Abstract
The insulin/insulin-like growth factor signaling pathway is evolutionarily conserved in animals, and is part of nutrient-sensing mechanisms that control growth, metabolism, reproduction, stress responses, and lifespan. In Drosophila, eight insulin-like peptides (DILP1-8) are known, six of which have been investigated in some detail, whereas expression and functions of DILP1 and DILP4 remain enigmatic. Here we demonstrate that dilp1/DILP1 is transiently expressed in brain insulin producing cells (IPCs) from early pupa until a few days of adult life. However, in adult female flies where diapause is triggered by low temperature and short days, within a time window 0–10h post-eclosion, the dilp1/DILP1 expression remains high for at least 9 weeks. The dilp1 mRNA level is increased in dilp2, 3, 5 and dilp6 mutant flies, indicating feedback regulation. Furthermore, the DILP1 expression in IPCs is regulated by short neuropeptide F, juvenile hormone and presence of larval adipocytes. Male dilp1 mutant flies display increased lifespan and reduced starvation resistance, whereas in female dilp1 mutants oviposition is reduced. Thus, DILP1 is expressed in non-feeding stages and in diapausing flies, is under feedback regulation and appears to play sex-specific functional roles.
Insulin and insulin-like growth factor (IGF) signaling (IIS) is part of a nutrient –sensing pathway that regulates multiple aspects of growth, metabolic homeostasis, stress responses, fecundity and lifespan in Drosophila and other organisms1,2,3,4,5,6. The IIS pathway is largely conserved over evolution1,7,8,9, and therefore Drosophila is an excellent genetically tractable model to investigate IIS mechanisms. In Drosophila eight insulin-like peptides (DILP1-8) have been identified1,4,10,11. Of these, six have been studied in some detail and their spatiotemporal expression patterns and several functions are known. In larvae and adults DILP2, 3 and 5 are produced by a set of 14 median neurosecretory cells in the brain, designated insulin-producing cells, IPCs1,12,13. These DILPs are thought to be released into the open circulation from axon terminations in the corpora cardiaca and corpora allata, as well as on the surface of the anterior intestine and aorta12,13,14,15, and have been found sufficient for regulation of stress resistance, fecundity, metabolic homeostasis and lifespan1,2,13,16,17,18. DILP6, an IGF-like peptide, is produced mainly in the fat body and regulates growth during non-feeding stages19,20. DILP7 is found in a set of about 20 neurons in the abdominal neuromeres of the ventral nerve cord of larvae and adults and plays roles in regulation of gut functions, tracheal growth and reproductive behavior21,22,23,24. Finally, DILP8 is released from the imaginal discs of larvae and can delay metamorphosis by inhibiting ecdysone biosynthesis, and thereby coordinates tissue growth with developmental timing10,11. In adult flies DILP8 and its G-protein-coupled receptor Lgr3 play a role in reproduction25. Much less is known about DILP1 and DILP4. According to modENCODE tissue expression transcriptome data26 and Slaidina et al.19 dilp1 appears to be expressed mainly in midpupal stages and dilp4 in the embryo. No functions have been revealed for these two DILPs and their cellular location is obscure, although in situ hybridization suggested dilp1 expression in the larval IPCs13,27. To fill this gap in knowledge we set out to analyze cellular expression and possible functions of DILP1.
Employing novel dilp1-Gal4 lines and antisera to DILP1 we found that the expression is in the brain IPCs in the pupa and newly eclosed adult fly. However, in newly-eclosed virgin female flies that had been subjected to low temperature and short photoperiod, and thus entered reproductive diapause28, we found that the dilp1/DILP1 expression remained high for at least 9 weeks of adult life. This expression declined after one week of recovery from diapause, and DILP1 expression could not be affected by exposure to diapause conditions or low temperature later in life. The DILP1 expression correlated to some extent with the persistence of larval/pupal fat body, and it can be regulated by other DILPs, the neuropeptide short neuropeptide F (sNPF), and inhibited by juvenile hormone. We found a male-specific effect of loss of dilp1 in normal lifespan and starvation resistance and in females oviposition was reduced in dilp1 mutants. Thus, the hitherto enigmatic DILP1 seems to play roles distinct from other DILPs and these appear to be partly sex-specific.
Results
Cellular expression pattern of dilp1 and DILP1
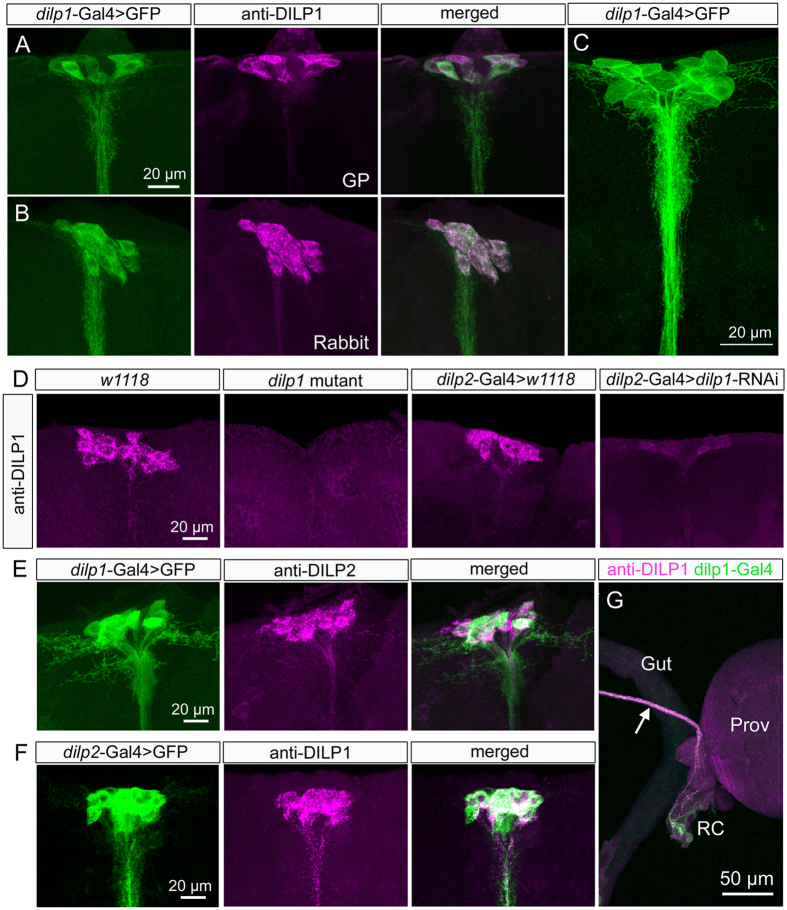
The Dilp1 precursor gene is unique among the eight Dilp genes with its lack of introns1,4,29. Otherwise the amino acid sequence of the precursor displays similarities to those of DILP2, 3 and 5 of the IPCs, and compared to DILP1 precursors of other Drosophila species display well-conserved regions corresponding to predicted A- and B-chains and a variable putative C-peptide sequence4 (Fig. S1). To obtain DILP1-specific antisera we, thus, selected a sequence of the Drosophila melanogaster C-peptide (Fig. S1) coupled to thyroglobulin for immunization. We also generated several dilp1-Gal4 drivers (see Methods), two of which are used here. As shown in Fig. 1A–C, the labeling with DILP1 antisera and dilp1-Gal4-driven GFP can be seen in the same set of neurosecretory cells in the pars intercerebralis of the brain of newly-eclosed flies. As specificity tests for the antisera we demonstrated that there is no immunolabeling in dilp1 mutant flies (Fig. 1D) and reduced immunolabeling after dilp1-RNAi using dilp2- and dilp5-Gal4 drivers (Figs 1D and S2A,B). We also observed that pre-immune sera and DILP1 antisera preabsorbed with antigen produced no immunolabeling in the brain. By applying antiserum to DILP1 on brains expressing dilp2-driven GFP (Fig. 1E,F), or applying anti-DILP2 to dilp1-driven GFP we could determine that DILP1/dilp1 is expressed in the bona fide IPCs, known to express DILP2, 3 and 51,13. The DILP1/dilp1 expression in IPCs is similar in in newly-eclosed males and females (see Fig. S2D,E).
Figure 1. DILP1 is expressed in IPCs in pars intercerebralis of the dorsal brain of newly-eclosed flies.
(A,B) Antisera to DILP1 produced in guinea pig (GP; A) and rabbit (B) label neurons identified by dilp1-Gal4-driven GFP. Although labeling intensity appears somewhat variable when the two markers are used simultaneously, it is clear that all IPCs coexpress the markers. (C) Higher resolution image of the 14 IPCs expressing dilp1-GFP. (D) DILP1 immunolabeling is not detected in the brain of dilp1-mutant flies. Also after dilp2-Gal4-driven dilp1-RNAi the DILP1 immunolabeling is strongly reduced. (E) To confirm that DILP1/dilp1 is co-expressed in DILP2 producing IPCs we applied anti-DILP2 to brains with dilp1-Gal4-driven GFP and reveal colocalization of markers. (F) Also the reverse experiment with dilp2-Gal4 >GFP and anti-DILP1 showed coexpression. (G) Details of axon terminations of neurons coexpressing DILP1-immunolabeling and dilp1-Gal4-GFP expression in the foregut structures proventriculus (Prov) and retrocerebral complex (RC; corpora cardiaca and corpora allata).
