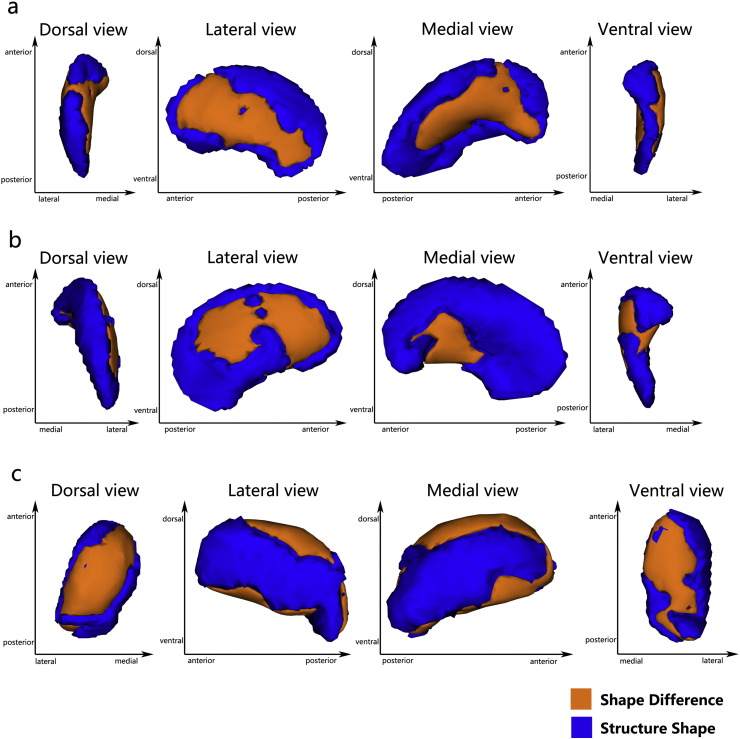
We observed between-group comparisons of vertex-wise shapes that showed significant regional shape deformation on the dorsolateral and ventromedial aspects of the bilateral putamen nucleus (left putamen, FWE-corrected, p = 0.036 < 0.05, x = − 15, y = 5, z = − 10; right putamen, FWE-corrected, p = 0.022 < 0.05, x = 23, y = 6, z = − 10) (Fig 1a, Fig 1b), and the dorsal and ventral aspects of the left thalamus (FWE-corrected, p = 0.014 < 0.05, x = − 16, y = − 32, z = − 4) (Fig 1c) in MDD patients. The other subcortical GM failed to show significant regional shape changes in patients compared to controls (see Supplementary Fig 1).
Fig. 1.
Vertex-wise comparison shows significant regional shape deformation on the dorsolateral and ventromedial aspects of the bilateral putamen Panel a, left putamen; Panel b, right putamen) and the dorsal and ventral aspects of the left thalamus Panel c in MDD patients, compared to HCs (family wise error-corrected, p < 0.05).
Supplementary Fig. 1.
Vertex-wise comparison on the other subcortical gray matter between MDD patients and HCs.
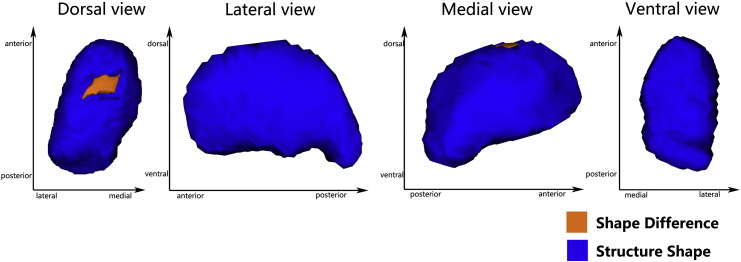
Meanwhile, there were significant negative correlations of HDRS scores with regional shape deformation on the dorsal side of the left thalamus (FWE-corrected, p = 0.044 < 0.05, x = − 9, y = − 14, z = 6) (Fig 2) in MDD patients. However, the other subcortical GM failed to have significant correlations of HDRS scores with the vertex-wise shape changes (see Supplementary Fig 2).
Fig. 2.
