Abstract
The potential long-term consequences of treatments delaying manifestations of neurodegenerative diseases have not been explored. Using Huntington disease (HD) data and Markov Chain Monte Carlo methods, we simulated the effects of therapies with equivalent effects on time to onset of HD and survival with HD. Our results suggest substantial potential trade-offs in effects of these therapies; significant delays in time to onset of HD were accompanied by significant prolongations of survival after onset of HD. Under a variety of assumptions, treatments delaying onset of HD result in some patients likely to have a greater increase in survival with manifest HD compared to delays in time to onset of HD. Our results suggest that future work in HD should be sensitive to the potential existence of such trade offs and that understanding the preferences of HD patients and the broader HD community will be increasingly important. Future research, trial design, and treatment strategies in HD and other mid-life onset neurodegenerative disorders should consider the possibility of trade-offs in long-term consequences of disease-modifying treatments.
Keywords: Huntington’s Disease, Monte Carlo Simulation, Outcomes Analysis, Simulation, Pre-symptomatic Treatment
Introduction
Disease modifying treatments for mid- to late-life onset neurodegenerative disorders may become a reality in the near future. There has been little effort to study the potential long-term consequences of such treatments, particularly therapies that delay the onset of conversion to manifest disease. Huntington disease (HD) is a mid-life onset dominant polyglutamine disorder with virtually complete penetrance, easily identified mutant allele carriers, good ability to predict age of conversion to manifest disease state, and reasonably well defined natural history.1,2 HD is an excellent target for treatments delaying onset of manifest disease and the subject of vigorous pre-clinical and clinical therapeutic research. While considerable attention has been paid to characterizing the pre-manifest period and the clinical features of manifest disease progression, relatively little attention has been paid to potential consequences of effective HD treatments. Preclinical research suggests that disease-modifying therapies based on suppression of mutant huntingtin gene expression are a viable treatment approach.3,4,5 Some of these therapies are now entering initial clinical trials.
Efficacious disease-modifying therapies for HD, however, may entail trade-offs. An effective disease-modifying treatment might plausibly both delay both onset of manifest HD and slow progression of HD, potentially leading to increased survival of HD patients. While delaying HD onset is undoubtedly desirable, increased survival with HD is not necessarily a desirable outcome for patients and their families. The potential existence of trade-offs between clearly desirable and potentially undesirable treatment effects suggests that patient-centered decision-making is important in pre-symptomatic HD treatment.6 Optimal decision procedures require understanding of patient and family values, and how treatments affect outcomes of interest to patients and their families. If such trade-offs exist, presymptomatic HD treatment decisions will necessitate a deliberative shared-decision making approach more similar to decisions to undergo genetic testing for HD than the decision to take an aspirin after a heart attack. With significant treatment experiments now underway, this is an appropriate time to address these issues, particularly as some promising treatments may produce lasting effects.5 Outcomes of interest to potential patients need to be enumerated now and trials designed to capture these outcomes.
To explore potential consequences of effective disease-modifying treatments of HD, we employed simulation methods and the best available data to estimate the effects of applying a hypothetically effective HD disease modifying treatment that affects time to onset of manifest disease and survival duration after disease onset in a realistic pre-manifest trial population. Formal modeling has the virtue of requiring explicit specification of crucial assumptions, facilitating identification of important questions related to treatment development.
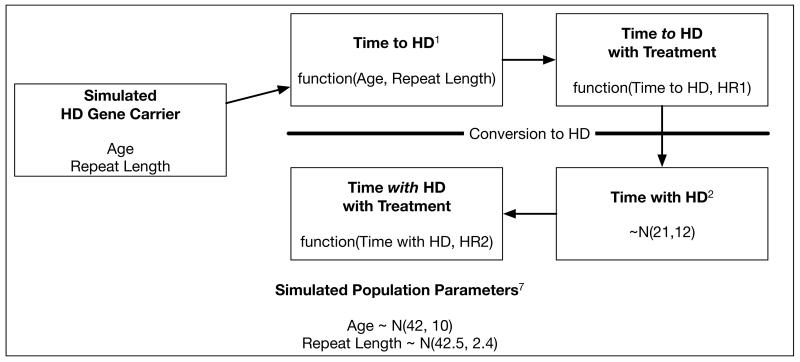
Simulation Model
We used Markov Chain Monte Carlo methods to build a simple simulation model to explore the impact of therapies with specified effect sizes on time to onset of HD and survival with HD (Figure). To this end, we first developed a simulated population of 10,000 pre-manifest HD patients with normal age (mean = 42, SD=10) and repeat length (mean 42.5, SD=2.4). These distributions are based on data from the Predict-HD study and assume that trial populations would be relatively similar to this population.7 Time to manifest HD was estimated for each individual using the Langbehn et al. model for predicting HD onset based on age at enrollment and repeat length.1 Time with HD was estimated by randomly drawing from a normal distribution (mean =21, SD=12) based on data from the National Research Roster for HD Patients and Families.2 Because these data show that patients with juvenile onset HD have less time with HD than patients with adult onset HD, we dropped individuals from the simulated population with age less than 20.