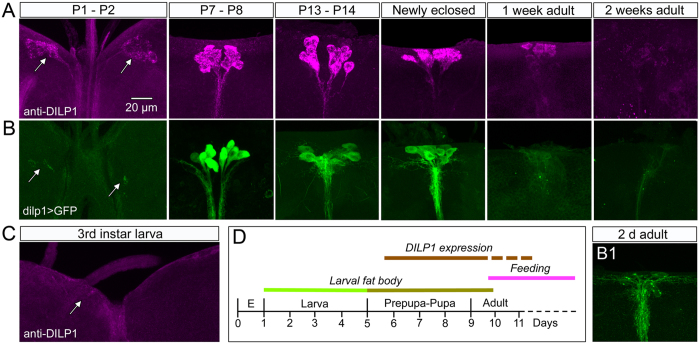
Next we screened tissues from different developmental stages and tissues (intestine, renal tubules, fat body and gonads) of Drosophila (Canton S) from first instar larvae to aged adult flies and found that only the brain IPCs express DILP1 immunolabeling or dilp1-GFP and that this expression is restricted to certain stages. In wild type flies kept under normal conditions, the DILP1 antiserum labels only the 14 IPCs with an onset in the early to mid-pupa (Fig. 2A). The DILP1 immunoreactivity started to decline after a few days of adult life and could not be detected in any neurons of 2-week-old flies (Fig. 2A). Also the dilp1-Gal4-driven GFP is detectable in IPCs from early to mid-pupae and fades to below detection after about one week of adult life (Fig. 2B). Thus, there is a close match between Dilp1-GFP expression and DILP1 immunolabeling both spatially and temporarily.
Figure 2. Temporal expression profile of DILP1 immunolabeling and dilp1-Gal4 expression in the brain.
(A) In Canton S flies DILP1 immunolabeling appears first in early pupal stages (P1-P2), remains strong in newly eclosed flies and starts fading in 1-week-old flies. (B) Dilp1-Gal4-driven GFP follows the same expression pattern until flies are newly eclosed. B1 At about 2–3 days of adult life GFP intensity starts fading. (C) In the larval brain no DILP1 or dilp1 expression could be detected. (D) Time course of DILP1 expression in relation to Drosophila development, presence of larval fat body and onset of feeding. We did not investigate DILP1 expression during embryonic (E) development.
DILP1 seems primarily produced in the IPCs and thus might be coreleased with DILP2, 3 and 5 in the anterior aorta and the retrocerebral complex located in the junction between the intestine and the crop duct (see Fig. 1G). Weaker DILP1 immunolabeling was also detected in branches of the IPCs within the brain dorsally in pars intercerebralis and more ventrally in the tritocerebrum.
The developmental expression of dilp1/DILP1 in the IPCs differs from that of DILP2, 3 and 5 immunolabeling, while the latter three can be detected in larvae, throughout pupal development and in both young and old adult flies (Figs S2C and S3). This time course of expression is also seen for transcripts of dilp2, 3 and 519. It can be noted that DILP3 and 5 are expressed also outside the brain IPCs in adult flies: DILP5 in ovaries and renal tubules, and DILP3 in midgut muscle cells16,30,31. However, the expression of the novel dilp5-Gal4 driver used here is restricted to the IPCs in larvae and adults (Fig. S2), and display a weak expression in muscle fibers of the anterior midgut of adults (not shown).
Our findings suggest that dilp1/DILP1 expression is regulated by factors different from those regulating the other peptides of the IPCs and, hence, that DILP1 peptide may serve unique functions. We therefore undertook an investigation of possible factors regulating DILP1 and functional roles of this peptide.
Dilp1/DILP1 expression is maintained high during adult diapause
Newly-eclosed virgin females of D. melanogaster can be experimentally triggered to enter reproductive diapause by low temperature provided that the day length is less than 16 h (16L:8D)32. Thus, diapause is usually experimentally induced at 11 °C with a slightly shorter photoperiod (10L:14D) and before 10 h post-eclosion28,33. This dormancy halts ovary development, decreases feeding, alters metabolism, increases stress resistance and drastically extends lifespan28. Here we monitored dilp1 and DILP1 expression in female flies (Canton S strain) kept in diapause conditions and sampled over a range of time points (up to 13 weeks), as well as after one week of recovery from three weeks of diapause. The recovery is obtained by transferring diapausing flies to 25 °C and 12L:12D28. We monitored relative dilp1 mRNA expression levels by using quantitative real time PCR (qPCR), and determined DILP1 expression by immunolabeling, as well as GFP fluorescence in dilp1-Gal4 >UAS-GFP flies.
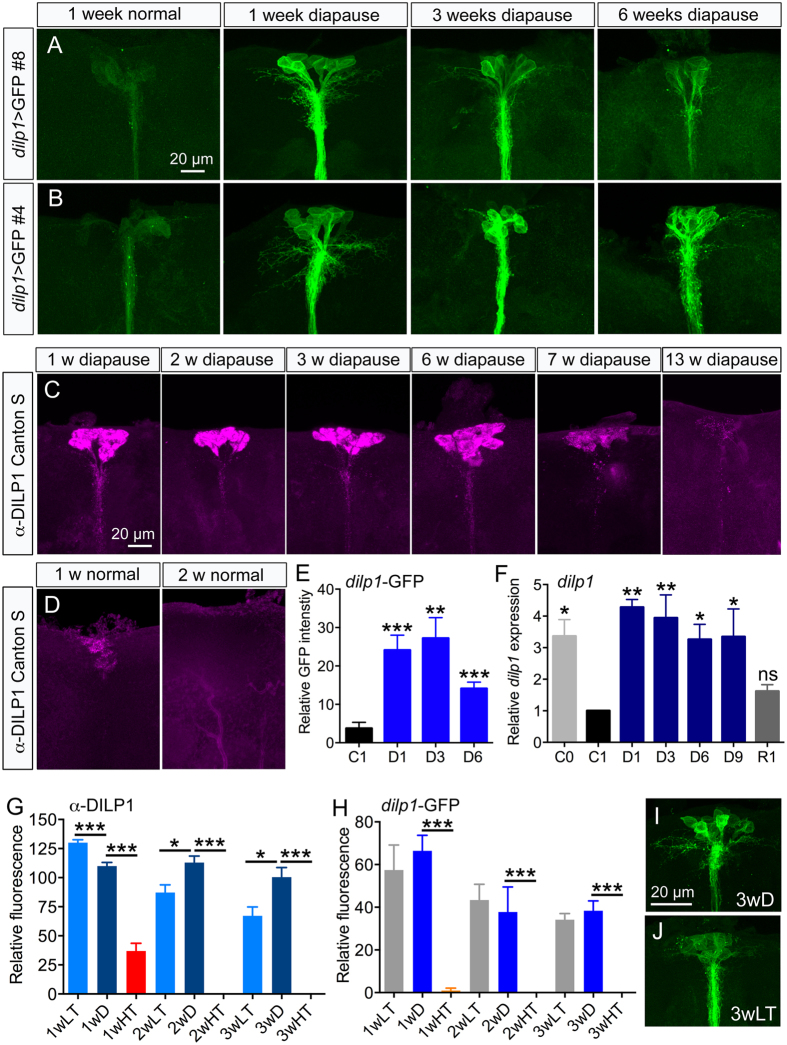
We used two different dilp1-Gal4 lines to drive UAS-mCD8-GFP and recorded GFP fluorescence levels in IPCs after diapause induction. Both fly lines displayed strong fluorescence after 1 and 3 weeks of diapause (Fig. 3A,B), and a slightly reduced, but still high level of fluorescence after 6 weeks of diapause (Fig. 3A,B). The dilp1-driven GFP in cell bodies of the IPCs is quantified in Fig. 3E. The Gal4 line #4 displayed the strongest fluorescence and is used in all subsequent experiments. Next we showed that the intensity of DILP1 immunofluorescence decreases in flies after 1 week in non-diapause conditions compared to that of newly eclosed flies (Fig. 3D). However, consistent with dilp1-Gal4 driven GFP fluorescence (Fig. 3A,B,E), flies subjected to diapause conditions maintained a strong intensity of DILP1 immunofluorescence over 6 weeks of diapause, and displayed a slight decrease only after 7 weeks and very weak, but detectable, expression after 13 weeks of diapause (Fig. 3C). These experiments show that dilp1/DILP1 expression is maintained in IPCs for at least 9 weeks of diapause conditions, whereas in control flies it is lost after about one week of adult life.
Figure 3. DILP1/dilp1 remains expressed during adult reproductive diapause.
(A,B) Two dilp1-Gal4 lines #8 (A) and #4 (B) were used for analysis of GFP expression during diapause. In contrast to 1w old flies kept at 25 °C and 12L:12D, GFP expression remains high over six weeks of diapause. (C) In Canton S flies DILP1 immunoreactivity could be clearly detected until 7 weeks of diapause. (D) Under normal conditions no DILP1 immunolabeling was detected after 2 weeks. (E) Relative dilp1-GFP fluorescence in cell bodies of IPCs in 1 week controls (C1) and flies diapausing for 1, 3 and 6 weeks (D1–D6). Data are presented as means ± S.E.M, n = 7–15 flies for each time point from three crosses (**p < 0.01, ***p < 0.001, compared to C1, unpaired Students’ t-test). (F) Relative Dilp1 transcript levels monitored by qPCR display a similar temporal profile. Newly-eclosed flies (C0) display high expression of dilp1, a decrease is seen in one-week-old flies kept in normal conditions (C1), whereas it remained high over 9 weeks of diapause (D1–D9). Flies that had recovered for 1 week (R1) after 3 weeks of diapause displayed control dilp1 levels (C1). Significance values are shown for comparisons with C1. Data are presented as means ± S.E.M; n = 3 replicates with 10–15 flies in each (*p < 0.05, **p < 0.01, ns – not significant, one way ANOVA followed by Dunnett’s multiple comparisons test). (G) DILP1 immunofluorescence levels in Canton S flies exposed to 11 °C and 12L:12D (LT) compared to 11 °C and 10L:14D (diapause conditions; D) and 25 °C and 10L:14D (HT) over 3 weeks. Both LT and D treatment induces high DILP1 levels, but not HT. Data are presented as means ± S.E.M, n = 7–12 flies from three replicates (**p < 0.01, ***p < 0.001, compared to D, unpaired Students’ t-test). (H) Using the same conditions and monitoring dilp1-GFP levels yield similar results. Data are presented as means ± S.E.M, n = 6–7 flies from three crosses (**p < 0.01, ***p < 0.001, compared to D, unpaired Students’ t-test). (I,J) Representative images of dilp1-GFP expression in flies kept three weeks in diapause conditions (3wD) and low temperature only (3wLT).