Vertex-wise comparison shows a significant negative correlation of HRDS scores with regional shape deformation on the dorsal side of the left thalamus in MDD patients (FWE-corrected, p < 0.05).
Supplementary Fig. 2.
Vertex-wise correlation of HRDS scores with regional shape deformation on the other subcortical gray matter in MDD patients.
3.5. Probabilistic tractography
The mean tracts originating from the mask of the bilateral putamen, which have a significant shape difference between the two groups, both ran posteriorly towards the temporal lobe and anteriorly towards the orbitofrontal gyrus (Fig 3a, Fig 3b). Meanwhile, the mean tracts originating from the left putamen also ran towards the dorsolateral prefrontal cortex (Fig 3a), and the mean tracts originating from the right putamen ran towards the primary and supplementary motor cortices (Fig 3c). These mean tracts did not have any significant difference in the mean MD, FA, RD, and AD values between MDD patients and controls (see Supplementary Table 3).
Fig. 3.
Probabilistic tractography shows the mean tracts originating from the area of shape difference in the bilateral putamen. The yellow part is the putamen structure and the red part is the area of shape deformation. The green part is the fibre bundle, which was generated from the area of shape deformation. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The mean tracts originated from the mask of the left thalamus, and had a significant shape difference between the two groups. The mean tracts ran posteriorly towards the temporal lobe and anteriorly towards the orbitofrontal cortex (Fig 4). Meanwhile, the mean tracts originated from the shape deformation area in the dorsal aspects of the left thalamus, having a significant negative correlation with HRDS scores (Fig 5). However, the mean tracts from the left thalamus also did not have any significant difference in the mean MD, FA, RD, and AD values between MDD patients and controls (see Supplementary Table 3).
Fig. 4.
Probabilistic tractography shows the mean tracts originating from the area of shape difference in the left thalamus (Fig 4). The blue part is the thalamus structure and the red part is the area of shape deformation. The green part is the fibre bundle, which was generated from the area of shape deformation. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Probabilistic tractography shows the mean tracts originating from the shape deformation area in the dorsal aspects of the left thalamus, which have a significant negative correlation with HRDS scores. The blue part is the thalamus structure. The red part is the area of shape deformation; the green part is the fibre bundle, which was generated from the area of shape deformation. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In the present study, structural changes in the subcortical grey matter were investigated in a homogenous group of untreated, first episode MDD patients relative to well-matched health controls using multiple methods, including voxel-based morphometry (VBM), and automated volumetric and vertex-based shape analyses. First, we observed significant volume reduction and shape deformation of the bilateral putamen and left thalamus in MDD patients. Second, we found that HDRS score changes were mainly related to shape deformation in the dorsal aspects of the left thalamus. In addition, vertex-wise analyses of shape differences proved to be more sensitive to subtle changes than was the VBM analysis, demonstrating significant shape differences of subcortical GM between MDD patients and controls. Furthermore, probabilistic tractography demonstrated that the area of shape deformation of the bilateral putamen and left thalamus had connections with the frontal and temporal lobes, which were proven to be related to major depression.
