Abstract
Background:
Oral cavity cancer is the most common cancer among rural India. There is a paucity of data for outcomes of operable oral cavity cancer from rural India. Use of maintenance metronomic may delay or avoid relapse.
Aim:
To evaluate outcomes of operable oral cavity carcinoma and evaluate impact of maintenance metronomic chemotherapy.
Objectives:
To evaluate disease-free survival (DFS), overall survival (OS), and factors affecting the outcome in operable oral cavity cancer.
Materials and Methods:
Data of patients diagnosed with oral cavity cancer registered between May 2008 and May 2014 were retrieved. Only those patients with operable oral cavity cancer and upfront definitive surgery were included in the study. Demographic profile, stage, tobacco consumption, adjuvant therapy, and pattern of failure were collected. Kaplan–Meir survival analysis was used to determine DFS and OS. Log-rank test was used to evaluate factors affecting outcome.
Results:
Median follow-up is 24 months. Out of 335 patients, 225 (67%) had advanced operable cancer with 42/225 (18%) and 183/225 (82%) as Stages III and IVA, respectively. Buccal mucosa was the most common subsite (178/335, 53%) followed by tongue (63/335, 19%). Ninety-two percent patients were addicted to smokeless tobacco, whereas 27% were smokers. Median DFS is 13 months with 2 years relative DFS 32%. Median OS is 30 months, with 2 years OS of 54%. Metronomic adjuvant oral chemotherapy was given in 130/225 (58%); Stage III and IVA patients with median of 14 months (3–18 months). Use of metronomic chemotherapy improved DFS (8 vs. 14 months, P = 0.22) and OS (14 vs. 26 months, P = 0.04).
Conclusion:
Oral cavity cancer is a major health care problem in rural India. Presentation at advanced stage leads to suboptimal outcomes. Benefit of metronomic maintenance chemotherapy in locally advanced oral cavity needs to be further evaluated prospectively.
Keywords: Locally advanced head and neck squamous cell carcinoma, maintenance metronomic, oral cavity
Introduction
Oral cavity cancer is the most common of all head neck cancers in India.[1] Age-adjusted incidence rates of oral cavity cancer are 20/100,000 population which accounts for over 30% of all cancers in India.[2] Early detection followed by optimized standard therapy offers the best chance of cure. Low socioeconomic status, lack of awareness, and poor accessibility to health care providers in rural India often leads to late presentation and hence, compromised outcomes.[3,4] Unlike in urban community, rural population consumes more smokeless tobacco which is linked to higher oral cavity cancers, hence posing a significant rural health care problem.[5,6] There is a lack of data regarding outcomes of oral cavity cancer from the rural population.
Metronomic chemotherapy is the low cost, low dose regular administration of drugs at shorter interval without interruption to avoid or delay the progression of disease.[7] We performed retrospective audit of resectable oral cavity cancer treated with standard of care surgery, radiotherapy, and chemotherapy in rural cancer center in India and analyzed the outcomes. We also present the impact of oral low-cost maintenance metronomic chemotherapy on the overall outcome of locally advanced resectable oral cavity cancer.
Materials and Methods
Case records of patients registered in our rural cancer center between May 2008 and May 2014 and diagnosed as squamous cell carcinoma of oral cavity were retrieved. Only patients who presented with resectable oral cavity carcinoma and were upfront operated were analyzed. Upfront resectability status of oral cavity was decided in a multidisciplinary joint meeting held weekly and attended by surgical oncologist, general surgeons, radiologist, radiation oncologist, and medical oncologist. Demographic profile including age at presentation, sex, subsite, extent, size, stage, comorbidities, tobacco and alcohol consumption, adjuvant radiation therapy, adjuvant chemotherapy, and patterns of failure including dates of progression and deaths were collected. Data of patients of locally advanced (Stage III and Stage IV) oral cavity cancer who received maintenance oral metronomic therapy after completion of standard adjuvant chemoradiotherapy were recorded separately. Follow-up data were collected from case records, patient home visits by social workers, and telephonic conversations. This retrospective audit was presented and approved by the Institutional Review Board and Ethic Committee.
