Abstract
Objective:
There are little data regarding safety and effectiveness of neoadjuvant chemotherapy (NACT) in patients who are considered unfit for receiving 3 weekly paclitaxel and carboplatin. The aim of this study was to examine the toxicity and response rates of weekly paclitaxel and carboplatin as NACT in such cohort of patients.
Methods:
Study population included advanced ovarian cancer patients who were unlikely to tolerate 3 weekly paclitaxel and carboplatin and hence received weekly paclitaxel (80 mg/m2) and carboplatin AUC-2 as NACT. The data regarding the baseline characteristics, chemotherapy tolerance, completion rates, toxicity (Common Terminology Criteria for Adverse Events version 4.02), and radiological response rates are presented. SPSS version 16 was used for analysis. Descriptive statistics is presented.
Results:
Eleven patients received this schedule. Nine patients completed nine cycles of NACT. Except one, all patients completed NACT with an average relative dose intensity of >0.8. There was no chemotherapy-related mortality. Grade 3–4 life-threatening complications were seen in two patients. The post NACT response rate was 100%.
Conclusions:
Weekly paclitaxel and carboplatin chemotherapy is safe and efficacious in patients who are unsuitable for 3 weekly paclitaxel and carboplatin chemotherapy schedules.
Keywords: Intolerable, metronomic, neoadjuvant chemotherapy, poor nutritional status, poor performance status
Introduction
In advanced epithelial ovarian cancers (Stage IIIc and IV), neoadjuvant chemotherapy (NACT) followed by interval cytoreductive surgery approach is noninferior to upfront surgery followed by adjuvant chemotherapy.[1,2] The chemotherapy schedule used in these studies was a 3 weekly schedule of paclitaxel and carboplatin and the inclusion criteria of these trials included patients with a WHO performance status (PS) 0–2, with adequate organ function and nutritional status.[1,2] However, frequently patients of ovarian cancer seen at our center have a poor PS, nutritional status or severe uncontrolled co-morbidities, making them ineligible for such 3 weekly schedules. There is a paucity of literature for the guidance of treatment in such situations. Frequently, practitioners choose either arbitrarily modified doses of chemotherapy (paclitaxel and carboplatin) or single-agent carboplatin.[3,4] However, in ovarian cancer that a decrease in dose intensity of the chemotherapy schedule leads to a corresponding decrease in survival.[5,6] In addition, the updated results of Japanese Oncology Group trial show the benefit of dose-dense schedule of paclitaxel with 3 weekly carboplatin over the standard 3 weekly paclitaxel and carboplatin schedule.[7]
In view of these results and certain Phase 2 studies showing a low risk of myelosuppression with the dose-dense weekly schedule of paclitaxel and carboplatin,[8] we reviewed our experience with this schedule in patients ineligible to receive standard 3 weekly paclitaxel and carboplatin. Our hypothesis was that weekly metronomic chemotherapy as NACT would be considered for further studies if in this study, we could deliver this schedule with an average relative dose intensity of 0.8 or more in at least 80% of our patients.
Methods
This was an IRB approved retrospective analysis. All patients with epithelial ovarian cancers seen at our center between August 2013 and April 2014 with below mention features were included.
Inclusion criteria
With two or more features mentioned below.
Age more than 65 years
Eastern Cooperative Oncology Group (ECOG) PS 2–3
Low serum albumin (below 3 g/dl)
Uncontrolled co-morbidity (any one of the following uncontrolled diabetes mellitus, Uncontrolled chronic obstructive pulmonary disease, ischemic heart disease within last 1 year, presence of active tuberculosis or Deep venous thrombosis)
Body mass index (BMI) equal to or below 16 kg/m2.
Exclusion criteria
Patients with seropositivity for HIV, hepatitis B surface antigen or hepatitis C virus
Patients not willing for close follow-up
Any previous treatment (surgery, radiation, or chemotherapy) except biopsy for the primary or regional tumor.