An important finding of our study is that the bilateral putamen and left thalamus volumes were reduced in first episode, untreated MDD patients compared to the controls. Our findings are consistent with several volumetric and advanced VBM studies in recent years, regarding volume reduction of the thalamus (Nugent et al., 2013, Webb et al., 2014). Firstly, it may be related to the improvement of morphological analysis method. Secondly, the thalamus is an integral part of the emotional salience network, emotion modulation network and cognitive/executive network (Yamamura et al., 2016). And the volume reduction of thalamus was considered to help account for the deficits in top-down regulation of negative emotions among individuals more prone to experiencing depressive symptoms (Webb et al., 2014). So the volume reduction of thalamus may be a potential early marker of MDD. Meanwhile, the putamen, part of striatum, has been associated with mood, cognitive processes, motivation and regulation of movement (Koolschijn et al., 2009). But there were just a few studies reported the volume reductions of putamen. A postmortem study have demonstrated putamen volumes reduced possibly in an unmedicated state of patients with MDD, compared with nonpsychiatric subjects (Baumann et al., 1999). And Andreescu et al. found that a later age of onset was also positively correlated with smaller volumes of putamen (Andreescu et al., 2008). In our studies, all patients were untreated, and the average age of onset is about 34 years old. Besides, the volumetric and shape analysis are considered to be more sensitive to subtle structural changes (Menke et al., 2014). So all the factors mentioned above could explain why the putamen have not been found in most studies. For the volume reduction of the putamen and thalamus, although robust pathological evidence was currently lacking, several studies have provided some insight into possible links with depression. For example, post-mortem studies have demonstrated increases of xanthine oxidase (XO) activity in the putamen and thalamus, possibly accounting for increased oxidative stress, which plays a key role in the aetiology of depression(Michel et al., 2010). Meanwhile, a recent PET study showed a decrease of serotonin in the putamen and thalamus, also playing an important role in the aetiology of depression (Meyer et al., 2006). In addition, greater iron deposition (Steffens et al., 1998) or lower extracellular dopamine (Meyer et al., 2001) of putamen nuclei, which might be associated with the MDD, were found in MDD patients compared to control subjects. Moreover, genetic polymorphism research also demonstrated that an epigenetic interaction of COMT Val158Met and MTHFR C677T polymorphisms may contribute to putamen volume differences between depressed and non-depressed subjects (Pan et al., 2009). All of the abnormal changes mentioned above may be blamed for volume reduction in the putamen and thalamus.
Meanwhile, the shape analysis demonstrated volume reductions in the dorsolateral and ventromedial aspects of the bilateral putamen nucleus in patients with MDD, leading to shape deformation of the putamen. Besides, probabilistic tractography demonstrated that the area of shape deformation in the bilateral putamen not only had connections with the orbitofrontal cortex (OFC) and temporal lobe, but also had connections with DLPFC in the left putamen. In addition, it was also connected with the primary and supplementary motor cortices in the right putamen. As part of the striatum, the putamen was considered to be composed of the limbic (the ventral putamen), associative (the dorsorostral putamen) and sensorimotor (the dorsocaudal putamen) subregions (Postuma and Dagher, 2006). According to previous studies, the ventral striatum, temporal lobe (including hippocampus and amygdala), dorsolateral prefrontal cortex (DLPFC), and orbitofrontal cortex (OFC) play a major role in emotional/motivational functions and reward processing (Kumar et al., 2008, Lacerda et al., 2004, Redgrave et al., 2010), and are involved in the fronto-striatal or fronto-limbic networks (Keating et al., 2012, Vai et al., 2015). These regions were also found to be associated with anhedonia and dejection (Heller et al., 2009), which are two core symptoms of MDD. Meanwhile, the sensorimotor striatum and the primary and supplementary motor cortices were implicated in motor output (Bracht et al., 2012, Redgrave et al., 2010), and were related with psychomotor retardation (Walther et al., 2012), a key feature of MDD. In addition, cognitive impairment was also found in MDD patients in several studies (Culpepper, 2015), and the associative striatum and DLPFC were found to play an essential role in the integration of cognitive functions and were implicated in the prefrontal cortex–basal ganglia circuit (Levy and Dubois, 2006). Therefore, based on these studies, we can consider that different regions of the putamen may have connections with the relative cortices and constitute a network that contributes to the relative symptoms in MDD. Although no structural changes in these cortices, and no difference in the mean FA, MD and RD values of these tracts connecting the putamen and relative cortices, were found in our studies, the shape deformation of the putamen, as a key node of these circuits. They may contribute to the symptoms in MDD and are involved in the striatum structural changes, as well as in the connections with DLPFC, OFC and parts of the limbic system. Thus, our findings were in accordance with the hypothesis of abnormality of the limbic-cortico-striatal-thalamic-cortical (LCSTC) circuits (Yeh et al., 2010) or limbic-cortico-striatal-pallidal-thalamic (LCSPT) network (Drevets et al., 2008, Sheline, 2000) in major depression.