We employed qPCR to quantify the dilp1 transcript levels in diapausing flies at different time points (Fig. 3F). After one week of diapause (D1 in Fig. 3F) flies displayed a 4-fold increase of dilp1 transcript level compared to one-week-old flies kept in control conditions. Note that recently hatched flies (C0 in Fig. 3F) also express higher levels of dilp1, as was shown earlier for dilp2, 3 and 528. The dilp1 levels remained high over the 9 weeks of diapause used for measurements. Flies that have been kept for 3 weeks in diapause and then placed in non-diapause conditions for 1-week were shown earlier to recover from diapause as determined by ovarian maturation and several other assays28. We monitored dilp1 transcript in flies that had recovered for one week after three weeks of diapause (R1 in Fig. 3F) and found that the level was back to that seen in one-week-old control flies (C1 in Fig. 3). Previous studies showed that also dilp2, 3, 5 and 6 were upregulated over 3 or 9 weeks of diapause28,34 and decreased to initial levels after one week or non-diapause conditions28.
Next we tested exposure of virgin flies to 11 °C with a 12L:12D light regime and 25 °C with 10L:14D and found that 11 °C with the slightly longer day length also induces diapause, as monitored by egg development (see Fig. 4), and leads to prolonged high expression of DILP1 (Fig. 3G,H). However, the short photoperiod had no effect on diapause or DILP1 expression when flies were kept at 25 °C (Fig. 3G–J; see also Figs S4 and S5). To test whether the increased DILP1 expression was due to a general response to low temperature we exposed three week old virgin flies to diapause conditions, to 11 °C alone (with 12L:12D), or to 25 °C with 10L:14D). We found that none of the treatments affected DILP1 levels in 3-week-old flies, i. e. no DILP1 immunolabeling was detected any of the flies (not shown). This is probably because treatments were given outside the diapause-induction time window, from eclosion up to 10 h posteclosion.
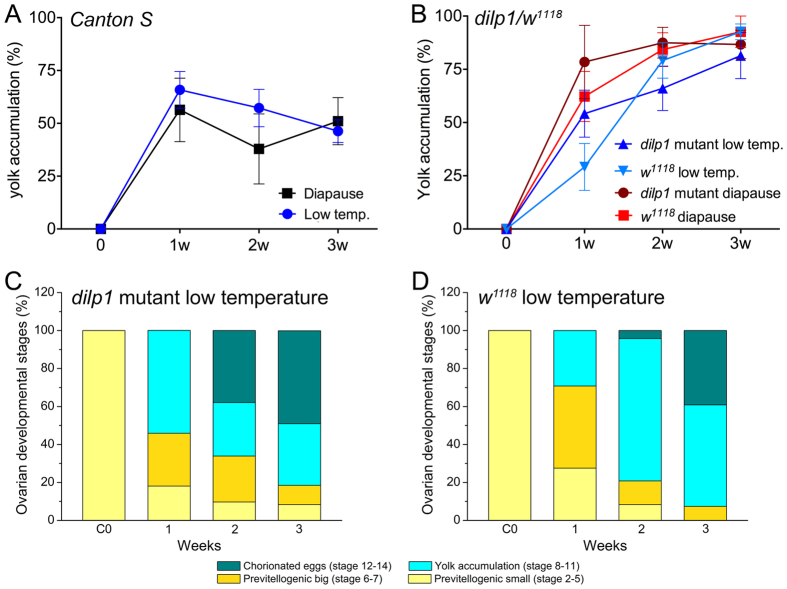
Figure 4. Diapause incidence is not affected in dilp1 mutant flies.
(A) Exposure to 11 °C with 12L:12D (Low temp) and diapause conditions induce similar effects on yolk accumulation in Canton S flies over three weeks and suggest that after 3wD about 50% of the flies have entered diapause. (B) In dilp1 mutant flies exposed to the same conditions the effect on yolk accumulation is less strong over 3 weeks than Canton S and does not differ significantly from control flies (w1118). Data are shown as means ± S.E.M, n = 7–14 flies from 4 independent replicates (ns – not significant, as assessed by two way ANOVA with Bonferroni’s multiple comparisons tests). Note that w1118 flies are much less prone to diapause than Canton S28. (C,D) Graphical representation of ovarian development showing defined stages of egg differentiation.
Since dilp1/DILP1 expression is increased and kept high during diapause, we asked whether genetic knockdown of dilp1 expression has an effect on diapause induction or maintenance. Thus, we examined diapause incidence in dilp1 mutant flies by monitoring the rate of vitellogenesis, which is commonly employed to phenotype reproductive diapause in Drosophila28,33,35. We compared yolk accumulation in ovaries of dilp1 mutant flies (in w1118 background) in recently hatched flies and over three weeks of diapause conditions compared to control flies (w1118). There was no significant difference in diapause incidence between mutants and controls when they were exposed to either diapause conditions or 11 °C with 12L:12D (Fig. 4A–D).
Interactions between DILP1 and other DILPs
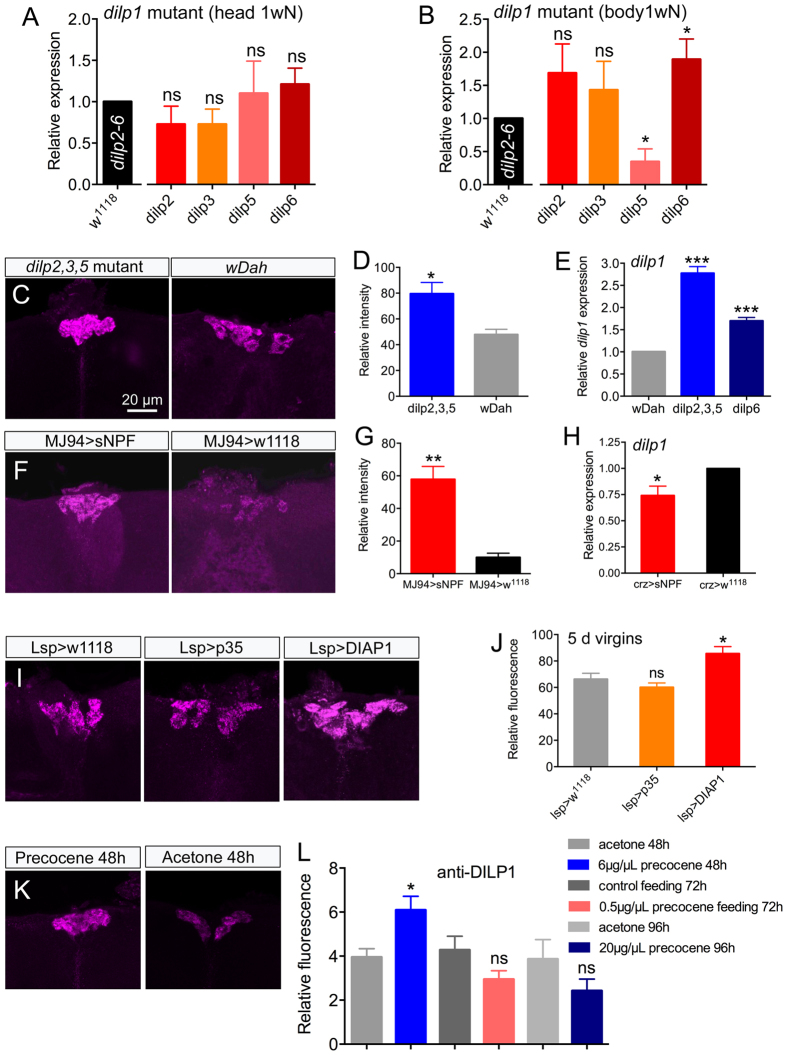
Expression of some of the DILPs can be regulated by feedback from other DILPs, and it has also been shown that loss of certain DILPs is functionally compensated by others4,36. Therefore, we tested the effects of loss of dilp1 on other dilp transcripts and also effects of loss of dilp2, 3, 5 and dilp6 on dilp1 expression. Extracts of heads of one-week-old dilp1 mutant flies did not show any alteration of dilp2, 3, 5 or 6 transcripts (Fig. 5A), whereas body extracts displayed increased dilp6 and reduced dilp5 expression (Fig. 5B). These results suggest that loss of dilp1 affects dilp6 in thoracic/abdominal fat body and dilp5 in ovaries or renal tubules, but has no significant effect on dilp transcription in IPCs. However, at the peptide level we found that DILP2 immunolabeling increased slightly in IPCs of dilp1 mutant flies (Fig. S6A,B), possibly suggesting decreased release of this peptide. As a comparison we monitored effects of loss of dilp1 on dilp transcripts in late pupae (stage P14) when dilp1 is normally high. At this stage dilp1 mutants displayed a slight decrease in dilp3 and dilp6 and no change in dilp2 and 5 (Fig. S2F).
Figure 5. Regulation of dilp1 levels and effects of dilp1 mutation on transcription of other dilps.