Intervention
NACT with the dose-dense weekly schedule of paclitaxel and carboplatin was offered to these patients after discussion in the Institutional tumor board. All patients were informed about the prognosis, and the option of treating with weekly chemotherapy was given after taking a written informed consent.
The chemotherapy offered was weekly paclitaxel (80 mg/m2) and carboplatin (AUC-2) with standard antiemetic prophylaxis. These patients were evaluated clinically before each weekly chemotherapy cycle and with contrast-enhanced computed tomography of thorax, abdomen, and pelvis after nine courses of the weekly schedule.
Data collection and analysis
The details of the basic demographic profile, staging details, NACT details, toxicity details (Common Terminology Criteria for Adverse Events [CTCAE] version 4.02), and response (RECIST version 1.1) were retrieved from the database in the MOG OPD and the case records for the purpose of this study. SPSS 16 was used for analysis, and descriptive statistics have been presented.
Results
Baseline details
Among 52 patients of ovarian cancer seen during this period, 20 patients were referred for NACT. Of these 11 patients (55% of patients referred for NACT) were found to be unsuitable for 3 weekly paclitaxel and carboplatin. The baseline characteristics of these 11 patients are shown in Table 1. The median age of these patients was 58 years (range 37–67 years). The reasons for consideration of weekly chemotherapy schedule are shown in Table 2.
Table 1.
Baseline features of patients
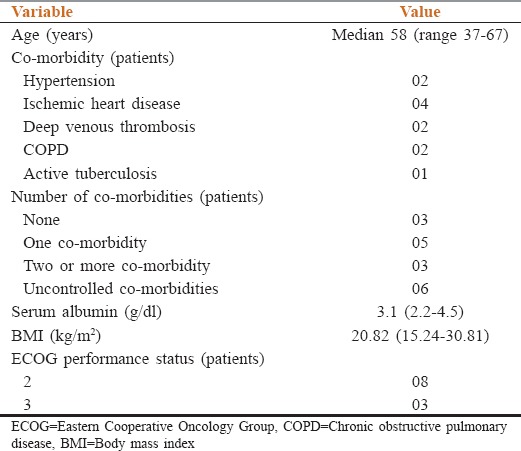
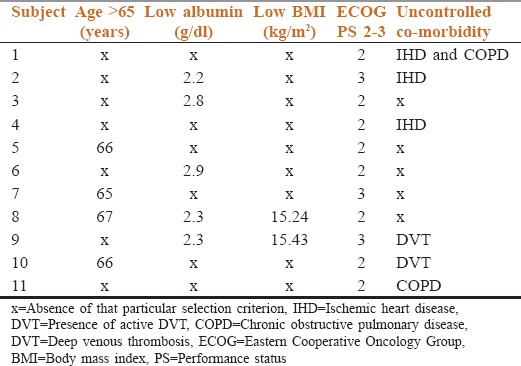
Table 2.
Details of selection criteria in each patient are shown
Staging details
The median CA 125 level was 661 (79–20228) U/mL. Stage IIIC disease was seen in eight patients whereas three patients had Stage IV disease. Pleural effusion at presentation was present in three patients, liver subcapsular deposits in five patients, and root of mesentery deposits in four patients. The median RECIST target size was 21.10 cm (14.84–40.90 cm).
Chemotherapy details
The median numbers of NACT cycles delivered were 9 (5–18). The proportion of patients who completed the planned course of NACT was 81.81% (9 patients out of 11). Two patients did not complete the planned course of nine cycles; both patients had Grade 3 sensory neuropathy. One patient developed it after five cycles while other developed it after eight cycles.
Delays in chemotherapy of more than 2 days were seen in eight patients. The reasons for the delay were logistical issue (public holiday/strike) in six patients while intolerable side effects were seen in two patients.