Within the left thalamus, local atrophy was also found in the dorsal and ventral aspects. But the previous studies failed to locate atrophy in the thalamus. Thus, the shape analysis is more sensitive to subtle structural changes than volumetric and VBM analysis. According to previous studies, sensory, motor and cognitive processes are organised and integrated in distinct nuclei of the thalamus, which have connections with specific cortical and subcortical regions (Alexander et al., 1986, Middleton and Strick, 2002). In addition, the thalamus was divided into seven sub-regions, which have connections with the prefrontal cortex, primary motor cortex, premotor cortex, occipital cortex, sensory cortex, temporal lobe, and parietal lobe separately (Behrens et al., 2003a, Behrens et al., 2003b). However, in our study, shape deformation of the left thalamus only had connections with the orbitofrontal cortex and temporal lobe, while the local atrophy area in the dorsal aspects of the left thalamus, which had a significantly negative correlation with HDRS scores, were connected with the temporal lobe. According to previous studies, the orbitofrontal cortex (OFC) played a major role in emotional/motivational functions (Lacerda et al., 2004). The temporal lobe, including the hippocampus and amygdala nucleus, which were most often mentioned in previous studies, was also considered to be implicated in the neural circuitry mediating major depressive disorder (van Eijndhoven et al., 2009). The amygdala is involved in the production of affective states and the hippocampus is involved in the regulation of affective states (Phillips et al., 2003). Meanwhile, hippocampus was also related to recollection memory and the memory of reward-associated rules (Campbell and MacQueen, 2004, Kaymak et al., 2010). In our study, no temporal lobe and orbitofrontal structural changes were found, and no difference in the mean FA, MD, and RD values of the mean tracts, which were connected with the orbitofrontal cortex, temporal lobe and thalamus, was found between the MDD patients and the controls. Thalamus is a complex sensory information node constituted by many nuclei. Several studies demonstrated that the thalamus connected the orbitofrontal cortex to negative emotion-generating limbic structures, such as the amygdala (Price and Drevets, 2010, Timbie and Barbas, 2015), and was related with the deficits in top-down regulation of negative emotions among individuals more prone to experiencing depressive symptoms (Webb et al., 2014). Meanwhile, structural changes of the dorsal aspects in the left thalamus also showed a negative correlation with HDRS scores. Therefore, the shape deformation area in the thalamus might contribute to clinical symptoms and depression severity in first episode, untreated MDD patients. Meanwhile, we found that only the left thalamus had volume and shape changes, and the cause of this result might be related to the dominant hemisphere. In addition, the reconstructed putamen fibre tracts were also projected to the thalamus (Leh et al., 2007). Our findings in the thalamus were also in accordance with the hypothesis of abnormality of the limbic-cortico-striatal-thalamic-cortical (LCSTC) circuits or the limbic-cortical-striatal- pallidal-thalamic (LCSPT) network.
However, in our study, we did not find any volume alternation in amygdala or hippocampus in first-episode MDD patients. In most previous studies, the structural changes of hippocampus and amygdala were the most consistent results in first-episode MDD patients (Frodl et al., 2002a, Frodl et al., 2002b, Frodl et al., 2003, van Eijndhoven et al., 2009, Zou et al., 2010). One reason may due to the relatively short duration of our sample of MDD. The other reason might be attributable to heterogeneity of MDD patients, such as differences in medication status, the number of episodes, age of onset and the sensitivity of volumetric and shape analyses to subtle structural changes. In our study, the effects of medication status were excluded. And recently, a large scale meta-analysis reported that the smaller hippocampus and amygdala volumes were associated with the age of onset and the number of episodes (Schmaal et al., 2015). Age of onset ≤ 21 was associated with a smaller hippocampus, and a trend towards smaller amygdala. But in our study, all of MDD patients were first-episode and the most of age of onset was > 21 years old. Besides, several research found that the volume deficit of hippocampus correlated with the length of illness (Colla et al., 2007, Frodl et al., 2008), and the hippocampal volume was lower in MDD patients with long-duration compared to patients with short-duration and HCs (Cheng et al., 2010). According to the clinical data, most of MDD patients in our study are short-duration. Therefore, we could consider that the all the factors mentioned above may contribute to explain the inconsistencies in hippocampus and amygdala.