Patient presenting with oral cavity ulcerative or ulceroproliferative lesion in tongue, buccal mucosa, gingivobuccal sulcus, hard palate, alveolus, and floor of mouth were evaluated first by general surgeons followed by surgical oncologist. Thorough clinical examination of neck was conducted to rule out neck nodal metastasis. Biopsy from the primary site with or without neck nodal fine needle aspiration cytology (FNAC) was conducted to confirm the diagnosis and stage. All patients were subjected to contrast-enhanced computed tomography (CT) scan of face-neck and chest X-ray to define the locoregional extent and metastasis. Upfront resectability of oral cavity cancer was decided predominantly by surgical oncologist in association with radiologist and general surgeons in weekly multidisciplinary clinic. All patients were operated within 10 days after decision to operate in multidisciplinary clinic.
All major surgical interventions for tongue (wide excision/hemiglossectomy, near total/total glossectomy), buccal mucosa (wide excision, marginal/segmental/hemimandibulectomy), alveolus/gingivobuccal sulcus (marginal/segmental/hemimandibulectomy), and lip/hard palate, floor of mouth (Wide excision) was done by a team of surgical oncologists and surgeons trained in head neck oncology. Detailed grossing, margin status, and histopathology reporting were done by oncopathologists. Patients received adjuvant radiotherapy with Cobalt 60 gamma rays, bilateral parallel opposed portal to face neck to a dose of 60 Gy in 30 fractions, 5 fraction per week with or without adjuvant chemotherapy cisplatin 30 mg/m2 with adequate hydration and premedications, once weekly for 6 weeks, as per standard guidelines accepted for adjuvant therapy. All patients were closely monitored for toxicity and weight loss at least weekly, and feeding with Ryle's tube was encouraged at the onset of moderate mucositis and odynophagia. Radiation therapy was initiated within 4–6 weeks of surgery after wound healing in majority of patients.
After completion of adjuvant chemoradiation in locally advanced Stage III and Stage IV, patients were referred to medical oncology outpatient department depending on individual dependent referral policy of surgical-radiation oncologists for maintenance metronomic chemotherapy. Metronomic chemotherapy comprised tablet methotrexate (15 mg/m2) once a week and capsule celecoxib 200 mg twice daily for a period of 18 months after completion of standard adjuvant therapy. Patients were clinically evaluated monthly with complete blood count and serum creatinine for first 3 months to access toxicity and compliance to therapy, thereafter assessment continued every 3 monthly. Detailed oral and neck clinical examination was performed every 3 monthly, and any clinical suspicion of recurrence/metastasis was confirmed with CT scans, FNAC of neck nodes, and chest X-ray as deemed necessary.
Disease-free survival (DFS) was defined as time interval from date of registration till date of recurrence or death from any cause. Overall survival (OS) was defined as time interval from date of registration till death from any cause. Median follow-up was calculated from date of registration to date of the last follow-up. All statistical analysis was carried out using SPSS software version 16 (SPSS Inc. Released 2007. SPSS for Windows, Chicago, SPSS Inc.). Kaplan–Meir curve was plotted for progression-free survival (PFS) and OS in months. Log-rank test was used to compare the PFS and OS in different groups.
Results
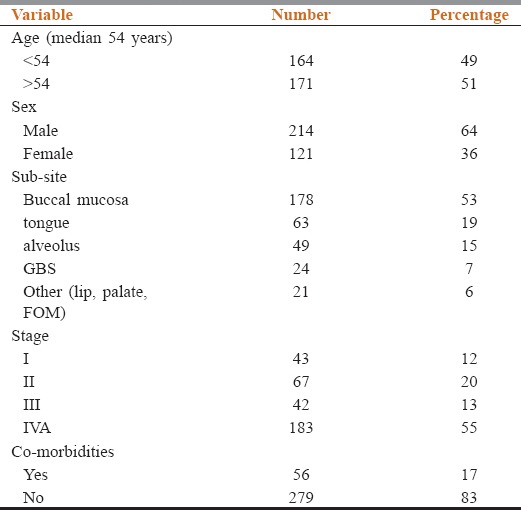
Between May 2008 and May 2014, 335 patients registered with confirmed diagnosis of squamous cell carcinoma of tongue, buccal mucosa, lip, alveolus, hard palate, floor of mouth, and gingivobuccal sulcus, who underwent major surgical procedure such as wide excision, mandibulectomy, and glossectomy with or without reconstructive surgeries were enrolled in this retrospective audit. Buccal mucosa was the most common site (n = 178, 53%) followed by carcinoma tongue (n = 63, 19%) and alveolus (n = 49, 15%). Out of 335 patients, 225 (67%) had advanced operable cancer with 42/225 (18%) and 183/225 (82%) as Stage III and IVA, respectively. Among 110/335 (33%) patients who had early operable oral cavity tumors, 43/110 (39%) were Stage I, and 67/110 (61%) were Stage II tumors, respectively. Median age at diagnosis is 54 years with 64% males. About 92% patients were addicted to smokeless tobacco (chewable tobacco powders and snuff, gutkha, pan masala, betel quid, areca nut, masheri, etc.), whereas 27% were smokers and 27% were chronic alcoholics [Table 1].
