Abstract
Introduction:
Acute myeloid leukemia (AML) in elderly patients differs biologically from that in younger patients and is known to have unfavorable chromosomal rearrangements, higher resistance, and lower tolerance to chemotherapy. In such circumstances, instead of giving full-blown chemotherapy, palliative metronomic chemotherapy (MCT) could be a treatment option.
Patients and Methods:
We performed a prospective pilot study of old AML patients (age >60 years) not amenable to curative treatment. Thirty-two patients were enrolled into the study and were treated with daily oral 6-mercaptopurine 75 mg/m2. The following inclusion criteria were used: age >60 years, nonpromyelocytic AML, the absence of uncontrolled comorbidities, and patient not amenable to curative treatment. Overall survival (OS) was calculated using Kaplan–Meier method and Cox regression analysis were used to calculate the hazards ratio of significant factors.
Results:
The median age of the patients was 69 years (range: 61–86 years) with male: female ratio of 2.5:1. About 59.4% of patients had Eastern Cooperative Oncology Group performance status of 2 while rest had the status of 3. The median OS was 6 months (95% confidence interval [CI]: 4.4–7.6). Males had median OS of 7 months (95% CI: 5.4–8.6) versus females with OS of 3 months (95% CI: 1.5–4.4; P = 0.008). There was no survival difference on the basis of baseline hemoglobin or French-American-British class. There were no Grade 4 toxicities and no episode of febrile neutropenia.
Conclusions:
MCT with oral 6-mercaptopurine is an attractive treatment option in elderly AML patients who are not amenable to curative therapy with minimal toxicities.
Keywords: 6-mercaptopurine, acute myeloid leukemia, elderly, metronomic chemotherapy
Introduction
Acute myeloid leukemia (AML) is a group of neoplastic disorders characterized by the proliferation and accumulation of immature hematopoietic cells in the bone marrow and blood. AML accounts for approximately 20% of acute leukemia in children and 80% of acute leukemia in adults.[1,2] The incidence of AML progressively increases with age and in adults over the age of 65 years, the incidence is approximately 30 times the incidence of AML in children.[3]
AML in elderly patients differs biologically from that in younger patients and is known to have unfavorable chromosomal rearrangements, higher resistance, and lower tolerance to chemotherapy. Old age is recognized as a risk factor for both the two major causes of therapeutic failure in AML: Treatment-related mortality and resistance to therapy.[4] Older patients tolerate less well-aggressive therapies due to poor performance status (PS), the presence of comorbid disease, decreased the ability of clearance of chemotherapy, and poor tolerance of systematic bacterial and fungal infections.[5] On the other hand, the disease in older patients shows an increased proportion of unfavorable karyotype (especially abnormalities of chromosomes 5 and 7 or complex chromosomal aberrations), the emergence of AML from an antecedent hematological disorder, the presence of dysplastic changes, the frequent expression of the multidrug resistance phenotype and the involvement of more primitive progenitors in the leukemic process, all of the above associated with increased resistance to treatment.[6]
In view of poor prognosis of elderly AML and toxicity of standard intravenous chemotherapy, metronomic chemotherapy (MCT) with daily oral drugs is an attractive option. The salient features of MCT include frequent chemotherapy administration without any interruption, preference for oral drugs, not using the maximal tolerated dose instead using a biological optimized dose, no application of hematopoietic growth factors, low incidence of treatment-related side effects, and potential for delayed development of resistance.
MCT has become quite popular in the past few years for relapsed/refractory disease in various solid malignancies.[7] The objective of this study was to estimate the overall survival (OS) in elderly AML patients on oral MCT and to calculate survival difference on the basis of various baseline characteristics.
Patients and Methods
We performed a prospective hospital-based pilot study enrolling old AML patients as per the following selection criteria: Age >60 years, nonpromyelocytic AML, the absence of uncontrolled comorbidites, and patient not amenable to curative treatment (the Eastern Cooperative Oncology Group [ECOG] PS ≥2 or refusal for standard treatment). Between July 2013 to June 2014, 32 consecutive patients fitting the selection criteria were enrolled into the study and were treated with daily oral 6-Mercaptopurine 75 mg/m2. AML-M3 and secondary AML (prior MDS or chemotherapy for other cancer) were not included in the study. Informed consent was obtained from the patients after clearly explaining all the alternative treatment options. The treatment was continued till patient's death, progressive disease, intolerable side effects, or the decision of patient or the family members. The primary endpoint of the study was the estimation of OS of the patients.