Several limitations of our study should be addressed. First, due to the relatively small sample size design, the results cannot be generalised to the general population. In addition, the impact of clinical variables, such as disease duration, age of onset, was also not evaluated. Future studies with a larger sample size of first episode, untreated MDD are necessary, and the relationship between clinical factors and neuroimaging results need to be clarified in the further researches. The second limitation involves the use of an automatic segmentation procedure. Although manual segmentation is time consuming and must be performed by a highly trained researcher, it is still viewed as the gold standard. Nevertheless, use of the automatic segmentation procedure has been extensively reported in peer-reviewed articles (Lee et al., 2014, Menke et al., 2014, Nemmi et al., 2015). Finally, in the end, although we discussed the relationship between subcortical GM structural changes and cognition, the evaluation of cognitive function was not carried out, and consequently, the exact relationships between structural changes of subcortical GM and cognitive functions remain speculative and were not addressed here.
5. Conclusion
Structural abnormalities in the putamen and thalamus may be present in the early stages of MDD, and subcortical GM plays an important role in the neural circuitry mediating MDD. Meanwhile, our study further confirmed the hypothesis of abnormality of the limbic-cortico-striatal-thalamic-cortical (LCSTC) circuits or limbic-cortical-striatal pallidal-thalamic (LCSPT) network in MDD patients, which may contribute to the pathophysiology of MDD.
The following are the supplementary data related to this article.
Normalised volumes of subcortical grey matter (mm3).
the correlations between volume changes of subcortical grey matter and HDRS scores in the MDD patients.
1. The mean FA value of the tracts originated from shape deformation area.
2. The mean MD value of the tracts originated from shape deformation area.
3. The mean RD value of the tracts originated from shape deformation area.
Conflict of interest
The authors declare no competing financial interests.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (Grant Agreement Number 81160171) and Yunnan Provincial Science and Technology Department & Kunming Medical University applied basic research (Grant Agreement Number 2013FB141). We would like to thank Jianhong Wang, Jiaqiang Zhang , Cirong Liu and Zhongping Zhang for their help.
References
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andreescu C., Butters M.A., Begley A., Rajji T., Wu M., Meltzer C.C., Reynolds C.F., 3rd, Aizenstein H. Gray matter changes in late life depression–a structural MRI analysis. Neuropsychopharmacology. 2008;33:2566–2572. doi: 10.1038/sj.npp.1301655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst J. Major Depression in 1998: Are We Providing Optimal Therapy? J. Clin. Psychiatry. 1999 [PubMed] [Google Scholar]
- Association, A.P. Practice guideline for the treatment of patients with major depressive disorder (revision) Am. J. Psychiatry. 2000;157:1–45. [PubMed] [Google Scholar]
- Baumann B., Danos P., Krell D., Diekmann S., Leschinger A., Stauch R., Wurthmann C., Bernstein H.G., Bogerts B. Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J. Neuropsychiatry Clin. Neurosci. 1999;11:71–78. doi: 10.1176/jnp.11.1.71. [DOI] [PubMed] [Google Scholar]
- Behrens T.E., Johansen-Berg H., Woolrich M.W., Smith S.M., Wheeler-Kingshott C.A., Boulby P.A., Barker G.J., Sillery E.L., Sheehan K., Ciccarelli O., Thompson A.J., Brady J.M., Matthews P.M. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Behrens T.E., Woolrich M.W., Jenkinson M., Johansen-Berg H., Nunes R.G., Clare S., Matthews P.M., Brady J.M., Smith S.M. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bora E., Fornito A., Pantelis C., Yücel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J. Affect. Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Bracht T., Federspiel A., Schnell S., Horn H., Höfle O., Wiest R., Dierks T., Strik W., Müller T.J., Walther S. 2012. Cortico-Cortical White Matter Motor Pathway Microstructure Is Related to Psychomotor Retardation in Major Depressive Disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S., MacQueen G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 2004;29:417. [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.-Q., Xu J., Chai P., Li H.-J., Luo C.-R., Yang T., Li L., Shan B.-C., Xu X.-F., Xu L. Brain volume alteration and the correlations with the clinical characteristics in drug-naive first-episode MDD patients: a voxel-based morphometry study. Neurosci. Lett. 2010;480:30–34. doi: 10.1016/j.neulet.2010.05.075. [DOI] [PubMed] [Google Scholar]
