Abstract
Background:
Topotecan is the standard second line agent used in relapsed small cell lung cancer (SCLC). However, the erratic availability and the cost of the drug has been a prohibitive factor for its use in second-line setting in India. Paclitaxel has shown antitumor activity in heavily pretreated patients with SCLC. Hence, this audit was performed to study the efficacy of weekly paclitaxel as a form of metronomic therapy in the second-line setting in SCLC.
Materials and Methods:
Fifty-seven patients of relapsed SCLC who presented to the thoracic medical oncology unit of Tata Memorial Centre, Mumbai between January 2011 and December 2015 were selected for this analysis. Weekly paclitaxel at a dose of 80 mg/m2 was administered until progression or development of intolerable side effects or patient refusal. Data regarding baseline demographics, previous treatment history, response rate, progression-free survival, overall survival (OS), and toxicity to weekly paclitaxel was extracted from a prospectively maintained database in the thoracic medical oncology unit and was analyzed using SPSS version 16 (IBM, New York, USA). Kaplan–Meier survival analysis was performed.
Results:
Median age of the cohort was 58 years (40–77 years). Etoposide with carboplatin was the regimen used in 40 patients (70.2%) whereas the remaining 17 patients received etoposide with cisplatin (29.8%). Eastern Cooperative Oncology Group performance status at relapse was 1 in 3 (5.3%), 2 in 49 (86.0%), and 3 in 5 (8.7%) patients. The response rate and clinical benefit rate were 9.1% (5 patients) and 52.7% (29 patients), respectively. Grade 3–4 toxicities were seen in 10.5% (6 patients). The median PFS was 145 days (95% confidence interval [CI]: 116.6–173.5 days) whereas the median OS was 168 days (95% CI: 112.5–223.5 days).
Conclusion:
Weekly paclitaxel as a second line agent in relapsed small cell cancer of the lung is a feasible and well-tolerated agent.
Keywords: Metronomic, palliative, second line, small cell lung cancer, weekly paclitaxel
Introduction
Extensive stage disease small cell lung cancer (SCLC) is a chemosensitive disease, but invariably relapses despite high response rates.[1] The treatment at relapse takes into account the performance status (PS) of the patient and the relapse-free interval (RFI) from prior chemotherapy.[2] Patients with Eastern Cooperative Oncology Group (ECOG) PS 0–2 are offered palliative chemotherapy while patients with PS 3–4 are generally treated with best supportive care. The chemotherapy offered in the second line is dictated by the RFI. Patients who have an RFI of 6 months or more can be rechallenged with the same regimen used in first-line setting while those with RFI between 3 and 6 months are treated with topotecan (either oral or intravenous [IV]) and those below 3 months with oral topotecan.[2] Topotecan is associated with modest improvements in survival and quality of life in these patients.[3,4,5] However, the cost of topotecan and its erratic availability have become an issue in the recent past in India and hence there was an unmet need for the development of alternate second line agents.
Paclitxael, when administered at 60–80 mg/m2 weekly doses is associated with antiangiogenic activity, and it increases the apoptotic activity in cancer cells.[6] It has shown to be of some activity in heavily pretreated small cell cancer patients. In a Phase 2 study from Japan, administration of weekly paclitaxel in the second line setting in SCLC was associated with a response rate of 35% and a median survival of 5.8 months.[7] The differences in pharmacogenomics of taxanes in Japanese patients and Western patients is a known fact, and hence, we wanted to confirm these results in our setting.[8,9,10] This audit was designed to study the median progression-free survival (PFS) and overall survival (OS) with weekly paclitaxel in the second line setting in SCLC patients.
Materials and Methods
Selection of cases
Patients of SCLC were selected from the lung cancer audit database subjected to satisfying all the below-mentioned selection criteria. The lung cancer audit is a multicentric, nationwide, Institutional Ethics Committee approved project funded by Indian Cooperative Oncology Network.
Recipient of second line regimen
Weekly paclitaxel as the second line regimen
ECOG PS 0–3
Treatment planned at Tata Memorial Hospital
Period January 2011–December 2015.
Treatment
All patients were treated with weekly paclitaxel regimen. Premedication with antihistamines and steroid, these patients received 80 mg/m2 of paclitaxel as 1 h infusion. The chemotherapy was continued until either disease progression, intolerable side effects or patient refusal, whichever occurred earlier. Postprogression, patients were treated in accordance with institutional standards.
Data extraction
Data regarding baseline demographics, previous treatment history, response rate, PFS, OS and toxicity to weekly paclitaxel were extracted from a prospectively maintained database in the Thoracic Medical Oncology Unit. The responses were coded in accordance with RECIST version 1.1 while the toxicity was coded in accordance with CTCAE version 4.03.