Figure.
Markov Chain Monte Carlo Model of HD Progressions.
HR1 — Treatment effect/hazard ratio of hypothetical treatment on time to HD.
HR2 — Treatment effect/hazard ratio of hypothetical treatment on time with HD.
The initial model instantiated the following assumptions:
-
1)
An effective treatment reduced both time to HD (HR1) and time with (HR2) HD with equal relative effects.
-
2)
The effective treatment does not change the clinical features of manifest HD, that is, HD has the same behavioral, cognitive, and motor deficits found presently in HD patients.
-
3)
The model does not include competing causes of mortality; model subjects die only from HD.
-
4)
The model population has the features of the PREDICT-HD study population.
To account for uncertainty in both time to HD and time with HD, we repeatedly sampled from the joint parameter space using Markov Chain Monte Carlo (MCMC) sampling implemented through JAGS and R (R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/).8 Sampling was repeated with the assumption that patients received an effective treatment that reduced both time to HD (HR1) and time with HD (HR2) with a hazard ratio (HR) of 0.8. Descriptive statistics were used to summarize the simulation results. To explore outcomes assuming therapies were more effective at delaying onset of HD than extending life with HD, sensitivity analysis was performed subsequently by repeating the primary analysis while holding the treatment effect for time to HD constant (HR1) and decreasing the treatment effect for time with HD (HR2).
Simulation Results
Before considering treatment effects, from our model we estimate that 69% of plausible candidates for a pre-manifest HD trial are likely to live a longer fraction of their remaining lives with manifest HD. Simulation results are stratified into quintiles on the basis of estimated time to onset of HD. For individuals in the first quintile of time to HD (mean age 52, mean repeat length 44), we estimate an expected ~2.7 years of time to onset of HD compared to ~21 years of time with HD. Individuals in the fifth quintile of time to HD are estimated to have almost 33 years of time to onset of HD compared to 21 years of time with HD (Table 1). Assuming that subjects receive a treatment with similar relative effects on time to onset of HD and time with HD (HR 0.8 for both), the overall mean gain in time to onset of HD is 2.7 (SD 2.9) years and the overall mean gain in time with HD is 5.3 (SD 3.0) years. Individuals in the first quintile of time to onset of HD are modeled to gain about 8 months in time to onset of HD vs. 5 years in time with HD. Individuals in the fifth quintile of time to HD would gain 8 years in time to onset of HD vs. 5 years in time with HD.
Table 1. Primary Simulation Results.
Baseline characteristics of the simulated population and time to and with HD as well as increase in time to and with HD with a treatment equally effective at increasing both (Hazard Ratio 0.8).
| Quintiles of Time to HD | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Age mean(SD) | 52.3 (7.0) | 45.6 (6.8) | 41.4 (6.8) | 38.0 (7.8) | 34.9 (7.9) |
| Repeat Length mean(SD) | 44.8 (1.8) | 43.5 (1.7) | 42.7 (1.6) | 41.8 (1.6) | 39.8 (1.7) |
| Time To HD mean(SD) | 2.7 (0.4) | 5.4 (1.2) | 10.7 (1.9) | 18.0 (2.5) | 32.6 (9.1) |
| Time with HD mean(SD) | 20.8 (11.7) | 21.0 (11.8) | 21.2 (12.4) | 20.9 (11.6) | 21.1 (12.0) |
|
% of Survival with HD mean(SD) |
85.7% | 66.2% | 59.8% | 46.2% | 36.1% |
|
Increase in Time to HD on Treatment mean(SD) |
0.7 (0.1) | 1.4 (0.3) | 2.7 (0.5) | 4.5 (0.6) | 8.1 (2.3) |
|
Increase in Time with HD on treatment mean(SD) |
5.2 (2.9) | 5.3 (2.9) | 5.3 (3.1) | 5.2 (2.9) | 5.3 (3.0) |
With sensitivity analysis, as the treatment effect on survival with HD was reduced with treatment effect of time to onset of HD unchanged, there was an expected decrease in survival with HD. Even for a treatment with a relatively disparate effect on time to onset of (HR 0.80) compared to time with HD (HR 0.95), however, approximately 40% of patients treated in this model population would experience greater increases in survival with HD than delays in HD onset (Table 2).