(A,B) qPCR analysis of one-week-old dilp1 mutant flies (kept at normal conditions) shows no effects on dilp2, 3, 5 or 6 transcripts in head extracts (A) whereas in whole body (without heads) dilp5 is downregulated and dilp6 upregulated (B). dilp levels are compared to control w1118 flies (the black bar shows control levels of dilp2, 3, 5 and 6; set to 1.0); presented as means ± S.E.M, three replicates with 15–20 heads/bodies per replicate (*p < 0.05, unpaired Students’ t-test). (C,D). In dilp2, 3, 5 mutant flies DILP1 immunofluorescence is upregulated compared to their controls wDahomey (wDah), presented as means ± S.E.M, n = 6–7 flies for each genotype from three crosses (*p < 0.05, unpaired Students’ t-test). (E) Also dilp1 is increased in head extracts of dilp2, 3, 5 and dilp641 mutant flies compared to controls (wDah); presented as means ± S.E.M, n = 3 replicates with 15–20 heads in each replicates for each genotype (***p < 0.001, unpaired Students’ t-test). (F,G) Ectopic expression of sNPF in sensory neurons (MJ94-Gal4 >UAS-sNPF) leads to increased DILP1 immunostaining in IPCs of 1-week old flies; presented as means ± S.E.M, n = 6–7 flies for each genotype from three crosses (**p < 0.01, unpaired Students’ t-test). (H) Over-expression of sNPF in corazonin-expressing neurons using a Crz-Gal4 decreases dilp1 transcript, but not DILP1 peptide levels (see Fig. S4C,D). (I,J) After blocking apoptosis in larval adipocytes by crossing Lsp-Gal4 flies to UAS-p35 of UAS-DIAP1 we extended survival of these adipocytes in the adult flies to determine effect on DILP1 immunolabeling in 5d virgin flies. Only Lsp >DIAP1 significantly increased DILP1 fluorescence in adult IPCs; presented as means ± S.E.M., n = 9–10 flies for each genotype from three crosses (*p < 0.05, unpaired Students’ t-test). (K,L) Precocene 1 applied (in acetone) on abdominal cuticle or fed to the flies halted ovary development. Only when applied topically for 48 h at 6 μg/μL it increased DILP1 immunofluorescence levels; presented as means ± S.E.M, n = 8–13 flies for each treatment from three replicates (*p < 0.05, as unpaired Students’ t test).
Next we analyzed dilp1 expression in one-week-old flies after loss of dilp2, 3, 5 in triple mutants. Indeed, the loss of the three other DILPs of the IPCs triggered a slight, but significant, increase in DILP1 immunofluorescence in IPCs (Fig. 5C,D), and a strong increase of dilp1 transcript (Fig. 5E). We also analyzed dilp1 transcript in dilp6 mutant flies, since an earlier study indicated that dilp1 and dilp6 display compensatory regulation19. The dilp1 transcript increased significantly in dilp6 mutants compared to control flies (Fig. 5E).
Regulation of DILP1 by short neuropeptide F (sNPF)
A few neuropeptides and neurotransmitters have been shown to act on the IPCs to regulate production and/or release of DILPs14,37,38. One of these, short neuropeptide F (sNPF), is also known to regulate feeding in Drosophila27,39. An earlier study has reported that mis-expression of short neuropeptide F (sNPF) in specific sensory neurons induces upregulation of dilp1 and dilp2 in the larval CNS27. Here we targeted expression of sNPF to the same set of sensory neurons using the MJ94-Gal4 and monitored DILP1 immunofluorescence in IPCs of one-week-old adult flies. The sNPF expression led to a significant increase of DILP1 immunofluorescence compared to control flies (Fig. 5F,G). Some of the sensory neurons of MJ94-Gal4 send their axonal projections to the pars intercerebralis close to the presumed dendrites of the IPCs (Fig. S6E), but it is not clear whether MJ94 expressing neurons act directly on IPCs. Nevertheless, sNPF from these neurons seems to regulate DILP1 production also in early stages of adult life.
Another study has shown that neurons in the pars lateralis, designated DLPs, express sNPF and impinge on the brain IPCs40. That study demonstrated that targeted knockdown of sNPF in the DLPs of adult male flies lead to diminished expression of dilp2 and dilp5 mRNA. Thus, we tested whether overexpression of sNPF in these neurons had an effect on dilp1 levels in female flies. We found that there was a very small, but significant, decrease in dilp1 transcript (Fig. 5H). However increased sNPF expression in DLPs did not result in a significant change in DILP1 immunolabeling (Fig. S6C,D), suggesting that DILP1 production, but not release, was affected.
Timing of dilp1/DILP1 expression with regulation of organismal development and differentiation and ovary maturation
Interestingly, the timing of the dilp1/DILP1 expression not only correlates with non-feeding stages, but also coincides with organismal remodeling during pupal development, adult eclosion, as well as physiological and reproductive maturation starting during the first day of adult life. The extensive tissue reorganization and growth during pupal development requires a reallocation of resources from nutrient stores in adipocytes and histolysing larval tissues; this requires activation of IIS and growth factor signaling19,20,41. Furthermore, during this period several hormonal signals are required to orchestrate tissue growth and differentiation42,43 and we thus sought evidence for links between DILP1 and other hormonally regulated events, including resource reallocation.
During the first day of adult life food intake is very low and steady state metabolite and hormone levels have not yet reached those seen in mature flies28. Another characteristic of the pupa and the first day of adult life is the presence of fat body cells derived from the larva44,45. These dissociated adipocytes constitute an energy and nutrient store during pupal development and also serve the newly eclosed adult until it is ready for flight, food search and feeding, which requires wing expansion and tanning of cuticle44,45. When no longer needed, the larval adipocytes undergo apoptosis the first days of adult life44. To test whether the presence of larval fat body is correlated with dilp1/DILP1 expression we delayed apoptosis in larval adipocytes by expressing anti-apoptosis genes (p35 and DIAP1) using an Lsp-Gal4 driver specific for fat body. When measuring DILP1 fluorescence in IPCs of 5 day old flies we found that expression in Lsp >DIAP1 flies was significantly higher than in controls and in Lsp >p35 flies (Fig. 5I,J). Thus, a slight effect of delaying adipocyte death can be seen on DILP1 expression. The apoptosis of larval adipocytes is ecdysone dependent44 and we investigated a possible link between DILP1 and ecdysone signaling by determining levels of prothoracicotropic hormone (PTTH) in brain lateral neurosecretory cells in dilp1 mutant flies. At adult eclosion PTTH triggers ecdysone production in the prothoracic glands and a few days post-eclosion the PTTH producing neurosecretory cells are no longer detectable46,47. In newly eclosed (1–3 hours after adult eclosion) dilp1 mutant flies the PTTH immunolabeling is significantly lower than in controls, suggesting increased PTTH release (Fig. S7).
We next tested whether ovarian maturation, which also occurs the first days of adult life, correlates with dilp1/DILP1 expression. Ovarian maturation and vitellogenesis depends on active juvenile hormone (JH) signaling. Exposing newly eclosed virgin flies to precocene 1, an inhibitor of JH biosynthesis in corpora allata, leads to inhibition of vitellogenesis and oocyte growth in ovaries48,49. We applied precocene 1 in acetone (6 μg/μL or 20 μg/μL) to the abdomen of newly eclosed virgin flies (Canton S) and monitored DILP1 immunofluorescence in 48 h and 96 h old flies compared to controls exposed to only acetone. Ovary development was arrested for both concentrations (not shown), but only the lower concentration led to increased DILP1 immunolevel (Fig. 5K,L). Feeding precocene to the flies (0.5 μg/μL in normal food) also resulted in blocked ovary development in 72 h old flies, but had no effect on DILP1 expression (Fig. 5K,L). Thus, there seems to be a dose-dependent effect of blocking JH signaling, but the relation of DILP1 to ovarian development is not clear. We also tried to prevent diapause (induce vitellogenesis) and diminish DILP1 expression by feeding or topical application of the JH analog Methoprene50. Thus, we tested (1) female flies (Canton S) that had been kept for two weeks in diapause with food containing 1.5 mM Methoprene, and (2) flies that after three weeks of diapause had 0.5 μL of 1 mM Methoprene in acetone applied to the abdomen and put back in diapause conditions for one week. As shown in Fig. S8 neither treatment altered the DILP1 expression in the IPCs.
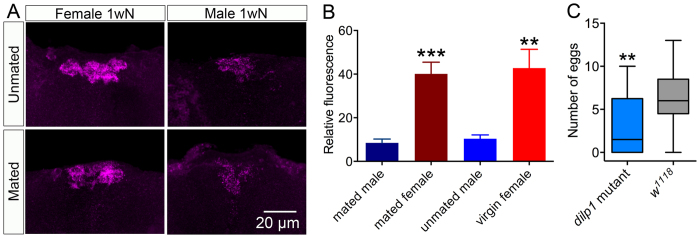
We asked whether mating had an effect on DILP1 expression in male and female flies. Thus, one-week-old mated flies were monitored, but no significant difference was detected in DILP1 labeling of IPCs in mated and unmated specimens (Fig. 6A). However in both states female flies had significantly stronger DILP1 expression in IPCs than males (Fig. 6A,B). This was not the case in newly-eclosed flies (Fig. S2D). To search for a role of DILP1 in ovary development we monitored the rate of oviposition in dilp1 mutant and control flies. The dilp1 mutant flies displayed a significantly reduced oviposition (Fig. 6C).
Figure 6. Roles of DILP1 in reproduction and fecundity.