The bone marrow diagnosis was classified as per French-American-British classification and diagnosis was further confirmed by flow cytometry. Revised recommendations of the International Working Group were followed for assessing the response to treatment.[8] All patients received blood and blood products along with supportive care when ever needed in addition to the prescribed treatment. The cost of treatment was extremely low (7.5 USD/month).
SPSS version 20.0 (IBM Corp., Armonk, NY, USA) statistical software was used for the statistical analysis. OS was calculated using Kaplan–Meier method and Cox regression analysis were used to calculate the hazards ratio (HR) of significant factors. Kaplan–Meier survival curves were drawn using the software and log-rank test was used to calculate the significance of factors influencing the OS.
Results
Table 1 shows the baseline characteristics of the patients at the time of enrollment into the study. The median age of the patients was 69 years (range 61–86 years) with male: female ratio of 2.5:1. 59.4% patients had ECOG PS of 2 while rest had the status of 3. Most of the patients (56.3%) were classified as French-American-British (FAB) class M2. Baseline hemoglobin at presentation was <5 g% in 46.9% and ≥5 g% in the rest. Total leukocyte count (TLC) at the presentation was <1 lakh/cmm in 40.6% patients and ≥1 lakh/cmm in 59.4% of the cases. Most of the patients (65.6%) had platelet count ≥20,000/cmm while <20,000/cmm in 34.4% of the patients. Table 1 depicts the results of Kaplan–Meier survival analysis on the basis of various subgroups. The median OS was 6 months (95% confidence interval [CI]: 4.4–7.6). The patients of age group 61–70 years had highest median OS of 8 months (95% CI: 6.9–9.0) while patients of 71–80 and 81–90 years had median OS of 3 (95% CI: 1.8–4.1) and 2 months (95% CI: 1.6–2.4; P < 0.001), respectively [Figure 1]. The OS for ECOG PS of 2 was 8 months (95% CI: 6.3–9.6) while PS of 3 had median OS of 5 months (95% CI: 2.7–7.2; P = 0.015). Males had median OS of 7 months (95% CI: 5.4–8.6) versus females with OS of 3 months (95% CI: 1.5–4.4; P = 0.008). There was no survival difference on the basis of baseline hemoglobin or FAB class. Patients with baseline TLC ≥1 lakh/cmm had significantly worse survival (median OS: 6 months) as compared to <1 lakh/cmm (median OS: 9 months; P = 0.011). The patients with platelet count ≥ 20,000/cmm at presentation had significantly better OS (median OS: 8 months) as compared to <20,000/cmm (4 months, P = 0.002). Cox regression analysis [Table 1] revealed HR of 6.752 (95% CI: 2.293–19.876; P = 0.001) for age group >80 years versus 61–70 years and HR of 2.785 (95% CI: 1.195–6.492; P = 0.018) for females versus males. Similarly, the HR for patients with TLC ≥ 1 lakh/cmm was 2.748 (95% CI: 1.151–6.557, P = 0.023) and platelet count ≥20,000/cmm was 0.275 (95% CI: 0.111–0.682, P = 0.005). There were no Grade 4 toxicities and no episode of febrile neutropenia attributable to treatment. No patient required treatment discontinuation due to toxicities of the treatment.
Table 1.