Dose reduction was required in two patients. The median dose reduction done was 25%. The causes of dose reduction were intolerable side effects in one patient and incomplete recovery of neutrophil count in the other patient. The median relative dose intensity of paclitaxel and carboplatin as NACT were 0.95 (0.55–1.00) and 0.95 (0.55–1.00), respectively. The median average relative dose intensity of the combination was 0.95 (0.55–1.00). Except one, all patients had received chemotherapy with an average relative dose intensity over 0.83.
Toxicity details
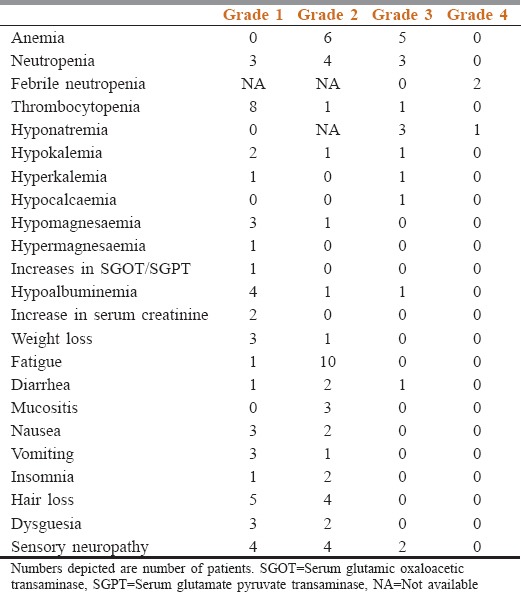
Toxicity recorded in accordance with CTCAE version 4.02 is shown in Table 3. The toxicity shown is the maximum toxicity seen during the whole NACT schedule. Toxicity-related admissions were required in four patients. The reason for toxicity-related admissions was febrile neutropenia in two patients, hyponatremia in one patient and diarrhea in one patient. The median number of admission per patient who required toxicity related admission was one (range: 1–3). Any Grade 3 or more toxicity was seen in six patients. Grade 3 and above life-threatening toxicities were seen in two patients. No treatment-related mortality was observed.
Table 3.
Toxicity details in accordance with Common Terminology Criteria for Adverse Events version 4.02
Efficacy of neoadjuvant chemotherapy
All patients had subjective symptomatic improvement. The radiological response rate as assessed by RECIST version 4.02 was CR in three patients and PR in rest eight patients. The median post chemotherapy RECIST target size was 7.6 cm (0–24.9 cm). The median proportional reduction in RECIST target size was 64.5% (33–100%).
Improvement in status
The improvement in PS was seen in all patients, improvement in BMI was seen patients 5 patients and an improvement in serum albumin was seen in 5 patients.
Discussion
In this study, 21.53% of patients were considered not suitable for 3 weekly paclitaxel and carboplatin. In a study by Jordan et al. 28.7% of patients with advanced ovarian cancer received nonstandard chemotherapy.[3] The nonstandard chemotherapy administered was mainly single agent carboplatin followed by variants of paclitaxel and carboplatin chemotherapy.[3] Unfortunately, these cohort patients who are considered unfit for 3 weekly paclitaxel and carboplatin are not eligible for most to randomized studies and hence there is a lack of guidance from the literature regarding treatment of these patients.
We tried to generate selection criteria for these patients. These selection criteria were drafted on the basis of evidence from literature, either showing that these patients were at risk of unacceptable toxicity after 3 weekly chemotherapy or patients with these features were routinely excluded from large studies of standard 3 weekly chemotherapy.