(A) There is a sex dimorphism in strength of DILP1 immunolabeling in IPCs of one-week-old mated and unmated flies. (B) Quantification of DILP1 immunolabeling in one-week-old mated and unmated flies, showing the significantly higher DILP1 expression in female IPCs in both mated and unmated flies. Data are presented as means ± S.E.M, n = 7–9 flies for each genotype from three independent replicates (***p < 0.001, **p < 0.01, ns – not significant, as assessed by unpaired Students’ t-test). (C) Box-Whisker graph showing that the number of eggs laid is significantly lower in dilp1 mutant than in control flies. Data are presented as medians ± range (where boxes show 25–75% percentile and the population median), n = 21–30 flies from three independent replicates (**p < 0.01, as assessed by Mann-Whitney test).
Effect of dilp1 mutation on lifespan and stress resistance
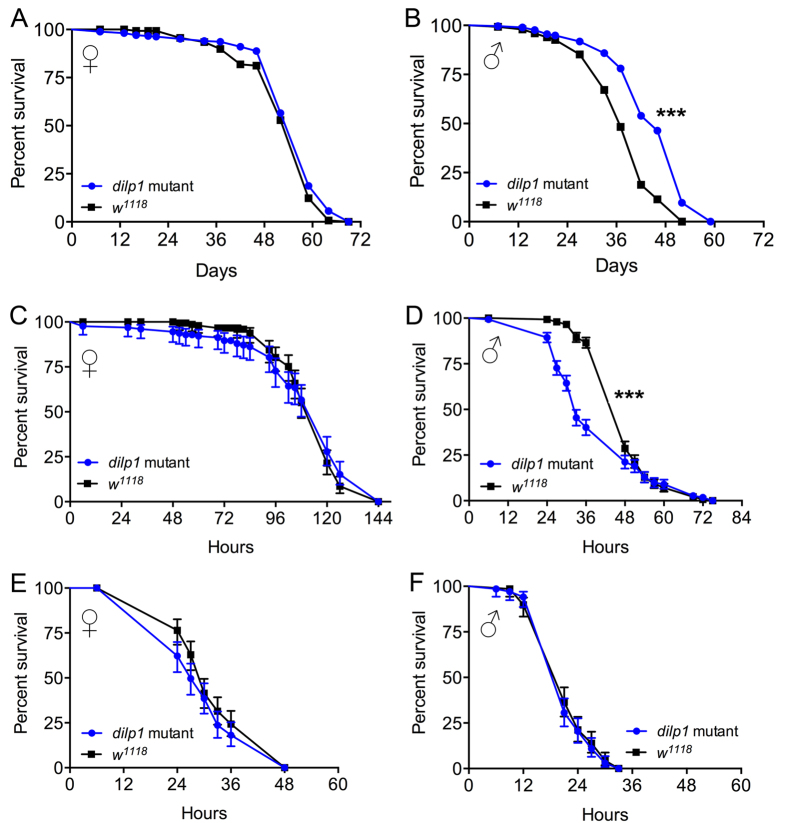
Diminished insulin signaling extends lifespan in Drosophila, and ablation of the brain IPCs or knockdown of dilp2 is sufficient to increase longevity2,4,51,52. We monitored longevity in male and female dilp1 mutant flies under normal feeding conditions and registered an increase of median lifespan in males only (Fig. 7A,B).
Figure 7. Male dilp1 mutant flies display altered lifespan and stress resistance.
(A,B) Lifespan is significantly increased in mated male dilp1 mutant flies compared to controls (w1118), whereas mated mutant females display no phenotype. Data are presented in survival curves, n = 291 and 149 flies for dilp1 mutant and w1118 (***p < 0.001, as assessed by Log-rank (Mantel-Cox) test). (C,D) Survival is drastically decreased only in mated male dilp1 mutant flies exposed to starvation. Data are presented in survival curve, n = 134 and 149 flies for dilp1 mutant and w1118 (***p < 0.0001, as assessed by Log-rank (Mantel-Cox) test). (E,F) Neither female nor male dilp1 mutants react to desiccation (dry starvation) by altered lifespan. Data are presented in survival curve, n = 128 and 141 flies for female dilp1 mutant and w1118 as well as n = 137 flies each for male dilp1 mutant and w1118 (***p < 0.0001, as assessed by Log-rank (Mantel-Cox) test).
Flies with ablated IPCs display an increased resistance to starvation2. To investigate possible DILP1 function in stress resistance, we tested responses to starvation and desiccation in dilp1 mutant flies. In these tests 4–5d old Dilp1 mutant flies were tested with w1118 flies as controls.
Before testing effect of starvation on survival, we monitored DILP1 immunolevels in one-day-old female flies (Canton S) that were starved for 24 h and found a significant decrease in immunolabeling (Fig. S6G), maybe suggesting increased DILP1 release. Next we showed that only male dilp1 mutant flies display reduced resistance to starvation, as seen in decreased survival, (Fig. 7C,D) and the response to desiccation was not affected in either sex (Fig. 7E,F). Thus, there may be a sexual dimorphism in DILP1 function in relation to lifespan and stress tolerance.
Discussion
Until now the spatiotemporal expression and function of DILP1 in Drosophila was largely unknown. Our study shows that under normal rearing conditions both dilp1 transcript and DILP1 peptide are expressed transiently by a set of 14 brain IPCs during pupal stages and the first days of adult life. However, a continuous high dilp1/DILP1 expression can be seen in IPCs of female flies that have entered reproductive diapause. The DILP1 expression, thus, coincides with the non-feeding stages of pupae and the very young fly44,45, and the strongly reduced feeding during adult diapause28. This temporal expression of DILP1 suggests a specific function of the peptide that may be related to development, growth or energy homeostasis characteristic of states of minimal nutrient intake.
The arrest of feeding at the end of the larval life is programmed and continues throughout pupal development. Thus, cell growth and proliferation during this stage occurs without global growth or gain of mass, using stored nutrients and supplies derived from histolysis of obsolete larval tissues19,41. The fat-body-derived DILP6 is known to promote tissue growth during non-feeding stages and dilp6 is induced by ecdysone in the wandering third instar larva19,20. Possibly DILP1 is functionally accessory to DILP6 during this non-feeding period, although a very minor growth promoting role was detected4. An additional possibility is a link to stages with no reproductive activity. Characteristic of Drosophila pupae, newly eclosed flies, and diapausing adults, is the immature ovaries and lack of vitellogenesis (see28). Thus, DILP1 down-regulation could correlate with both the metabolic transition during the first days of adult life and the onset of sexual maturation. Indeed, we found that dilp1 mutant flies display a reduced early oviposition, suggesting a role in egg development. This is consistent with the importance of intact systemic insulin signaling in the control of ovarian germ line stem cells53.
To further understand DILP1 function we undertook an analysis of factors regulating its expression and effects of manipulations of DILP1 production. It has been shown that loss of certain DILPs can be compensated by upregulation of others4,19,36. We tested the effect of loss of dilp2, 3, 5 and dilp6 using mutant flies4 and found that the dilp1 transcript was elevated in both mutants. Conversely, we detected a slight but significant increase in dilp6 expression and a reduced dilp5 expression in the body, but not heads, of 1-week-old dilp1 mutant flies. These findings suggest that loss of dilp1 may affect dilp5 transcription in ovaries or renal tubules and dilp6 in adipocytes of the body. Apparently the loss of dilp1 did not affect dilp2 and dilp3 levels (or dilp5 in the head), suggesting that in the IPCs there are no detectable compensatory transcriptional mechanisms for lack of dilp1 in adult flies. The upregulation of dilp1/DILP1 after loss of dilp2, 3, 5 or dilp6 may be reminiscent of the situation during diapause where the dilp1 transcript is elevated but systemic insulin signaling appears to be generally downregulated, probably due to diminished DILP release (see28,34,54).
Previous studies have shown that a few neuropeptides and neurotransmitters act on the IPCs to alter their activity and/or production of DILPs (summarized in14,37,55,56). One of the peptides acting on IPCs is sNPF, released from DLP neurons, that triggers increased levels of dilp2 and dilp5 transcripts in the adult brain40. In another study sNPF was ectopically targeted to sensory neurons of larvae using the MJ94-Gal4 and it was shown that dilp1 and dilp2 transcripts were elevated27. When we targeted sNPF to MJ94 neurons there was an increase in DILP1 immunolabeling of the IPCs of one-week-old flies, but we noted no effect on peptide levels when targeting DLP neurons. However, measuring dilp1 transcript we noted a slight decrease after sNPF overexpression in DLP neurons. These findings suggest that sNPF signaling is involved in regulation of DILP1/dilp1 expression. Earlier work has suggested multiple functional roles of sNPF, including regulation of food intake, modulation of food odor responses, fine control of locomotor behavior, and learning and memory57,58,59,60,61,62.
There might, hence, be a link between the role of sNPF in feeding and food odor processing and its action on DILP1 production and/or release in the IPCs. This regulation can perhaps be extended to taste inputs since flies lacking gustatory bristles due to a null mutation in the gene Pox neuro (Poxn) were shown to live longer and in females display increased levels of dilp1, dilp3 and dilp663. The MJ94-Gal4 line used in our study includes gustatory receptors64,65 and may, thus, account for the effect of sNPF overexpression on dilp1 levels. The study of Poxn mutants revealed a female-specific upregulation of dilp1, dilp3 and dilp6 and a male downregulation of dilp3 and dilp5, suggesting a sex-specific influence of taste on lifespan mediated by different DILPs63.