Baseline characteristics along with Kaplan–Meier and Cox regression survival analysis for the subgroups of elderly acute myeloid leukemia patients
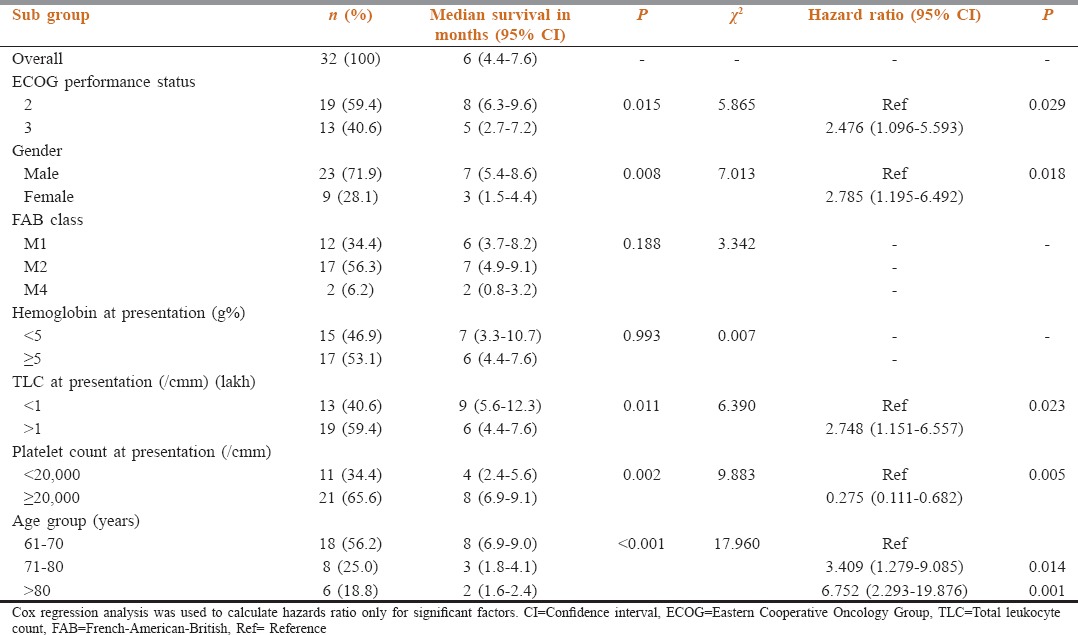
Figure 1.
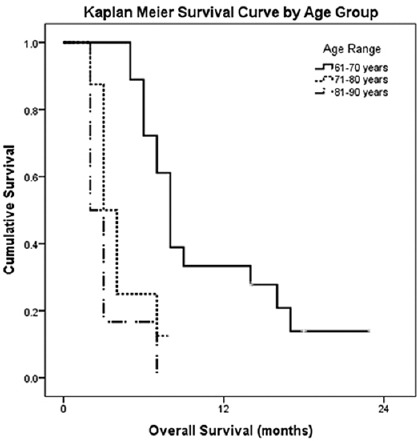
Kaplan–Meier survival analysis of elderly acute myeloid leukemia patients by age group
Discussion
MCT is based on more frequent and low-dose drug administration as compared to conventional chemotherapy.[9] MCT targets tumor angiogenesis which is necessary for tumor growth and metastasis. The anti-angiogenic activity of MCT has been demonstrated in vivo, and several other mechanisms of action have also been recognized. These include selective inhibition of proliferation and/or induction of apoptosis of activated endothelial cells, selective inhibition of endothelial cell migration, increase in the expression level of the endogenous angiogenesis inhibitor thrombospondin-1, and sustained decrease in levels and viability of bone marrow-derived endothelial progenitor cells.[10] One more proposed mechanism is the induction of senescence in tumors achieved by repetitive, low-dose regimens of cytostatic drugs including oral 6-mercaptopurine.[11]
AML in elderly patients has been recognized to be a disease of very poor prognosis and lower tolerance to conventional treatment options. Thus, there have been some recent reports of the trial of MCT in treatment of elderly AML.[12,13] In most of these reports, there has been the use of low-dose cytarabine which requires subcutaneous administration 2 times a day and given 4 days in a week.[14] However, the basic principle of MCT is to prefer oral drug which can be given continuously without any interruption. Keeping this in mind, oral 6-mercatopurine was given in our trial continuously without any scheduled interruption.
In our study, 59.4% of patients had ECOG PS of 2 while rest had the status of 3. As per the guidelines of the National Comprehensive Cancer Network, low-intensity therapy, or palliation only is recommended for older AML patients with ECOG > 2.[8] Thus, the patient profile was suitable for noncurative intent treatment. The median age of the patients was 69 years with the range being 61–86 years. In Surveillance, Epidemiology, and End Results (SEER) analysis of 5480 older AML patients by Oran and Weisdorf,[15] the median age was 78 years, range 65–93 years and the median OS was 2 months in the untreated group versus 6 months in the treated group (P < 0.01). This data matches with the results of our study with median OS being 6 months with oral MCT. Thus, oral 6-mercaptopurine achieved similar results as with other standard treatment in this patient profile.