Data analysis
SPSS version 16 (IBM, New York, USA) was used for analysis. Descriptive statistics were performed. Continuous variables were expressed with median and its respective interquartile range whereas noncontinuous variables were expressed in proportions with its respective 95% confidence interval (CI) interval. Kaplan–Meier survival estimation method was used for time to event calculation. PFS was calculated from the date of the first cycle of weekly paclitaxel to the date of progression or death (whichever was earlier). Patients who did not have events (progression or death) were censored during the PFS estimation. OS was calculated from the date of the first cycle of weekly paclitaxel to the date of death. Patients who did not have an event (death) were censored during the OS estimation.
Results
Patient characteristics
The median age of the cohort was 58 years (IQR 53–65 years). There were 49 males (86.0%) and 8 females (14.0%). 51 patients (89.5%) had a previous history of smoking. ECOG PS at relapse was 1 in 3 (5.3%), 2 in 49 (86.0%), and 3 in 5 (8.7%) patients.
Fifty patients (87.7%) had extensive disease at baseline diagnosis, and the remaining 7 (12.3%) had limited stage disease. All patients were exposed previously to etoposide and platinum. The platinum used was cisplatin in 17 patients (29.8%) and carboplatin in 40 patients (70.2%). Previous radiation at local tumor site was received by seven patients (12.3%). The median RFI postprior chemotherapy was 216 days (IQR 63–532 days). 19 patients (33.3%) had RFI below 6 months, whereas 38 patients (66.7%) had RFI beyond 6 months.
Response
The response was evaluated in 55 patients. The response post-2 months (8 weeks) of weekly paclitaxel was partial response in five patients (9.1%, n = 55), stable disease in 24 patients (43.6% n = 55), and progressive disease in 26 patients (47.3%, n = 55). The response rate was 9.1% (95% CI: 3.4–20.7%, n = 55). The clinical benefit rate at 2 months was 52.7% (95% CI: 39.8–65.3%, n = 55). The response rate was 5.3% in patients with RFI below 6 months while it was 11.1% in patients with RFI above 6 months (Fisher's exact test, two-tailed P = 0.655).
Toxicity
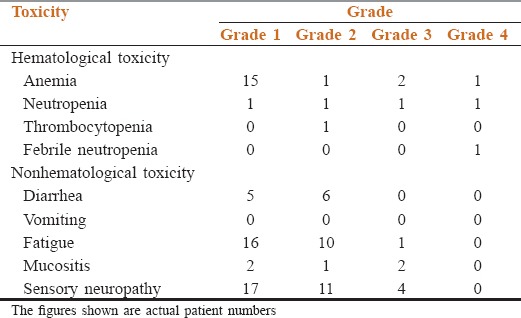
The median number of cycles of weekly paclitaxel received was 8 (IQR 6–14) cycles. Toxicity related cessation of treatment was required in ten patients (17.5%). The reason for stoppage was Grade 3–4 toxicities in four patients (7.0%) and deterioration in PS in six patients (10.5%). The cumulative incidence of Grade 3–4 toxicities was 10.5% (6 patients). The details about toxicity are shown in Table 1.
Table 1.
The toxicity details
Outcomes
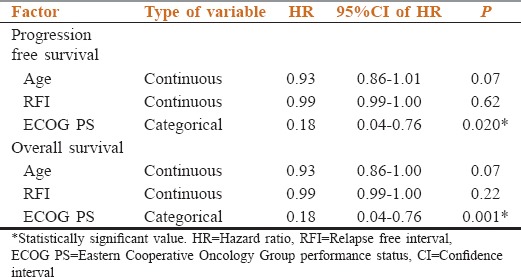
The median PFS was 145 days (95% CI: 116.6–173.5 days) while the median OS was 168 days (95% CI: 112.5–223.5 days). Out of the tested prognostic factors, the impact of age, ECOG PS at relapse and RFI on PFS and OS is shown in Table 2. Poor ECOG PS (3–4) had significantly adverse impact on PFS and OS.
Table 2.
The impact of different factors on outcomes
Discussion
Topotecan is the only Food and Drug Administration-approved second-line agent for palliative chemotherapy in SCLC.[2] In the study carried out by Pawel et al., IV topotecan was associated with a response rate of 24.3%, median PFS of 13.3 weeks, and median OS of 25.0 weeks.[4] A study by O’Brien et al., in patients with RFI below 90 days was associated with a response rate of 7.0% and median OS of 25.3 weeks. In comparison to these data, this study had overall response rate of 9.1%, median PFS of 145 days (20.7 weeks), and median OS of 168 days (24.0 weeks).[5] Similar data with weekly paclitaxel showing a response rate of 23.8% and median OS of 5.8 months (23.2 weeks) was published by Yamamoto et al.[7] Both our data and data from Japan by Yamamoto et al., testify the efficacy of weekly paclitaxel in this setting.