Table 2. Sensitivity Analysis.
Assuming that a treatment has a constant effect on the time to HD (HR 0.8, top row), the increase in time with HD is shown with decreasing effect sizes on survival with HD as the HR increases from 0.80 to 0.95.
| Quintiles of Time to HD | ||||||
|---|---|---|---|---|---|---|
| Hazard Ratio for Survival with HD |
1 | 2 | 3 | 4 | 5 | |
|
Increase in Time to HD on Treatment mean(SD) |
0.7 (0.1) |
1.4 (0.3) |
2.7 (0.5) |
4.5 (0.6) |
8.1 (2.3) |
|
|
Increase in Time with HD on treatment mean(SD) |
0.80 | 5.2 (2.9) |
5.3 (2.9) |
5.3 (3.1) |
5.2 (2.9) |
5.3 (3.0) |
|
Increase in Time with HD on treatment mean(SD) |
0.85 | 3.7 (2.1) |
3.7 (2.1) |
3.7 (2.2) |
3.7 (2.1) |
3.7 (2.1) |
|
Increase in Time with HD on treatment mean(SD) |
0.90 | 2.3 (1.3) |
2.3 (1.3) |
2.4 (1.4) |
2.3 (1.3) |
2.3 (1.3) |
|
Increase in Time with HD on treatment mean(SD) |
0.95 | 1.1 (0.6) |
1.1 (0.6) |
1.1 (0.7) |
1.1 (0.6) |
1.1 (0.6) |
Discussion
Our results suggest significant trade-offs accompanying effective disease-modifying treatments for HD. In our simulations, delaying onset of HD was accompanied often by larger extensions of time with HD in a substantial fraction of this simulated patient population. These results suggest that unless effective disease-modifying therapies have no effect on extending HD survival, effective treatment may be accompanied by potentially undesirable consequences of extending time with HD in a sizable proportion of treated patients and also of increasing HD prevalence.
In terms of designing disease-modifying trials for HD, our simulation results suggest that attention should be paid to assessing treatment effects on both time to onset of HD and progression of HD. Accumulating data in both pre-manifest and manifest HD subjects will be necessary to fully gauge the consequences of HD treatments. Trials aiming to modify onset of HD should incorporate long-term follow-up in at least of subset of subjects. Serial randomization using SMART clinical trials designs (e.g., rerandomizing at onset of HD) might be considered to measure the effects of therapies on time to HD and time with HD.9
Given the possibility that effective disease-modifying treatments will prolong survival of HD, our results suggest that increased attention should be devoted to developing effective treatments for disabling clinical features of HD such as its psychiatric and cognitive impairments. While it may be the case that effective presymptomatic therapies would reduce disease severity with manifest HD, particularly given the prevalence of problematic psychicatric symptoms in some patients with mild HD., Consequently, potential treatments that delay disease onset and ameliorate important clinical features of manifest HD would be particularly desirable and attention should be paid to beneficial symptomatic effects of potentially disease-modifying interventions in trials.
Our modeling approach is admittedly simple and has several limitations. It is plausible, however, that our primary conclusion — trade offs between delays to HD onset and survival with HD — would obtain under a variety of assumptions. The most limiting assumptions of our approach are that successful disease modifying therapies will 1. affect time to onset of HD and survival with HD equally. The primary pathogenic process(es) in HD may trigger self-sustaining secondary pathologic cascades that drive progression of manifest disease. CAG repeat number strongly influences age of onset of HD but its relationship to manifest disease progression is controversial with some analyses describing only a weak relationship (for concise review, see Ravina et al.).10 These results are consistent with dissociation of processes driving onset of HD and progression of HD. Analysis of CARE-HD trial data, however, indicates that CAG repeat number, a major determinant of age of onset of manifest HD, is also a significant driver of disease progression.10 This result is consistent with underlying uniformity of processes determining age of HD onset and speed of HD progression. Our sensitivity analyses explored decoupling of processes driving onset of and progression of HD. In simulations modeling treatments with significantly larger effects in delaying onset of HD than slowing progression of HD, a substantial number of simulated patients experienced greater prolongation of survival with HD than delay in onset of HD.