In our study, the patients of age group 61–70 years had highest median OS of 8 months as compared to 71–80 and 81–90 years having median OS of 3 and 2 months, respectively (P < 0.001). The HR for age group >80 years versus 61–70 years was estimated to be 3.190 in our study. This data are also in agreement with SEER analysis in which significant prolongation in median OS by 6 months in those aged 65–69 years (10 vs. 4 months, P < 0.01) with HR of 3.1 for patients of >80 years age as compared to reference age of 65–69 years.[15] Thus, the data suggest that patients of relatively younger age have a better prognosis.
In our study, the median OS for ECOG PS of 2 was 8 months as compared to 5 months for PS of 3. This could be explained regarding higher comorbidities in patients with poor ECOG PS. Another striking finding in our study was significantly poor prognosis of elderly female patients (median OS: 3 months) as compared to males (median OS: 7 months, P = 0.008). Although the number of female patients in our study was small (nine patients), it could indicate unfavorable cytogenetics in females. However, our study was underpowered to exactly comment on the cause of survival difference on the basis of gender. The survival analysis of our study suggests that patients with higher baseline TLC and lower baseline platelet count had significantly worse survival. Such features indicate more severe involvement of bone marrow by the leukemic cells leading to poorer prognosis. There was no survival difference on the basis of baseline hemoglobin or FAB class.
There are some important limitations of this study. The study enrolled a small number of patients and detailed cytogenetics with all prognostic markers could not be performed due to financial constraints. Although the study design was prospective, randomization could not be performed in the absence of a standard arm in the study. Despite these limitations, this study indicates toward the benefit of cheap oral metronomic treatment in elderly AML patients in a limited resource setting.
Conclusions
MCT with oral 6-mercaptopurine is an attractive treatment option in elderly AML patients who are not amenable to curative therapy with minimal toxicities. Randomized multicenter study involving a larger number of patients is required to quantify the exact benefits of this regimen.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ghosh S, Shinde SC, Kumaran GS, Sapre RS, Dhond SR, Badrinath Y, et al. Haematologic and immunophenotypic profile of acute myeloid leukemia: An experience of Tata Memorial Hospital. Indian J Cancer. 2003;40:71–6. [PubMed] [Google Scholar]
- 2.Weinstein HJ. In: Acute myeloid leukemia. Childhood Leukemias. Pui CH, editor. UK: Cambridge University Press; 1999. pp. 322–35. [Google Scholar]
- 3.Bhatia S, Neglia JP. Epidemiology of childhood acute myelogenous leukemia. J Pediatr Hematol Oncol. 1995;17:94–100. doi: 10.1097/00043426-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Klepin HD, Balducci L. Acute myelogenous leukemia in older adults. Oncologist. 2009;14:222–32. doi: 10.1634/theoncologist.2008-0224. [DOI] [PubMed] [Google Scholar]
- 5.Heinemann V, Jehn U. Acute myeloid leukemia in the elderly: Biological features and search for adequate treatment. Ann Hematol. 1991;63:179–88. doi: 10.1007/BF01703440. [DOI] [PubMed] [Google Scholar]
- 6.Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM, et al. Acute myeloid leukemia in the elderly: Assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89:3323–9. [PubMed] [Google Scholar]
- 7.Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: New rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–65. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell MR, Appelbaum FR, Baer MR, Byrd JC, Coutre SE, Damon LE, et al. Acute myeloid leukemia clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4:16–36. doi: 10.6004/jnccn.2006.0004. [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Scharovsky OG, Mainetti LE, Rozados VR. Metronomic chemotherapy: Changing the paradigm that more is better. Curr Oncol. 2009;16:7–15. doi: 10.3747/co.v16i2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewald JA, Desotelle JA, Wilding G, Jarrard DF. Therapy-induced senescence in cancer. J Natl Cancer Inst. 2010;102:1536–46. doi: 10.1093/jnci/djq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 13.Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–24. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 14.Bashir Y, Geelani S, Bashir N, Mir SA, Mushtaq M, Jan MA, et al. Role of low dose cytarabine in elderly patients with acute myeloid leukemia: An experience. South Asian J Cancer. 2015;4:4–6. doi: 10.4103/2278-330X.149918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica. 2012;97:1916–24. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]