An intriguing finding in our study is that flies kept in reproductive diapause display a sustained high dilp1/DILP1 expression in IPCs until the dormancy is interrupted. Adult diapause is characterized by halted reproduction, a drastic reduction of food intake, accompanied by a shift in energy metabolism towards increased nutrient stores, as well as increased stress tolerance, and upregulated immune defense28,34,54. In Drosophila diapause can only be triggered in newly eclosed flies before 10 h of adult life28,32. Flies at this stage share several features with the late pupae in addition to the lack of food intake and high expression of DILP1/dilp128,44,45. For instance, both pupae and adult flies that are less than 24 h old display presence of fat body cells derived from the larva44,45. These dissociated adipocytes constitute an energy and nutrient store during pupal development and also serve the newly eclosed adult until it is ready for flight, food search and feeding, which requires wing expansion and tanning of cuticle44,45. The presence of larval fat body cells is extended somewhat in diapausing flies. Thus, we tested whether genetic blocking of apoptosis in larval fat body in non-diapausing flies has an effect on DILP1 expression. Similar to the study of Aguila et al.44 we could only extend the presence of larval adipocytes a few days, but found that DILP1 expression was increased in 5d old flies compared to controls.
The sustained DILP1 expression during diapause is not likely to be due only to the presence of larval fat body since this is no longer detectable after 3 weeks of diapause (unpublished observations). Since ovaries do not mature during diapause we tested whether some ovary-derived factor might be involved in the regulation of dilp1/DILP1 expression. By treating newly eclosed flies with precocene 1, an inhibitor of juvenile hormone biosynthesis, we halted ovary maturation and observed a dose-dependent increase in DILP1 expression in young flies. However, since we did not obtain clear evidence for long term effects of larval fat body or ovary maturation in keeping the DILP1 expression high, we suggest that during diapause the DILP1 level in IPCs remains high due to the altered metabolic state and endocrine signaling (including diminished general IIS) characteristic of dormancy28. It can be noted that loss of dilp1 does not result in drastically altered diapause incidence, probably due to redundant functions of other DILPs. However, dilp1 mutant flies display reduced egg-laying, indicating a role of the peptide in ovary development, maybe accessory to DILP2 which was shown to control germ line stem cells in Drosophila ovaries53.
In our work we found that dilp1 mutant male flies display increased median lifespan under normal conditions, and also reduced resistance to starvation. This is similar to the effects of deletion of the IPCs, which renders flies more long-lived2, but the reduced starvation resistance in dilp1 mutants is opposite of findings for IPC ablation and dilp6 mutants2,19. Our experiments herein do not provide any clues to this sex dimorphism in the role of dilp1. It is noteworthy that deletion of single dilps, except dilp2, has little effect on fly physiology4. Thus, dilp1 mutants display unexpectedly strong effects on adult male physiology, in spite of the dilp1/DILP1 expression peaking during pupal stages. One possible explanation is that loss of dilp1 seems to have very limited effects on compensatory expression of dilp2, 3 and 5 in IPCs and has rather small effects on dilp5 and 6 transcripts in the body. In other words, compensatory interactions between dilp1 and other dilps work primarily one-way whereby dilp1 levels are affected by knockout of dilp2, 3, 5 and dilp6. This is different for dilp2 mutants, which display increased dilp3 and dilp5 transcript4,36. Another aspect of interest is that dilp1/DILP1 expression is very weak in flies older than one week (kept at 25 °C) and yet we see effects on lifespan and stress responses in older male dilp1 mutant flies. Therefore it is possible that the adult phenotypes are consequences of developmental effects induced by lack of DILP1 signaling in the pupa and/or first 1–2 days of adult life, similar to findings for DILP619.
In summary, our study is the first to determine the spatial-temporal expression pattern of DILP1. The expression appears to be primarily in the brain IPCs where DILP1 is colocalized with DILP2, 3, 5 and the CCK-like peptide drosulfakinin (DSK)1,13,66. In contrast to DILP2, 3, 5, 6 and 7, we find that under normal conditions DILP1 is produced by only in the pupa and first few days of adult life. However during adult reproductive diapause DILP1 levels remain high. These findings indicate that DILP1 has a unique function that may be relevant in stages where food intake is low or absent and where sexual maturity has not been reached. Analysis of dilp1 mutant flies reveals that the peptide is important in formation of adult male responses to starvation and general longevity, and in female ovary maturation.
Methods
Fly lines and husbandry
As wild type and control flies we used Drosophila melanogaster of the strains Canton S and w1118, respectively, from The Bloomington Drosophila Stock Center (BDSC), Bloomington, IN. Three mutant strains were employed: dilp1 (transcript null allele), dilp641 (small deletion covering first exon; loss of functional allele) and dilp2, 3, 5 mutants4, kindly provided by S. Grönke (Cologne, Germany). As controls for dilp1 mutant, we used w1118 and for dilp641 and dilp2, 3, 5 mutants controls were wDahomy, dependent on mutant background, all from S. Grönke. We utilized MJ94-Gal4 (from L. Griffith, Waltham, MA), UAS-dilp1-RNAi from The Vienna Drosophila Resource Center, Vienna (VDRC), UAS-2xsNPF (from K. Yu, Daejon, Korea), dilp2-Gal4 (from E. Rulifson, Stanford, CA), Crz-Gal4 (from J.H. Park, Knoxville, TN), Lsp-Gal4 (BDSC), Ppl-Gal4 (from M.J. Pankratz, Bonn, Germany), UAS-mCD8-GFP, UAS-DIAP1 and UAS-p35 (from BDSC). The production of two sets of dilp-Gal4 lines is described in the next section.
Parental flies for Gal4-UAS crossings were reared on BDSC food medium with 1.5 g/L nipagin (http://flystocks.bio.indiana.edu/Fly_Work/media-recipes/bloomfood.htm). Canton S flies and progeny of Gal4-UAS crossings were (if not otherwise indicated) raised on food medium containing 100 g/L sucrose (S), 50 g/L yeast (Y), 12 g/L agar, 3 mL/L propionic acid and 3 g/L nipagin (2S:1Y food medium). Flies were kept either under normal conditions with 12:12 Light:Dark cycle at 25 °C or under diapause conditions with 10:14 Light:Dark cycle at 11 °C. In the starvation and desiccation experiments on dilp1 mutants food medium containing 2S:1Y was used for raising flies.
Production of dilp1-Gal4 and dilp5-Gal4 lines
In both the dilp1 and dilp5 genes the transcription start site is very close the predicted TATA box, making it difficult to amplify the putative promoters in a single PCR reaction. We thus used two sequential PCR reactions. For the first PCR reaction primers for dilp1 were
dilp1-1(5′-GGACAGAATTCACCGTCAGCGGATAATCGTA-3′) and
dilp1-2 (5′-TGCTGGCTAAACATCTTGGA-3′)
and for dilp5
dilp5-1 (5′-GGACAGAATTCGGCTCTGTTCGGGATTGATA-3′) and
dilp5-2 (5′-ATCATTGCCTTGCTGGAACT-3′).
The products of these PCR reactions were then reamplified by primers dilp1-1 and dilp1–3 (5′-GGATCGGATCCTTGGATATGCAGTGAATGCTC-3′) and dilp5-1 and dilp5-3 (5′-GGATCGGATCCTTGCTGGAACTGCTGCTG-3′) respectively. After gel purification and digestion with Eco RI and Bam HI the putative promoters were cloned into the P[pAKH-GAL4] vector67, from which the AKH promoter had been removed with the same enzymes. Sequencing confirmed that the transgenes had the intended sequences. Embryos were injected by thebestgene.com yielding 10 independent transformants for dilp1 and 10 for dilp5.
Two transformants (#4 and #8) for dilp1 were selected after screening GFP expression patterns driven by dilp1-Gal4 throughout larval, pupal, newly eclosed and 1-week-old fly stages. Similarly, two transformants for dilp5 were selected for further use here (only few experiments).
Production of DILP1 antisera
For generation of antisera we selected a sequence from the C-peptide of D. melanogaster DILP1 synthesized with a cysteine in the N-terminus: CEVQDDSSMWQTLDGAGYS. This peptide was conjugated to maleimid-coupled thyroglobulin via the SH group of the N-terminal cysteine and the conjugate used for immunization of two rabbits and two guinea pigs. The production of the antigen and the immunization were performed by Pineda Antibody Service (Berlin, Germany). Preimmune sera were collected from each animal prior to immunization.
Other antisera and immunocytochemistry
We used rabbit antisera to A-chains of DILP2 and DILP330 and C-peptide of DILP531 at a dilution of 1:2000. A rabbit antiserum to Drosophila prothoracicotropic hormone (PTTH68), was kindly provided by Dr. P. Leopold (Nice, France) and applied at 1:500. The central nervous system of third instar larvae, different pupal stages and adult CNS, as well as various tissues (fat body, gut, ovaries) were fixed in ice-cold 4% paraformaldehyde (4% PFA) in 0.1 M sodium phosphate buffer (PB; pH 7.4) for 2–4 h. After washing with PB 3 × 15 min, tissues were dissected in PB. All tissues were washed finally in 0.01 M phosphate buffered saline (PBS) with 0.25% Triton-X (PBS-Tx) for 15 min. Tissues were then incubated in primary antibodies for 24–48 h at 4 °C with gentle agitation. After washes (4 × 15 min) in PBS-Tx, secondary antibodies were applied for overnight or 48 h at 4 °C. Tissues were then washed in PBS-Tx 7 × 10 min, rinsed in 0.01 M PBS and mounted with 80% glycerol in 0.01 M PBS. Rabbit and mouse anti-GFP (1:1000) were used (Invitrogen, Carlsbad, CA). The following secondary antibodies (1:1000) were employed for detection: goat anti-rabbit Alexa 546, goat anti-rabbit Alexa 488, goat anti-mouse Alexa 488, goat anti-mouse Alexa 546 (all from Invitrogen), Cy3-tagged goat anti-guinea pig antiserum (Jackson ImmunoResearch, West Grove, PA).