- Colla M., Kronenberg G., Deuschle M., Meichel K., Hagen T., Bohrer M., Heuser I. Hippocampal volume reduction and HPA-system activity in major depression. J. Psychiatr. Res. 2007;41:553–560. doi: 10.1016/j.jpsychires.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Culpepper L. Impact of untreated major depressive disorder on cognition and daily function. J. Clin. Psychiatry. 2015;76:901. doi: 10.4088/JCP.13086tx4c. [DOI] [PubMed] [Google Scholar]
- Douaud G., Smith S., Jenkinson M., Behrens T., Johansen-Berg H., Vickers J., James S., Voets N., Watkins K., Matthews P.M., James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Furey M.L. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T., Meisenzahl E., Zetzsche T., Bottlender R., Born C., Groll C., Jäger M., Leinsinger G., Hahn K., Möller H.-J. Enlargement of the amygdala in patients with a first episode of major depression. Biol. Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- Frodl T., Meisenzahl E.M., Zetzsche T., Born C., Groll C., Jager M., Leinsinger G., Bottlender R., Hahn K., Moller H.J. Hippocampal changes in patients with a first episode of major depression. Am. J. Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- Frodl T., Meisenzahl E.M., Zetzsche T., Born C., Jäger M., Groll C., Bottlender R., Leinsinger G., Möller H.-J. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol. Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Frodl T.S., Koutsouleris N., Bottlender R., Born C., Jäger M., Scupin I., Reiser M., Möller H.-J., Meisenzahl E.M. Depression-related variation in brain morphology over 3 years: effects of stress? Arch. Gen. Psychiatry. 2008;65:1156–1165. doi: 10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Haruno M., Kuroda T., Doya K., Toyama K., Kimura M., Samejima K., Imamizu H., Kawato M. A neural correlate of reward-based behavioral learning in caudate nucleus: a functional magnetic resonance imaging study of a stochastic decision task. J. Neurosci. 2004;24:1660–1665. doi: 10.1523/JNEUROSCI.3417-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A.S., Johnstone T., Shackman A.J., Light S.N., Peterson M.J., Kolden G.G., Kalin N.H., Davidson R.J. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc. Natl. Acad. Sci. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kaymak S.U., Demir B., Şentürk S., Tatar I., Aldur M.M., Uluğ B. Hippocampus, glucocorticoids and neurocognitive functions in patients with first-episode major depressive disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2010;260:217–223. doi: 10.1007/s00406-009-0045-x. [DOI] [PubMed] [Google Scholar]
- Kaymaz N., Os J.v., Loonen A.J., Nolen W.A. Evidence that patients with single versus recurrent depressive episodes are differentially sensitive to treatment discontinuation: a meta-analysis of placebo-controlled randomized trials. J. Clin. Psychiatry. 2008;69:1423. doi: 10.4088/jcp.v69n0910. [DOI] [PubMed] [Google Scholar]
- Keating C., Tilbrook A.J., Rossell S.L., Enticott P.G., Fitzgerald P.B. Reward processing in anorexia nervosa. Neuropsychologia. 2012;50:567–575. doi: 10.1016/j.neuropsychologia.2012.01.036. [DOI] [PubMed] [Google Scholar]
