Abstract
Definitive haematopoiesis occurs during the lifetime of an individual, which continuously replenishes all blood and immune cells. During embryonic development, haematopoietic stem cell (HSC) formation is tightly controlled by growth factors, signalling molecules and transcription factors. But little is known about roles of the cytochrome P450 (CYP) 2 family member in the haematopoiesis. Here we report characterization and functional studies of Cyp2aa9, a novel zebrafish Cyp2 family member. And demonstrate that the cyp2aa9 is required for the HSC formation and homeostasis. Knockdown of cyp2aa9 by antisense morpholino oligos resulted the definitive HSC development is defective and the Wnt/β-catenin activity becomes reduced. The impaired HSC formation caused by cyp2aa9 morpholino can be rescued by administration of PGE2 through the cAMP/PKA pathway. Furthermore, the in vivo PGE2 level decreases in the cyp2aa9 morphants, and none of the PGE2 precursors is able to rescue phenotypes in the Cyp2aa9-deficient embryos. Taken together, these data indicate that Cyp2aa9 is functional in the step of PGE2 synthesis from PGH2, thus promoting Wnt activation and definitive HSC development.
Haematopoietic stem cell (HSC), which are a self-renewing population of cells that continuously replenish all blood and immune cells during fetal and adult life1, first form in the definitive wave of haematopoiesis during vertebrate embryogenesis2. In mice, the original pool of HSCs is established in a complex developmental process that involves several anatomical sites3. The aorto-gonads-mesonephros (AGM) region where clusters of haematopoietic cells are found to associate with the ventral wall of dorsal aorta (VDA) has been widely believed as the initial site for HSC production4.
Zebrafish has been recognized as a powerful model organism for the study of haematopoiesis owing to its embryological and genetic advantages5. During the definitive wave, HSCs first emerge from the VDA at 28 hours post fertilization (hpf)6,7. These HSCs migrate to the caudal haematopoietic tissue (CHT) from two days post fertilization (dpf) on8,9. By 3 dpf, lymphopoiesis occurs in the thymus. One day later, the HSCs migrate to the kidney marrow, which is analogous to the bone marrow in mammals10,11,12.
The prostaglandins (PG), which is synthesized from arachidonic acid (AA), is an evolutionarily conserved regulator of HSCs13. Following the phospholipase-mediated release from phospholipids of the cell membrane, AA is sequentially converted to prostaglandin precursors G2 (PGG2) and H2 (PGH2) by cyclooxygenases Cox1/214,15. These precursors are used to synthesize numerous prostanoids, including prostaglandin E2 (PGE2). In a recent chemical screen, PGE2 has been identified to regulate the HSCs homeostasis16. Further studies indicate that PGE2 can directly regulate the Wnt activity in vivo through the cAMP/PKA pathway17,18. The genetic interaction of PGE2 and the Wnt pathway has been demonstrated as a crucial signalling that regulates induction as well as homeostasis of HSCs17.
Cytochrome P450 (CYP) enzymes are involved in numerous detoxication and synthetic processes including generation of potent lipid mediators from endogenous substrates19. Biological functions of many CYPs become understood through identification of their substrates21. CYP isozymes can oxidize a spectrum of n-6 and n-3 polyunsaturated fatty acids (PUFA), such as retinoic acid, linoleic acid, eicosapentaenoic acid, docosahexenoic acid, and AA20. However, roles of CYP2s in the embryonic development remain largely unknown.
In this study, we have conducted a morpholino screen for all the 47 cyp2 family genes in zebrafish to explore their functions in embryogenesis. In the cyp2aa9 morphants, definitive HSC development is defective and the Wnt/β-catenin activity becomes reduced. The impaired HSC formation caused by cyp2aa9 morpholino can be rescued by administration of PGE2 through the cAMP/PKA pathway. Furthermore, the PGE2 synthesis decreases in the cyp2aa9 morphants, and none of the PGE2 precursors is able to rescue phenotypes in the Cyp2aa9-deficient embryos. So, we conclude that Cyp2aa9 functions at the step of PGH2 to PGE2 conversion, thus promoting Wnt activation and definitive HSC development.