Quantitative real-time PCR (qPCR)
Total RNA was extracted from whole flies or separated heads and bodies of experimental virgin female flies using Trizol-chloroform (Sigma-Aldrich) from three independent biological replicates with 15–25 flies in each replicate. Quality and concentration of the RNA were determined with a NanoDrop 2000 spectrophotometer (Thermo Scientific). For cDNA synthesis reactions 2 μg of total RNA, 0.4 μL random hexamer primer (Thermo Scientific) and 2 μl of M-MuLV reversible transcriptase (Thermo Scientific) were used. The cDNA was then applied for quantitative real-time PCR (qPCR) using a StepOnePlus™ System (Applied Biosystem, USA) instrument and SensiFAST SYBR Hi-ROX Kit (Bioline) according to the manufacturer. For each sample triplicate reactions of the total volume of 20 μl were conducted with a primer concentration of 400 nM and 4 μl of diluted 1:10 cDNA template. The mRNA levels were normalized to rp49 levels in the same samples. Relative expression values were determined by the 2−ΔΔCt method69.
We used the following primers for qPCR (all displayed 5′–3′):
dilp1 F: CGGAAACCACAAACTCTGCG
dilp1 R :CCCAGCAAGCTTTCACGTTT
dilp2 F: AGCAAGCCTTTGTCCTTCATCTC
dilp2 R: ACACCATACTCAGCACCTCGTTG;
dilp3 F: TGTGTGTATGGCTTCAACGCAATG
dilp3 R: CACTCAACAGTCTTTCCAGCAGGG;
dilp5 F: GAGGCACCTTGGGCCTATTC
dilp5 R: CATGTGGTGAGATTCGG
dilp6 F: CCCTTGGCGATGTATTTCCCAACA
dilp6 R: CCGACTTGCAGCACAAATCGGTTA
rp49 F: ATCGGTTACGGATCGAACAA
rp49 R: GACAATCTCCTTGCGCTTCT
Diapause induction and analysis of ovarian development
Diapause was induced by keeping virgin female flies, collected 4–6 h post-eclosion, at 11 °C and a slightly shorter photoperiod (10L:14D)28,33. At each investigated time point of diapause (as well as in control and recovery groups) ovarian development was monitored microscopically and used as a criterion for degree of reproductive diapause as proposed earlier33,48,70. Ovaries were dissected in 0.1 M phosphate saline buffer (pH 7.4) and their developmental stages assessed by using the combined criteria of several investigations28,33,48,71,72. Stages of ovarian development were divided into four groups: small previtellogenic ovaries (stage 2–5), big previtellogenic ovaries (stage 6–8), accumulated yolk (stage 9–11) as well as several chorionated eggs (stage 12–14). Recovery from diapause was monitored one week after transferring flies from diapause conditions to 25 °C and 12L:12D; this period is sufficient for onset of vitellogenesis and reversal of hormonal and metabolic status back to pre-diapause values28. Results are displayed as percentage (%) of ovaries in different development stages, or as incidence (%) of yolk accumulation in analyzed ovaries. Data are shown as means of 4 independent replicates with 7–14 flies in each replicate.
Stress assays
For starvation resistance experiments, 4–5d-old flies were placed in vials containing 0.5% aqueous agarose for starvation (no access to food) in an incubator at 25 °C with 12:12 h Light:Dark (LD) conditions and controlled humidity. Each vial contained 15 flies to avoid a crowded environment. Dead flies were counted in every 12 hours. For desiccation resistance the experiment was the same, except that flies obtained no food and no water.
All these experiments were performed in three replicates with at least 30 flies of each genotype per replicate. Survival curves were produced in Prism GraphPad 6.0.
Precocene 1 and methoprene application and feeding
Precocene 1 (Sigma-Aldrich, catalog no. 195855-1G) was diluted in acetone with final concentrations 6 μg/μL and 20 μg/μL and applied on the abdomens of newly eclosed virgin flies with a small brush (5 μL was applied to each abdomen). After precocene treatment flies were checked for recovery (locomotion, wing expansion and flipping). For 96 h treatment precocene 1 was applied a second time after 48 h. Control flies were exposed to acetone alone. As a second approach newly eclosed virgin flies were fed precocene 1 mixed into food medium (final concentration 0.5 μg/μL). Control flies were fed food medium alone.
Methoprene (Sigma-Aldrich, St Louis, MO, PESTANAL 33375, racemic mixture), a JH analog50, was diluted in acetone with final concentrations of 1.5 mM or 1 mM. For topical application 0.5 μL of 1 mM methoprene was quickly applied on the abdomens of virgin flies after three weeks of diapause and flies were transferred back into vials and the diapause incubator as soon as possible to minimize the interruption of diapause. Flies after one week in diapause were taken for immunohistochemistry. For feeding methoprene 2S:1Y food medium supplemented with 1.5 mM methoprene was given to newly eclosed virgin flies kept for two weeks in diapause.
Oviposition
For oviposition, the number of eggs laid by individual female flies within 16 h was counted. Individual pairs of newly eclosed males and females were mated for 6 days. Egg numbers from 21–30 pairs of flies were counted 16 hours after fly pairs were placed into separate food vials.
Statistical Analysis
All statistical analyses were performed using Prism GraphPad 5.0. Survival data were analyzed by Log rank test with Mantel-Cox posttest. For the remaining experiments, data was first checked with Shapiro-Wilk normality test and then analyzed with unpaired Students’ t test or ANOVA with Dunnett’s or Sidak’s multiple comparisons tests. If data was not normally distributed, non-parametric tests, Mann Whitney or Kruskal-Wallis test was performed.
Additional Information
How to cite this article: Liu, Y. et al. Drosophila insulin-like peptide 1 (DILP1) is transiently expressed during non-feeding stages and reproductive dormancy. Sci. Rep. 6, 26620; doi: 10.1038/srep26620 (2016).
Supplementary Material
Acknowledgments
We thank Dr. Olga Kubrak for valuable advice on qPCR and diapause experiments. We are grateful to Drs L. Griffith, S. Grönke, P. Leopold, M. Pankratz, J.H. Park and K. Yu for providing flies and reagents. Also the following organizations are thanked for flies and reagents: The Bloomington Drosophila Stock Center, Bloomington, IN, and The Vienna Drosophila Resource Center, Vienna, Austria. Stina Höglund and the Imaging Facility at Stockholm University (IFSU) are acknowledged for maintenance of the confocal microscopes. This study was supported by the Swedish Research Council (VR; 621-2010-5742) and the Knut and Alice Wallenberg Foundation (KAW2012.0058). We thank Dr Sören Nylin for support.
Footnotes
Author Contributions Y.L., S.L. and D.R.N. designed the study and analyzed data, Y.L. and S.L. performed experiments, J.A.V. generated novel transgenic flies, Y.L. and D.R.N. wrote the manuscript, all authors read, edited and approved the final manuscript, and D.R.N. supervised the study and obtained funding.