Table 1.
Demographic details
Adjuvant radiotherapy was advised after thorough evaluation of postoperative histopathological report and preoperative clinicoradiological finding in 225 patients as per accepted standard indication of adjuvant therapy. Out of 225 patients, 225 (96%) patients started the advised adjuvant radiotherapy, whereas 196/217 (90%) patients completed the prescribed dose of 60 Gy/30 fractions of radiotherapy. Concurrent cisplatin 30 mg/m2 was given along with adjuvant radiotherapy in 110/225 (49%) of Stage III and Stage IVA patients with a median number of cycles of 4 (range 2–6). Depending on referral pattern to department of medical oncology, after completion of adjuvant radiotherapy, locally advanced Stage III and Stage IVA patients were advised to initiate oral metronomic chemotherapy for a maximum period of 18 months, as to extend the possible beneficial effect of metronomic chemotherapy during the peak recurrence period of 6–18 months of adjuvant therapy. Out of 225 Stage III and Stage IV patients, 130 (58%) patients received metronomic chemotherapy for a median duration of 14 months (range 3–18 months). None of the patients discontinued oral metronomic chemotherapy due to toxicity. Dose reduction of methotrexate as per mucositis and tolerance was done as per discretion of treating oncologist, avoiding any change in schedule and frequency of administration.
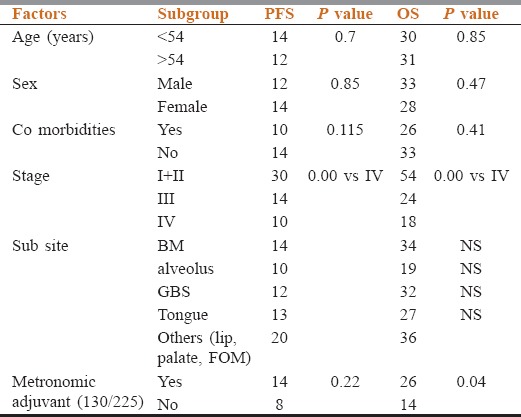
Median follow-up is 24 months. Median DFS is 13 months, whereas 1 year and 2 years DFS are 55% and 32%, respectively [Figure 1]. Median OS is 30 months, whereas 1 year and 2 years OS are 74% and 54%, respectively [Figure 2]. Early stage oral cavity cancer fared better with higher DFS (30 vs. 10 months, P = 0.01) and OS (54 vs. 18 months, P = 0.01), compared to Stage IVA patients. Use of oral maintenance metronomic chemotherapy after completion of standard adjuvant chemoradiotherapy in locally advanced Stage III and Stage IVA, improved DFS (8 vs. 14 months, P = 0.22) and OS significantly. (14 vs. 26 months, P = 0.04) [Figures 3 and 4]. There was no statistically significant difference in outcome irrespective of age, sex, comorbidities and several subsites of oral cavity [Table 2]. Among the available various pattern of relapse recorded, local relapse: 82/127 (65%), regional: 14/127 (11%), local with regional: 12/127 (9%), and metastasis: 19/127 (15%).
Figure 1.