Another potential limitation of our model is that we do not correct for competing causes of mortality. In this relatively young projected population, competing mortality effects should be negligible, but with substantial delays in age of onset of manifest HD, competing causes of mortality may remove patients from the population prior to HD onset. This effect would constitute an virtual cure and was seen in simulated subjects with the largest effects of treatment in delaying onset of HD (Table 1- quintile 5); those who were farthest from onset of manifest HD at time of treatment initiation. These results may inform use of an effective treatment. An intuitively attractive corollary is that earlier treatment is better, suggesting that treatment should be initiated in early adult life. This potential treatment strategy demands effective therapies with excellent tolerability for decades, and as it would be applied in normal individuals, will have to possess low toxicities. A more radical potential implication is that a rational treatment strategy would be to treat until onset of manifest disease, then cease treatment. It is possible as well that interventions delaying onset and prolonging survival of manifest HD could “buy time” for affected individuals while effective symptomatic therapies are developed.
Most important, our simulation results indicate the presence of significant trade-offs in the development and application of disease-modifying treatments for HD. The potential existence of undesirable effects on HD survival and prevalence suggest that more effort should be devoted to defining the criteria for successful treatment of HD. While delaying onset to manifest HD is certainly an unalloyed good, pre-symptomatic treatments may have less obviously positive consequences such as increased survival with HD, institutionalization, resource use, and prevalence. While discussions of this kind are informed by scientific assessments, this is not a scientific problem. Discussions about the criteria for successful treatments for HD confront potentially difficult questions of values and quality of life. These discussions must focus on patients with manifest HD, pre-manifest HD mutant allele carriers, and the unaffected family members who bear the burden of caring for HD patients. It will be crucial to understand the preferences of these individuals with respect to the possible trade-offs that arise from pre-symptomatic HD treatment. The HD research community should take the lead in initiating these essential discussions and developing the patient-centered shared-decision making strategies necessary to optimize decisions in the context of trade offs. This is an opportunity for the HD community to establish standards to guide the adoption of treatments rather than passively accept the consequences of novel technologies. As similar issues will likely arise for presymptomatic disease-modifying therapies for other neurodegenerative disorders, this opportunity has to the potential to establish procedures of broad utility to the larger biomedical research community.
Acknowledgments
Financial Disclosures
Dr. Albin is employed by the University of Michigan and funded by NIH grants P50 NS091856, R56 NS082941, R21 NS088302, and R25 NS089450. He serves on the DSMBs for the PRIDE-HD and LEGATO studies. Dr. Burke is employed by the University of Michigan, is funded by NIH grants K08 NS082597 and R01 MD008879 and has received honoraria from the American Academy of Neurology for the development of educational materials.
References
- 1.Langbehn DR, Brinkman RR, Falush D, et al. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clinical Genetics. 2004;65(4):267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 2.Foroud T, Gray J, Ivashina J, Conneally PM. Differences in duration of Huntington’s disease based on age at onset. J Neurol Neurosurg Psychiatr. 1999;66(1):52–56. doi: 10.1136/jnnp.66.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 2000;101(1):57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- 4.Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, Pytel KA, Artates JW, Weiss A, Cheng SH, Shihabuddin LS, Hung G, Bennett CF, Cleveland DW. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron. 2012;74(6):1031–44. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBride JL, Pitzer MR, Boudreau RL, Dufour B, Hobbs T, Ojeda SR, Davidson BL. Preclinical safety of RNAi-mediated HTT suppression in the rhesus macaque as a potential therapy for Huntington’s disease. Mol Ther. 2011;19(12):2152–62. doi: 10.1038/mt.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. New Eng J Med. 2012;366(9):780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 7.Paulsen JS, Hayden M, Stout JC, et al. Preparing for preventive clinical trials: the Predict-HD study. Arch Neurol. 2006;63(6):883–890. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- 8.Plummer M. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. 2003.
- 9.Almirall D, Compton SN, Gunlicks-Stoessel M, et al. Designing a pilot sequential multiple assignment randomized trial for developing an adaptive treatment strategy. Statist. Med. 2012;31(17):1887–1902. doi: 10.1002/sim.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravina B, Romer M, Constantinescu R, et al. The relationship between CAG repeat length and clinical progression in Huntington’s disease. Mov Disord. 2008;23(9):1223–1227. doi: 10.1002/mds.21988. [DOI] [PubMed] [Google Scholar]