References
- Brogiolo W. et al. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol 11, 213–21 (2001). [DOI] [PubMed] [Google Scholar]
- Broughton S. J. et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA 102, 3105–10 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M., Bartke A. & Antebi A. The endocrine regulation of aging by insulin-like signals. Science 299, 1346–51 (2003). [DOI] [PubMed] [Google Scholar]
- Grönke S., Clarke D. F., Broughton S., Andrews T. D. & Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet 6, e1000857 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivonen J. M. & Partridge L. Endocrine regulation of aging and reproduction in Drosophila. Mol Cell Endocrinol 299, 39–50 (2009). [DOI] [PubMed] [Google Scholar]
- Fontana L., Partridge L. & Longo V. D. Extending healthy life span–from yeast to humans. Science 328, 321–6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleman A. A. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem J 425, 13–26 (2010). [DOI] [PubMed] [Google Scholar]
- Garofalo R. S. Genetic analysis of insulin signaling in Drosophila. Trends Endocrinol Metab 13, 156–62 (2002). [DOI] [PubMed] [Google Scholar]
- Antonova Y., Arik A. J., Moore W., Riehle M. R. & Brown M. R. Insulin-like peptides: Structure, Signaling, and Function. In Insect Endocrinology (ed. Gilbert L. I.) 63–92 (Elsevier/Academic Press, New York, 2012). [Google Scholar]
- Garelli A., Gontijo A. M., Miguela V., Caparros E. & Dominguez M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 336, 579–82 (2012). [DOI] [PubMed] [Google Scholar]
- Colombani J., Andersen D. S. & Leopold P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 336, 582–5 (2012). [DOI] [PubMed] [Google Scholar]
- Cao C. & Brown M. R. Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res 304, 317–21 (2001). [DOI] [PubMed] [Google Scholar]
- Rulifson E. J., Kim S. K. & Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–20 (2002). [DOI] [PubMed] [Google Scholar]
- Nässel D. R., Kubrak O. I., Liu Y., Luo J. & Lushchak O. V. Factors that regulate insulin producing cells and their output in Drosophila. Front Physiol 4, 252 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. et al. A genetic strategy to measure circulating Drosophila insulin reveals genes regulating insulin production and secretion. PLoS genetics 10, e1004555 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya T., Galic M., Belawat P., Nairz K. & Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol 12, 1293–300 (2002). [DOI] [PubMed] [Google Scholar]
- Padmanabha D. & Baker K. D. Drosophila gains traction as a repurposed tool to investigate metabolism. Trends Endocrinol Metabol 25, 489–554 (2014). [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E. & Perrimon N. Modeling metabolic homeostasis and nutrient sensing in Drosophila: implications for aging and metabolic diseases. Dis Model Mech 7, 343–50 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaidina M., Delanoue R., Grönke S., Partridge L. & Leopold P. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell 17, 874–84 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N. et al. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev Cell 17, 885–91 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Aliaga I., Thor S. & Gould A. P. Postmitotic specification of Drosophila insulinergic neurons from pioneer neurons. PLoS Biol 6, e58 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. H., Belawat P., Hafen E., Jan L. Y. & Jan Y. N. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319, 1679–83 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognigni P., Bailey A. P. & Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab 13, 92–104 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linneweber G. A. et al. Neuronal control of metabolism through nutrient-dependent modulation of tracheal branching. Cell 156, 69–83 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. W., Luo S. D., Dias B. G., Texada M. J. & Baker B. S. Sex-specific regulation of Lgr3 in Drosophila neurons. Proc Natl Acad Sci USA 113, E1256–1265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley B. R. et al. The developmental transcriptome of Drosophila melanogaster. Nature 471, 473–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S. et al. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat Cell Biol 10, 468–475 (2008). [DOI] [PubMed] [Google Scholar]
- Kubrak O. I., Kucerova L., Theopold U. & Nässel D. R. The sleeping beauty: how reproductive diapause affects hormone signaling, metabolism, immune response and somatic maintenance in Drosophila melanogaster. PLoS ONE 9, e113051 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao-Rico S. & Aguade M. Molecular evolution of the ligands of the insulin-signaling pathway: dilp genes in the genus Drosophila. Mol Biol Evol 28, 1557–60 (2011). [DOI] [PubMed] [Google Scholar]
- Veenstra J. A., Agricola H. J. & Sellami A. Regulatory peptides in fruit fly midgut. Cell Tissue Res 334, 499–516 (2008). [DOI] [PubMed] [Google Scholar]
- Söderberg J. A., Birse R. T. & Nässel D. R. Insulin production and signaling in renal tubules of Drosophila is under control of tachykinin-related peptide and regulates stress resistance. PLoS ONE 6, e19866 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders D. S. & Gilbert L. I. Regulation of ovarian diapause in Drosophila melanogaster by photoperiod and low moderately temperature. J Insect Physiol 36, 195–200 (1990). [Google Scholar]
- Saunders D. S., Henrich V. C. & Gilbert L. I. Induction of diapause in Drosophila melanogaster: photoperiodic regulation and the impact of arrhythmic clock mutations on time measurement. Proc Natl Acad Sci USA 86, 3748–52 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucerova L. et al. Slowed aging during reproductive dormancy is reflected in genome-wide transcriptome changes in Drosophila melanogaster. BMC Genomics 17, 50 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders D. S. The circadian basis of ovarian diapause regulation in Drosophila melanogaster: is the period gene causally involved in photoperiodic time measurement? J Biol Rhythms 5, 315–31 (1990). [DOI] [PubMed] [Google Scholar]
- Broughton S. et al. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE 3, e3721 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nässel D. R., Liu Y. & Luo J. Insulin/IGF signaling and its regulation in Drosophila. Gen Comp Endocrinol 221, 255–266 (2015). [DOI] [PubMed] [Google Scholar]
- Sano H. et al. The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of Drosophila melanogaster. PLoS genetics 11, e1005209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., You K. H., Choo J. K., Han Y. M. & Yu K. Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem 279, 50781–9 (2004). [DOI] [PubMed] [Google Scholar]
- Kapan N., Lushchak O. V., Luo J. & Nässel D. R. Identified peptidergic neurons in the Drosophila brain regulate insulin-producing cells, stress responses and metabolism by coexpressed short neuropeptide F and corazonin. Cell Mol Life Sci 69, 4051–4066 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninov N., Manjon C. & Martin-Blanco E. Dynamic control of cell cycle and growth coupling by ecdysone, EGFR, and PI3K signaling in Drosophila histoblasts. PLoS biology 7, e1000079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewitz K. F., Yamanaka N. & O’Connor M. B. Developmental checkpoints and feedback circuits time insect maturation. Curr Top Dev Biol 103, 1–33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. H. & Ewer J. Neural and Hormonal Control of Postecdysial Behaviorsin Insects. Annu Rev Entomol 59, 363–381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguila J. R., Suszko J., Gibbs A. G. & Hoshizaki D. K. The role of larval fat cells in adult Drosophila melanogaster. J Exp Biol 210, 956–63 (2007). [DOI] [PubMed] [Google Scholar]
- Hoshizaki D. K. Fat-cell development. In Complete Molecular Insect Science, Vol. 2 (eds. Gilbert L. I., Iatrou K. & Gill S.) 315–345 (Elsevier, Berlin, 2005). [Google Scholar]
- McBrayer Z. et al. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell 13, 857–71 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Liu Y. & Nässel D. R. Insulin/IGF-regulated size scaling of neuroendocrine cells expressing the bHLH transcription factor Dimmed in Drosophila. PLoS genetics 9, e1004052 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders D. S., Richard D. S., Applebaum S. W., Ma M. & Gilbert L. I. Photoperiodic diapause in Drosophila melanogaster involves a block to the juvenile hormone regulation of ovarian maturation. Gen Comp Endocrinol 79, 174–84 (1990). [DOI] [PubMed] [Google Scholar]
- Landers M. H. & Happ G. M. Precocene inhibition of vitellogenesis in Drosophila melanogaster. Experentia 36, 619–620 (1980). [Google Scholar]
- Riddiford L. M. & Ashburner M. Effects of juvenile hormone mimics on larval development and metamorphosis of Drosophila melanogaster. Gen Comp Endocrinol 82, 172–83 (1991). [DOI] [PubMed] [Google Scholar]
- Tatar M. et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292, 107–10 (2001). [DOI] [PubMed] [Google Scholar]
- Clancy D. J. et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292, 104–6 (2001). [DOI] [PubMed] [Google Scholar]
- Hsu H. J. & Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc Natl Acad Sci USA 106, 1117–21 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn D. A. & Denlinger D. L. Energetics of insect diapause. Ann Rev Entomol 56, 103–21 (2011). [DOI] [PubMed] [Google Scholar]
- Okamoto N. & Yamanaka N. Nutrition-dependent control of insect development by insulin-like peptides. Curr Opin Insect Sci 11, 21–30 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nässel D. R. & Vanden Broeck J. Insulin/IGF signaling in Drosophila and other insects: factors that regulate production, release and post-release action of the insulin-like peptides. Cell Mol Life Sci 73, 271–290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nässel D. R., Enell L. E., Santos J. G., Wegener C. & Johard H. A. A large population of diverse neurons in the Drosophila central nervous system expresses short neuropeptide F, suggesting multiple distributed peptide functions. BMC Neurosci 9, 90 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nässel D. R. & Wegener C. A comparative review of short and long neuropeptide F signaling in invertebrates: Any similarities to vertebrate neuropeptide Y signaling? Peptides 32, 1335–55 (2011). [DOI] [PubMed] [Google Scholar]
- Knapek S., Kahsai L., Winther A. M., Tanimoto H. & Nässel D. R. Short neuropeptide F acts as a functional neuromodulator for olfactory memory in Kenyon cells of Drosophila mushroom bodies. J Neurosci 33, 5340–5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root C. M., Ko K. I., Jafari A. & Wang J. W. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell 145, 133–44 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak O. V., Carlsson M. A. & Nässel D. R. Food odors trigger an endocrine response that affects food ingestion and metabolism. Cell Mol Life Sci 72, 3143–55 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahsai L., Martin J. R. & Winther Å. M. Neuropeptides in the Drosophila central complex in modulation of locomotor behavior. J Exp Biol 213, 2256–65 (2010). [DOI] [PubMed] [Google Scholar]
- Ostojic I. et al. Positive and negative gustatory inputs affect Drosophila lifespan partly in parallel to dFOXO signaling. Proc Natl Acad Sci USA 111, 8143–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner M. A. & Griffith L. C. Mapping of the anatomical circuit of CaM kinase-dependent courtship conditioning in Drosophila. Learning & memory 6, 177–92 (1999). [PMC free article] [PubMed] [Google Scholar]
- Melcher C. & Pankratz M. J. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol 3, e305 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderberg J. A., Carlsson M. A. & Nässel D. R. Insulin-Producing Cells in the Drosophila Brain also Express Satiety-Inducing Cholecystokinin-Like Peptide, Drosulfakinin. Front Endocrinol 3, 109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabel G., Martin J. R., Chidami S., Veenstra J. A. & Rosay P. AKH-producing neuroendocrine cell ablation decreases trehalose and induces behavioral changes in Drosophila. Am J Physiol Regul Integr Comp Physiol 288, R531–8 (2005). [DOI] [PubMed] [Google Scholar]
- Yamanaka N. et al. Neuroendocrine control of Drosophila larval light preference. Science 341, 1113–6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25, 402–8 (2001). [DOI] [PubMed] [Google Scholar]
- Tatar M. & Yin C. Slow aging during insect reproductive diapause: why butterflies, grasshoppers and flies are like worms. Experimental gerontology 36, 723–38 (2001). [DOI] [PubMed] [Google Scholar]
- Dapples C. C. & King R. C. The development of the nucleolus of the ovarian nurse cell of Drosophila melanogaster. Z Zellforsch Mikrosk Anat 103, 34–47 (1970). [DOI] [PubMed] [Google Scholar]
- Shimada Y., Burn K. M., Niwa R. & Cooley L. Reversible response of protein localization and microtubule organization to nutrient stress during Drosophila early oogenesis. Dev Biol 355, 250–62 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.