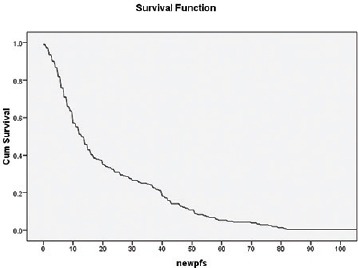
Overall DFS
Figure 2.
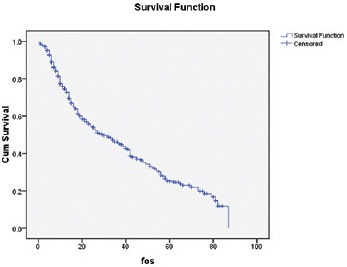
Overall OS
Figure 3.
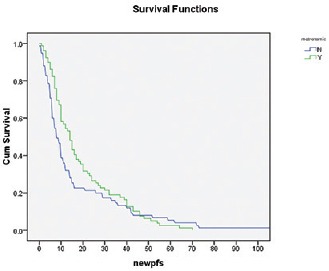
The impact of metronomic on DFS
Figure 4.
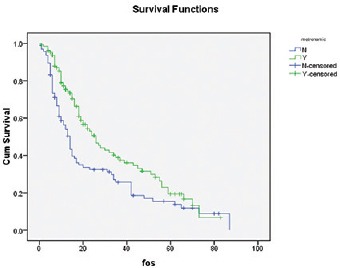
The impact of metronomic on OS
Table 2.
Prognostic factors
Discussion
Locally advanced oral cavity cancer is the most common presentation seen among head neck cancers in rural India mainly due to lack of awareness, suboptimal local healthcare facilities, and delayed presentation and referral.[3,4] Standard therapy for locally advanced oral cavity carcinoma is optimal surgical resection with negative margins followed by adjuvant therapy.[8] Postoperative radiotherapy with or without chemotherapy is the accepted standard of care after resection of locally advanced carcinomas, as these patients are higher risk for locoregional relapse.[9] Despite of such intensive adjuvant therapy, more than 50% of patients relapse with 2 years and succumb to disease in another 8–9 months of relapse.[8,9] Hence, there is an unmet need to introduce novel therapy after adjuvant treatment which could delay or avoid the relapses with easy of accessibility and affordability, especially in India where oral cavity cancer is the major healthcare problem.[1,2]
We conducted a retrospective audit of all resectable oral cavity patients registered in our rural hospital database from May 2008 till May 2014. Median age at presentation in rural India is 54 years which is older at compared to urban population (45 years).[10] Smoking pattern in rural populace differs with 27% smokers and 92% patients were smokeless tobacco consumers, compared to 32% and 74%, respectively, in metropolitan city.[11] Similarly, buccal mucosa was the most common subsite (53%) rather than tongue (urban) which could be due to habit of placing tobacco quid in gingivobuccal sulcus leading to prolonged irritant and carcinogenic effect.[12] Unlike in cities, where the gender presentation is heavily skewed to 5:1; male:female, there is a higher representation of women in rural India (1.7:1).[11] This could be because of higher use of masheri and tobacco among rural women and relative higher migration of young men to bigger cities for earning livelihood. More than two-third of patients presented with locally advanced Stage III and Stage IVA oral cavity cancer.