Results
HSC development is defective in the cyp2aa9 deficient embryos
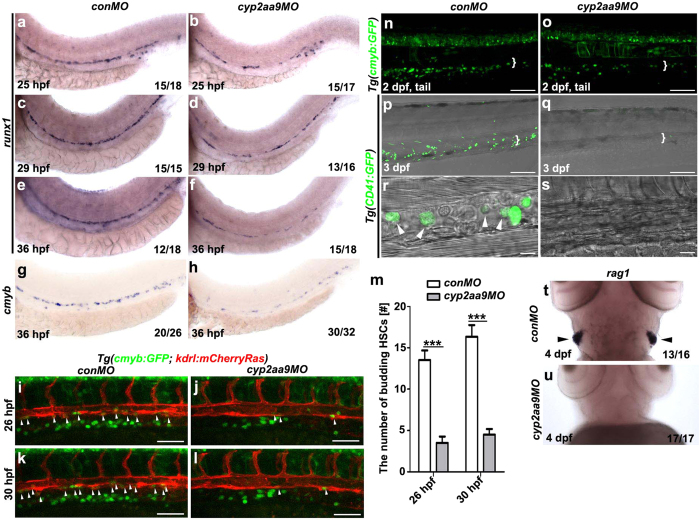
In a morpholino screen to explore roles of CYP2s in embryogenesis, we found that a morpholino oligo against the ATG-region of cyp2aa9 (cyp2aa9MO) led to defects in definitive haematopoiesis. After injection of cyp2aa9MO, the precursors of definitive HSCs marked by runx1 became decreased in the VDA from 25 hpf to 36 hpf (Fig. 1a–f). Expression of cmyb, which is enriched between the dorsal aorta and posterior cardinal vein as an indication of definitive HSC formation, was down-regulated in the cyp2aa9 morphants at 36 hpf (Fig. 1g,h). Under the Tg(cmyb: GFP, kdrl:mCherryRas) transgenic background, real-time imaging showed that the number of cmyb+ HSCs budding from the VDA dramatically reduced in the cyp2aa9 morphant from 26 hpf to 30 hpf (Fig. 1i–m, and see Supplementary Videos 1 to 2). By 2 dpf, the majority of the VDA-derived HSCs seed the CHT, an intermediate haematopoietic site analogous to the mouse fetal liver2. Under the Tg(cmyb:GFP) transgenic background, injection of cyp2aa9MO led to reduced HSCs in the CHT at 2 dpf (Fig. 1n,o, brace). At 3 dpf, the round-shaped HSCs were hardly detected in the CHT of the cyp2aa9 morphants under the Tg(CD41: GFP) background (Fig. 1p–s). Generation of T-lymphocytes requires HSCs as precursors, providing a useful readout of HSCs22. Consistent with loss of HSCs in the CHT at the early stage (Fig. 1p–s), expression of the T-lymphocyte marker rag1 in the thymus was nearly absent in the cyp2aa9 morphants at 4 dpf (Fig. 1t,u). Taken together, these results demonstrated that loss of Cyp2aa9 impairs formation of HSCs during embryonic development.
Figure 1. cyp2aa9 is required for the HSCs formation.
(a–f) In the cyp2aa9 morphants, the expression of the definitive HSC precursors marker runx1 became decreased in the ventral floor of the dorsal aorta from 25 hpf to 36 hpf. (g,h) The number of cmyb-expressing HSCs was significantly reduced in cyp2aa9 morphants at 36 hpf. (i–l) In contrast to the control embryos at 26 hpf (i, n = 22/24) and 30 hpf (k, n = 23/24), the number of HSCs undergoing budding process from VDA (arrowheads) dramatically reduced in the cyp2aa9 morphant (j, n = 21/25; l, n = 21/25; Scale bar, 50 μm). (m) Quantifications of the number of HSCs undergoing budding process (n = 8, mean ± SD, ***p < 0.001, Student’s t-test). (n,o) In CHT (white brackets), the number of cmyb:GFP-positive cells was significantly reduced in cyp2aa9 morphants (27.6 ± 5.3, mean ± s.e.m., n = 6) comparing with the control (58.2 ± 4.1, mean ± s.e.m., n = 6). Scale bar, 100 μm. (p–s) Fluorecently labelled HSCs and DIC image showed the round-shaped HSCs (white arrowheads) were hardly detected in the CHT (white brackets) of cyp2aa9 morphants under the Tg(CD41:GFP) background (p, n = 24/24; q, n = 25/29; scale bar, 100 μm). CHT regions were zoomed in and showed in the (r,s) (scale bar, 10 μm). (t,u) The expression of T-lymphocyte marker rag1 in the thymus (arrowheads) dramatically decreased in the cyp2aa9 morphants. (a–s) Lateral views, anterior left, dorsal up. (t,u) Ventral views, anterior up.