Elderly ovarian cancer patients are generally subjected to suboptimal chemotherapy. A report from M D Anderson cancer center highlighted the reduced use of combination platinum therapy with increasing age. While 83% of patients between age 70 and 79 years received combination platinum-based chemotherapy in this study, the same was delivered to only 41% of the patients with age >80 years.[9] Similarly, in an analysis of elderly patients above the age of 65 years, it was shown that old age lead to a delay in initiation of chemotherapy and this delay lead to a decrease in survival.[10] Analysis from OVCAD consortium too showed that elderly patients were more likely to receive suboptimal treatment as compared to younger patients (60% vs. 30%).[11] Giving 3 weekly schedule in elderly patients has been associated with increase in treatment-related mortality. A prospective trial by Matulonis et al. evaluating 3 weekly paclitaxel and carboplatin in elderly patients had to be prematurely closed as out of 12 patients, 3 had treatment-related mortality.[12]
Similarly, patients with ECOG PS 2 or more have a higher risk of developing chemotherapy toxicity.[10,13] In fact, PS 3–4 patients and patients with uncontrolled co-morbidities were routinely excluded from large studies.[1,2] Nutritional status is also a predictor of chemotherapy toxicity.[13,14] A low serum albumin and low BMI has been shown to be associated with an increase in chemotherapy toxicity.[13]
There are certain important observations generated from our study. The schedule of weekly paclitaxel and carboplatin was well-tolerated in patients who were considered unfit for receiving 3 weekly chemotherapy. The average relative dose intensity was above 0.83 in all, but one patient and no treatment-related mortality was observed. Life-threatening toxicities were also seen in only 18.18% of patients. Ours is probably the first report highlighting the safety of this schedule in NACT setting. There are a few reports in literature attesting the safety of this schedule in the adjuvant setting. Report of use of weekly paclitaxel and carboplatin by Safra et al. as adjuvant treatment highlighted the attenuated toxicity of such weekly regimens.[8] Palaia et al. in his audit of elderly patients also concluded that elderly patients receiving weekly therapy tolerated it better.[15] Similarly, in MITO-7 study, weekly paclitaxel and carboplatin adjuvant schedule was better tolerated, with a lower level of hematological toxicity, particularly febrile neutropenia, than the 3 weekly schedule.[16]
The regimen had a response rate of 100%, with 27.27% of patients having a complete response. These patients had advanced tumors with a median RECIST target size of 21 cm. This response rate compares favorably with those reported in the MITO-5 study and OVCTAVIA study.[17,18] We feel that these better results than these studies may have been achieved as a result of a higher dose of paclitaxel, i.e., 80 mg/m2. Another important aspect of NACT schedule was the improvement in PS and nutritional status seen in this study. This improvement in the general condition of patients is important in our setting where patients are often considered unfit for surgery due to malnutrition and poor PS.
This study is not without its weaknesses. The limited number of patients and the subjective nature of the selection criteria limit our ability as to draw any firm conclusions. However the primary endpoint of this study was met, and we would be considering this schedule safe for further studies in advanced ovarian cancers in our population.
Conclusion
Weekly paclitaxel and carboplatin schedule was well-tolerated, feasible, and showed 100% response in advanced ovarian cancers patients who were unfit for 3 weekly chemotherapy. The encouraging findings need to be confirmed in larger prospective studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
All staffs of Malabar cancer center.
References
- 1.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–53. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 2.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener HC, Lopes T, et al. Chemotherapy or upfront surgery for newly diagnosed advanced ovarian cancer: Results from the MRC CHORUS trial. [Last cited on 2014 Jul 07];J Clin Oncol. 2013 31(Suppl) Abstr 5500. Available from: http://www.meetinglibrary.asco.org/content/112631–132 . [Google Scholar]