A peculiar finding in our study which differs from previously published data is the low response rates. In general, metronomic chemotherapy regimens work by disease stabilization and hence low response rates are the norm.[11] However, the Japanese study used nearly the same regimen and had higher response rates. The probable reason for this may be the way response rates were assessed. The response in our setting was assessed by RECIST version 1.1 while response in the study reported by Yamamoto et al., was done by WHO criteria.[7] Discrepancy in the response assessment done by WHO and RECIST criteria is known in literature.[12]
CAV was traditionally used as a second line agent in SCLC. One of the factors which makes Topotecan feasible in comparison to CAV regimen as a second line agent is its limited toxicity. Cumulative Grade 4 neutropenia was seen in 37.8% of patients who received IV topotecan as against 51.4% in CAV regimen (P < 0.01).[4] The study reported by O’Brien et al., with oral topotecan was associated with a Grades 3 and 4 neutropenia of 61% similar to that seen with CAV regimen.[5] The corresponding rates of Grades 3–4 neutropenia in our study was 3.5%. The overall toxicity cumulative incidence of Grades 3–4 toxicity was 10.5%. This is a heartening fact that metronomic chemotherapy in this palliative setting was relatively toxicity-free in comparison to toxicities reported in other studies.[4,5]
The study was not without its pitfalls. This was a single center, small, retrospective analysis, and quality of life data was not maintained. These factors limit the wide applicability of this data. However, it does not deny the fact that metronomic weekly paclitaxel regimen in SCLC in second line has promise and needs further evaluation.
Conclusion
Weekly Paclitaxel as a second line agent in relapsed small cell cancer of lung is feasible and is a well-tolerated agent. In addition, it shows promising results which are comparable to topotecan.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Alvarado-Luna G, Morales-Espinosa D. Treatment for small cell lung cancer, where are we now.-a review? Transl Lung Cancer Res. 2016;5:26–38. doi: 10.3978/j.issn.2218-6751.2016.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalemkerian GP, Akerley W, Bogner P, Borghaei H, Chow LQ, Downey RJ, et al. Small cell lung cancer. J Natl Compr Canc Netw. 2013;11:78–98. doi: 10.6004/jnccn.2013.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horita N, Yamamoto M, Sato T, Tsukahara T, Nagakura H, Tashiro K, et al. Topotecan for relapsed small-cell lung cancer: Systematic review and meta-analysis of 1347 patients. Sci Rep. 2015;5:15437. doi: 10.1038/srep15437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Pawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–67. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien ME, Ciuleanu TE, Tsekov H, Shparyk Y, Cuceviá B, Juhasz G, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441–7. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 6.Marchetti P, Urien S, Cappellini GA, Ronzino G, Ficorella C. Weekly administration of paclitaxel: Theoretical and clinical basis. Crit Rev Oncol Hematol. 2002;44(Suppl):S3–13. doi: 10.1016/s1040-8428(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto N, Tsurutani J, Yoshimura N, Asai G, Moriyama A, Nakagawa K, et al. Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res. 2006;26:777–81. [PubMed] [Google Scholar]
- 8.Kenmotsu H, Tanigawara Y. Pharmacokinetics, dynamics and toxicity of docetaxel: Why the Japanese dose differs from the Western dose. Cancer Sci. 2015;106:497–504. doi: 10.1111/cas.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low SK, Chung S, Takahashi A, Zembutsu H, Mushiroda T, Kubo M, et al. Genome-wide association study of chemotherapeutic agent-induced severe neutropenia/leucopenia for patients in Biobank Japan. Cancer Sci. 2013;104:1074–82. doi: 10.1111/cas.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katori N, Sai K, Saito Y, Fukushima-Uesaka H, Kurose K, Yomota C, et al. Genetic variations of orosomucoid genes associated with serum alpha-1-acid glycoprotein level and the pharmacokinetics of paclitaxel in Japanese cancer patients. J Pharm Sci. 2011;100:4546–59. doi: 10.1002/jps.22648. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal A, Purandare N, Shah S, Puranik A, Banavali S, Rangarajan V. Response assessment in metronomic chemotherapy: RECIST or PERCIST? Indian J Nucl Med. 2014;29:74–80. doi: 10.4103/0972-3919.130285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn SH, Garewal HS, Dragovich T. Discrepancy in the assessment of tumor response in patients with pancreatic cancer: WHO versus RECIST criteria. J BUON. 2008;13:359–62. [PubMed] [Google Scholar]