- Koolschijn P.C., van Haren N.E., Lensvelt-Mulders G.J., Hulshoff Pol H.E., Kahn R.S. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Waiter G., Ahearn T., Milders M., Reid I., Steele J. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–2093. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- Lacerda A.L., Keshavan M.S., Hardan A.Y., Yorbik O., Brambilla P., Sassi R.B., Nicoletti M., Mallinger A.G., Frank E., Kupfer D.J. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol. Psychiatry. 2004;55:353–358. doi: 10.1016/j.biopsych.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Lai C.H. Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry Res. 2013;211:37–46. doi: 10.1016/j.pscychresns.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Lee H.M., Kwon K.Y., Kim M.J., Jang J.W., Suh S.I., Koh S.B., Kim J.H. Subcortical grey matter changes in untreated, early stage Parkinson's disease without dementia. Parkinsonism Relat. Disord. 2014;20:622–626. doi: 10.1016/j.parkreldis.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Leh S.E., Ptito A., Chakravarty M.M., Strafella A.P. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci. Lett. 2007;419:113–118. doi: 10.1016/j.neulet.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R., Dubois B. Apathy and the functional anatomy of the prefrontal cortex–basal ganglia circuits. Cereb. Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Malykhin N.V., Carter R., Hegadoren K.M., Seres P., Coupland N.J. Fronto-limbic volumetric changes in major depressive disorder. J. Affect. Disord. 2012;136:1104–1113. doi: 10.1016/j.jad.2011.10.038. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S. Limbic-cortical dysregulation: a proposed model of depression. J. Neuropsychiatry Clin. Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Menke R.A., Szewczyk-Krolikowski K., Jbabdi S., Jenkinson M., Talbot K., Mackay C.E., Hu M. Comprehensive morphometry of subcortical grey matter structures in early-stage Parkinson's disease. Hum. Brain Mapp. 2014;35:1681–1690. doi: 10.1002/hbm.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J.H., Krüger S., Wilson A.A., Christensen B.K., Goulding V.S., Schaffer A., Minifie C., Houle S., Hussey D., Kennedy S.H. Lower dopamine transporter binding potential in striatum during depression. Neuroreport. 2001;12:4121–4125. doi: 10.1097/00001756-200112210-00052. [DOI] [PubMed] [Google Scholar]
- Meyer J.H., McNeely H.E., Sagrati S., Boovariwala A., Martin K., Verhoeff N.P.L., Wilson A.A., Houle S. Elevated putamen D 2 receptor binding potential in major depression with motor retardation: an [11 C] raclopride positron emission tomography study. Am. J. Psychiatr. 2006;163:1594–1602. doi: 10.1176/ajp.2006.163.9.1594. [DOI] [PubMed] [Google Scholar]
- Michel T.M., Camara S., Tatschner T., Frangou S., Sheldrick A.J., Riederer P., Grünblatt E. Increased xanthine oxidase in the thalamus and putamen in depression. World J. Biol. Psychiatry. 2010;11:314–320. doi: 10.3109/15622970802123695. [DOI] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cereb. Cortex. 2002;12:926–935. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- Nemmi F., Sabatini U., Rascol O., Peran P. Parkinson's disease and local atrophy in subcortical nuclei: insight from shape analysis. Neurobiol. Aging. 2015;36:424–433. doi: 10.1016/j.neurobiolaging.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Nugent A.C., Davis R.M., Zarate C.A., Drevets W.C. Reduced thalamic volumes in major depressive disorder. Psychiatry Res. Neuroimaging. 2013;213:179–185. doi: 10.1016/j.pscychresns.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C.C., McQuoid D.R., Taylor W.D., Payne M.E., Ashley-Koch A., Steffens D.C. Association analysis of the COMT/MTHFR genes and geriatric depression: an MRI study of the putamen. Int. J. Geriatr. Psychopharmacol. 2009;24:847–855. doi: 10.1002/gps.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Postuma R.B., Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb. Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes F., Hermans D., Williams J.M.G., Demyttenaere K., Sabbe B., Pieters G., Eelen P. Is overgeneral autobiographical memory an isolated memory phenomenon in major depression? Memory. 2006;14:584–594. doi: 10.1080/09658210600624614. [DOI] [PubMed] [Google Scholar]