Metronomic chemotherapy is the chronic administration of chemotherapy at low, minimally toxic doses on a frequent schedule of administration, with no prolonged drug-free breaks.[13,14] The success of metronomic therapy relies on three main mechanisms: continuous administration, activation of cancer immunology, and antiangiogenic effects.[15] Cyclooxygenase-2 (COX-2) is commonly overexpressed in a variety of premalignant conditions and head and neck squamous cell carcinoma (HNSCC).[16] Celecoxib proved effective for treating HNSCC through the suppression of cell proliferation and the induction of apoptosis in a COX-2 - independent manner.[17] Metronomic chemotherapy in recurrent head neck carcinoma has shown good disease control rates with effective palliation, minimal toxicity, and preserved quality of life.[10,12,18,19] Recently, a phase II randomized trial of metronomic chemotherapy in recurrent head neck carcinoma showed better survival outcomes compared to standard intravenous chemotherapy with significantly less toxicity, better compliance, and early pain resolution with preserved quality of life.[10,11] When used as maintenance after standard adjuvant therapy, metronomic methotrexate, and celecoxib improved DFS without any Grade III or IV toxicities though the studied population was small.[20]
We used oral methotrexate (15 mg/m2) once a week and celecoxib 200 mg twice a day daily in patients of Stage III/IVA oral cavity carcinoma after completion of adjuvant therapy, which were referred to medical oncologist by the referral consultant. Due to inherent institutional referral policy, 130 out of 225 (58%) such eligible patients received above maintenance therapy for a duration of 18 months (median 14 months). This cohort of patients when compared to other Stage III/IVA (95/225) patients who did not receive maintenance therapy, maintenance therapy improved DFS (8 vs. 14 months, P = 0.22) and OS significantly (14 vs. 26 months, P = 0.04). None of the patients receiving metronomic therapy received blood transfusion, admissions due to toxicity or had febrile neutropenia. None of the patients discontinued therapy due to toxicity. Hence, chronic low dose administration of oral chemotherapy in locally advanced oral cavity carcinoma after completion of adjuvant therapy was associated with fewer relapses with minimal toxicity and survival benefit at the cost of <10 $/month. This could be a boon for low- and middle-income countries such as India, where oral cavity carcinoma is the most common head neck cancer, and more than half of the patients relapse early despite standard adjuvant therapy due to advanced stage at presentation.[21] After extensive literature search, currently, only one registered trial is investigating the question of maintenance metronomic chemotherapy in HNSCC who achieve a complete response and is actively recruiting patients (NCT00855881).[22]
Being retrospective, we do not rule out any selection bias and inadvertent omission of patients which might have inflated the outcome. Only patients whose case records were found were eventually entered into our database and analyzed, which might not have included all patients with oral cavity carcinoma treated or referred in our institute. As it was a retrospective study and compared groups were not randomized, confounding of treatment effects and referral bias for maintenance therapy cannot be certainly ruled out. Till date, as per the best knowledge of the authors, no other large study has been reported which tests the merit of using maintenance oral metronomic therapy after adjuvant treatment in locally advanced oral cavity carcinoma. However, this result can be used for hypothesis generation, and a prospective randomized trial must be planned to answer this question more conclusively in near future.
Oral cavity cancer is a major health care problem in rural India due to various smokeless tobacco preparations. Low socioeconomic status, lack of awareness, and poor accessibility to health care providers often leads to late presentation and hence, compromised outcomes. Despite curative surgery followed by standard adjuvant therapy, considerable numbers of patients relapse, and succumb to disease within first 2 years in locally advanced oral cavity cancer. Using low-cost oral maintenance metronomic therapy after completion of standard adjuvant therapy avoids and delay relapse and improves survival with minimal toxicity. Benefit of metronomic maintenance chemotherapy in locally advanced oral cavity needs to be further evaluated prospectively in a randomized trial.
Conclusion
Oral cavity cancer is a major health care problem in rural India. Presentation at advanced stage leads to suboptimal outcomes. Benefit of metronomic maintenance chemotherapy in locally advanced oral cavity needs to be further evaluated prospectively.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Elango JK, Gangadharan P, Sumithra S, Kuriakose MA. Trends of head and neck cancers in urban and rural India. Asian Pac J Cancer Prev. 2006;7:108–12. [PubMed] [Google Scholar]