- 3.Jordan S, Steer C, DeFazio A, Quinn M, Obermair A, Friedlander M, et al. Patterns of chemotherapy treatment for women with invasive epithelial ovarian cancer – A population-based study. Gynecol Oncol. 2013;129:310–7. doi: 10.1016/j.ygyno.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Piura B, Meirovitz M. Weekly carboplatin in a frail elderly woman with advanced peritoneal carcinoma. Arch Gynecol Obstet. 2005;273:192–4. doi: 10.1007/s00404-005-0024-z. [DOI] [PubMed] [Google Scholar]
- 5.Fauci JM, Whitworth JM, Schneider KE, Subramaniam A, Zhang B, Frederick PJ, et al. Prognostic significance of the relative dose intensity of chemotherapy in primary treatment of epithelial ovarian cancer. Gynecol Oncol. 2011;122:532–5. doi: 10.1016/j.ygyno.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Hanna RK, Poniewierski MS, Laskey RA, Lopez MA, Shafer A, Van Le L, et al. Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi-agent chemotherapy for epithelial ovarian cancer. Gynecol Oncol. 2013;129:74–80. doi: 10.1016/j.ygyno.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol. 2013;14:1020–6. doi: 10.1016/S1470-2045(13)70363-2. [DOI] [PubMed] [Google Scholar]
- 8.Safra T, Menczer J, Bernstein RM, Shpigel S, Matcejevsky D, Inbar MJ, et al. Combined weekly carboplatin and paclitaxel as primary treatment of advanced epithelial ovarian carcinoma. Gynecol Oncol. 2009;114:215–8. doi: 10.1016/j.ygyno.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Uyar D, Frasure HE, Markman M, von Gruenigen VE. Treatment patterns by decade of life in elderly women (> or =70 years of age) with ovarian cancer. Gynecol Oncol. 2005;98:403–8. doi: 10.1016/j.ygyno.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Wright J, Doan T, McBride R, Jacobson J, Hershman D. Variability in chemotherapy delivery for elderly women with advanced stage ovarian cancer and its impact on survival. Br J Cancer. 2008;98:1197–203. doi: 10.1038/sj.bjc.6604298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trillsch F, Woelber L, Eulenburg C, Braicu I, Lambrechts S, Chekerov R, et al. Treatment reality in elderly patients with advanced ovarian cancer: A prospective analysis of the OVCAD consortium. J Ovarian Res. 2013;6:42. doi: 10.1186/1757-2215-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matulonis UA, Krag KJ, Krasner CN, Atkinson T, Horowitz NS, Lee H, et al. Phase II prospective study of paclitaxel and carboplatin in older patients with newly diagnosed müllerian tumors. Gynecol Oncol. 2009;112:394–9. doi: 10.1016/j.ygyno.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Freyer G, Tew WP, Moore KN. Treatment and trials: Ovarian cancer in older women. Am Soc Clin Oncol Educ Book. 2013:227–35. doi: 10.14694/EdBook_AM.2013.33.227. [DOI] [PubMed] [Google Scholar]
- 14.Geisler JP, Linnemeier GC, Thomas AJ, Manahan KJ. Nutritional assessment using prealbumin as an objective criterion to determine whom should not undergo primary radical cytoreductive surgery for ovarian cancer. Gynecol Oncol. 2007;106:128–31. doi: 10.1016/j.ygyno.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Palaia I, Loprete E, Musella A, Marchetti C, Di Donato V, Bellati F, et al. Chemotherapy in elderly patients with gynecological cancer. Oncology. 2013;85:168–72. doi: 10.1159/000350859. [DOI] [PubMed] [Google Scholar]
- 16.Pignata S, Scambia G, Katsaros D, Gallo C, Pujade-Lauraine E, De Placido S, et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014;15:396–405. doi: 10.1016/S1470-2045(14)70049-X. [DOI] [PubMed] [Google Scholar]
- 17.Pignata S, Breda E, Scambia G, Pisano C, Zagonel V, Lorusso D, et al. A phase II study of weekly carboplatin and paclitaxel as first-line treatment of elderly patients with advanced ovarian cancer. A Multicentre Italian Trial in Ovarian cancer (MITO-5) study. Crit Rev Oncol Hematol. 2008;66:229–36. doi: 10.1016/j.critrevonc.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Martin A, Gladieff L, Tholander B, Stroyakovsky D, Gore M, Scambia G, et al. Efficacy and safety results from OCTAVIA, a single-arm phase II study evaluating front-line bevacizumab, carboplatin and weekly paclitaxel for ovarian cancer. Eur J Cancer. 2013;49:3831–8. doi: 10.1016/j.ejca.2013.08.002. [DOI] [PubMed] [Google Scholar]