- Redgrave P., Coizet V., Comoli E., McHaffie J.G., Leriche M., Vautrelle N., Hayes L.M., Overton P. Interactions between the midbrain superior colliculus and the basal ganglia. Front. Neuroanat. 2010;4 doi: 10.3389/fnana.2010.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L., Veltman D.J., van Erp T.G., Sämann P., Frodl T., Jahanshad N., Loehrer E., Tiemeier H., Hofman A., Niessen W. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol. Psychiatry. 2015 doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Blasco M., Portella M.J., Gómez-Ansón B., de Diego-Adeliño J., Vives-Gilabert Y., Puigdemont D., Granell E., Santos A., Álvarez E., Pérez V. Effects of illness duration and treatment resistance on grey matter abnormalities in major depression. Br. J. Psychiatry. 2013;202:434–440. doi: 10.1192/bjp.bp.112.116228. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol. Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Steffens D.C., Tupler L.A., Ranga K., Krishnan R. Magnetic resonance imaging signal hypointensity and iron content of putamen nuclei in elderly depressed patients. Psychiatry Res. Neuroimaging. 1998;83:95–103. doi: 10.1016/s0925-4927(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Štepán-Buksakowska I., Szabó N., Horínek D., Tóth E., Hort J., Warner J., Charvát F., Vécsei L., Rocek M., Kincses Z.T. Cortical and subcortical atrophy in Alzheimer disease: parallel atrophy of thalamus and hippocampus. Alzheimer Dis. Assoc. Disord. 2014;28:65–72. doi: 10.1097/WAD.0b013e318299d3d6. [DOI] [PubMed] [Google Scholar]
- Sullivan P.F., Neale M.C., Kendler K.S. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Timbie C., Barbas H. Pathways for emotions: specializations in the Amygdalar, Mediodorsal thalamic, and posterior orbitofrontal network. J. Neurosci. 2015;35:11976–11987. doi: 10.1523/JNEUROSCI.2157-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vai B., Poletti S., Radaelli D., Dallaspezia S., Bulgarelli C., Locatelli C., Bollettini I., Falini A., Colombo C., Smeraldi E. Successful antidepressant chronotherapeutics enhance fronto-limbic neural responses and connectivity in bipolar depression. Psychiatry Res. Neuroimaging. 2015;233:243–253. doi: 10.1016/j.pscychresns.2015.07.015. [DOI] [PubMed] [Google Scholar]
- van Eijndhoven P., van Wingen G., van Oijen K., Rijpkema M., Goraj B., Verkes R.J., Voshaar R.O., Fernández G., Buitelaar J., Tendolkar I. Amygdala volume marks the acute state in the early course of depression. Biol. Psychiatry. 2009;65:812–818. doi: 10.1016/j.biopsych.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Walther S., Hügli S., Höfle O., Federspiel A., Horn H., Bracht T., Wiest R., Strik W., Müller T.J. Frontal white matter integrity is related to psychomotor retardation in major depression. Neurobiol. Dis. 2012;47:13–19. doi: 10.1016/j.nbd.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Webb C.A., Weber M., Mundy E.A., Killgore W.D. Reduced gray matter volume in the anterior cingulate, orbitofrontal cortex and thalamus as a function of mild depressive symptoms: a voxel-based morphometric analysis. Psychol. Med. 2014;44:2833–2843. doi: 10.1017/S0033291714000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura T., Okamoto Y., Okada G., Takaishi Y., Takamura M., Mantani A., Kurata A., Otagaki Y., Yamashita H., Yamawaki S. Association of thalamic hyperactivity with treatment-resistant depression and poor response in early treatment for major depression: a resting-state fMRI study using fractional amplitude of low-frequency fluctuations. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh P.-H., Zhu H., Nicoletti M.A., Hatch J.P., Brambilla P., Soares J.C. Structural equation modeling and principal component analysis of gray matter volumes in major depressive and bipolar disorders: differences in latent volumetric structure. Psychiatry Res. Neuroimaging. 2010;184:177–185. doi: 10.1016/j.pscychresns.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K., Deng W., Li T., Zhang B., Jiang L., Huang C., Sun X., Sun X. Changes of brain morphometry in first-episode, drug-naïve, non–late-life adult patients with major depression: an optimized voxel-based morphometry study. Biol. Psychiatry. 2010;67:186–188. doi: 10.1016/j.biopsych.2009.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Normalised volumes of subcortical grey matter (mm3).
the correlations between volume changes of subcortical grey matter and HDRS scores in the MDD patients.
1. The mean FA value of the tracts originated from shape deformation area.
2. The mean MD value of the tracts originated from shape deformation area.
3. The mean RD value of the tracts originated from shape deformation area.