- 2.Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S, Mathew B, et al. Effect of screening on oral cancer mortality in Kerala, India: A cluster-randomised controlled trial. Lancet. 2005;365:1927–33. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S, Heller RF, Pandey U, Tewari V, Bala N, Oanh KT. Delay in presentation of oral cancer: A multifactor analytical study. Natl Med J India. 2001;14:13–7. [PubMed] [Google Scholar]
- 4.Conway DI, Petticrew M, Marlborough H, Berthiller J, Hashibe M, Macpherson LM. Socioeconomic inequalities and oral cancer risk: A systematic review and meta-analysis of case-control studies. Int J Cancer. 2008;122:2811–9. doi: 10.1002/ijc.23430. [DOI] [PubMed] [Google Scholar]
- 5.Sunny L, Yeole BB, Hakama M, Shiri R, Sastry PS, Mathews S, et al. Oral cancers in Mumbai, India: A fifteen years perspective with respect to incidence trend and cumulative risk. Asian Pac J Cancer Prev. 2004;5:294–300. [PubMed] [Google Scholar]
- 6.Thorat RV, Panse NS, Budukh AM, Dinshaw KA, Nene BM, Jayant K. Prevalence of tobacco use and tobacco-dependent cancers in males in the rural cancer registry population at Barshi, India. Asian Pac J Cancer Prev. 2009;10:1167–70. [PubMed] [Google Scholar]
- 7.De Felice F, Musio D, Tombolini V. Head and neck cancer: Metronomic chemotherapy. BMC Cancer. 2015;15:677. doi: 10.1186/s12885-015-1705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 9.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–44. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 10.Noronha V, Joshi A, Marfatia S, Patil V, Juvekar S, Arya S, et al. Health-related quality of life in patients with metastatic, relapsed, or inoperable squamous cell carcinoma of the head and neck in India. Support Care Cancer. 2016;24:1595–602. doi: 10.1007/s00520-015-2937-9. [DOI] [PubMed] [Google Scholar]
- 11.Patil VM, Noronha V, Joshi A, Muddu VK, Dhumal S, Bhosale B, et al. A prospective randomized phase II study comparing metronomic chemotherapy with chemotherapy (single agent cisplatin), in patients with metastatic, relapsed or inoperable squamous cell carcinoma of head and neck. Oral Oncol. 2015;51:279–86. doi: 10.1016/j.oraloncology.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Patil VM, Noronha V, Joshi A, Pinninti R, Dhumal S, Bhattacharjee A, et al. Metronomic chemotherapy in platinum-insensitive failures and/or early failures postmultimodality management in oral cancers. Indian J Med Paediatr Oncol. 2015;36:161–5. doi: 10.4103/0971-5851.166725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: Metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045–7. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O’Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–86. [PubMed] [Google Scholar]
- 15.Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: New rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–65. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 16.Ko SH, Choi GJ, Lee JH, Han YA, Lim SJ, Kim SH. Differential effects of selective cyclooxygenase-2 inhibitors in inhibiting proliferation and induction of apoptosis in oral squamous cell carcinoma. Oncol Rep. 2008;19:425–33. [PubMed] [Google Scholar]
- 17.Bock JM, Menon SG, Sinclair LL, Bedford NS, Goswami PC, Domann FE, et al. Celecoxib toxicity is cell cycle phase specific. Cancer Res. 2007;67:3801–8. doi: 10.1158/0008-5472.CAN-06-3780. [DOI] [PubMed] [Google Scholar]
- 18.Patil V, Noronha V, D’cruz AK, Banavali SD, Prabhash K. Metronomic chemotherapy in advanced oral cancers. J Cancer Res Ther. 2012;8(Suppl 1):S106–10. doi: 10.4103/0973-1482.92223. [DOI] [PubMed] [Google Scholar]
- 19.Patil V, Noronha V, Krishna V, Joshi A, Prabhash K. Oral metronomic chemotherapy in recurrent, metastatic and locally advanced head and neck cancers. Clin Oncol (R Coll Radiol) 2013;25:388. doi: 10.1016/j.clon.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Pai PS, Vaidya AD, Prabhash K, Banavali SD. Oral metronomic scheduling of anticancer therapy-based treatment compared to existing standard of care in locally advanced oral squamous cell cancers: A matched-pair analysis. Indian J Cancer. 2013;50:135–41. doi: 10.4103/0019-509X.117024. [DOI] [PubMed] [Google Scholar]
- 21.André N, Banavali S, Snihur Y, Pasquier E. Has the time come for metronomics in low-income and middle-income countries? Lancet Oncol. 2013;14:e239–48. doi: 10.1016/S1470-2045(13)70056-1. [DOI] [PubMed] [Google Scholar]
- 22.Metronomic Chemotherapy with Tegafur/Uracil for Head and Neck Squamous Cell Carcinoma. [Last accessed on 2016 Apr 03]. Available from: http://www.clinicaltrials.gov/ct2/show/study/NCT00855881?show_desc=Y#desc .