To confirm the efficiency and specificity of the morpholino approach, cyp2aa9MO was co-injected with the cyp2aa9-GFP mRNA encoding the fusion protein. Expression of Cyp2aa9-GFP was specifically knocked down by the cyp2aa9MO, but not by a control morpholino (conMO) (See Supplementary Fig. S1a,b). To exclude the p53-mediated off-target effects of the morpholino which caused apoptosis23, a morpholino against p53 (p53MO) was co-injected with cyp2aa9MO and no extra apoptosis induced by cyp2aa9MO was observed (See Supplementary Fig. S1c–e). Furthermore, although injection of the a morpholino-resistant cyp2aa9 mRNA alone was ineffective to the body shape and HSC formation, it rescued the down-regulated cmyb expression in the cyp2aa9 morphants (See Supplementary Fig. S2). These results confirm the efficiency and specificity of cyp2aa9MO, excluding the off-target or developmental delay effects. In situ hybridizations indicated diffused expression of cyp2aa9 in the mesoderm and tail region at 20 hpf and 36 hpf (See Supplementary Fig. S3, arrowheads), spatially and temporally correlated with the budding of recognizable HSCs from VDA.
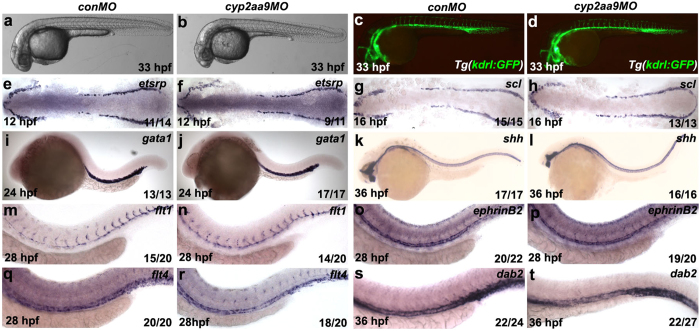
To further exclude that the impaired HSC development in the cyp2aa9 morphants is secondary to early developmental defects, we examined the integrity of haematopoietic and surrounding tissues according to morphology and expression of markers24. cyp2aa9 morphants were morphologically normal at 33 hpf (Fig. 2a,b). And the cyp2aa9 morphants displayed intact and functional vasculature as shown by beating hearts and circulating primitive non-HSC-derived erythroid cells under the Tg(kdrl:GFP) and Tg(gata1:DsRed) background (Fig. 2c,d; See Supplementary Fig S1f,g). Primitive haematopoiesis (etsrp, scl, gata1; Fig. 2e–j)25,26,27, notochord development (shh; Fig. 2k,l), dorsal aorta (flt1, ephrinB2; Fig. 2m–p)28,29, and the posterior cardinal vein (flt4, dab2; Fig. 2q–t)28 appear to be normal in the cyp2aa9 morphants. Thus, defects in HSC formation caused by cyp2aa9MO were specific and not due to wholesale failures in the specification of primitive haematopoietic cells or the nearby tissues.
Figure 2. The non-HSC tissues are unaffected in the cyp2aa9 morphants.
(a–d) The cyp2aa9 morphants in bright-field were morphologically normal (a, n = 110/110; b, n = 125/130) and with intact vasculature at 33 hpf under the Tg(kdrl:GFP) background (c, n = 21/21; d, n = 21/25). (e–t) The cyp2aa9 morphants displayed normal expressions of the following tissue-specific markers: primitive haematopoiesis (etsrp, e,f; scl, g,h; gata1, i,j), notochord (shh, k,l), dorsal aorta (flt1, m,n; ephrinB2, o,p) and posterior cardinal vein (flt4, q,r; dab2, s,t). (a–d,i–t) Lateral views, anterior left, dorsal up. (e–h) Dorsal views, anterior left.
Cyp2aa9 regulates HSC formation and Wnt activity through the PGE2/cAMP/PKA pathway
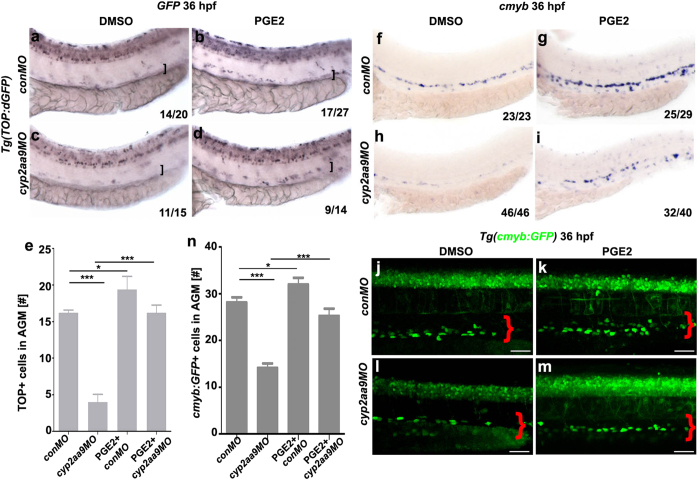
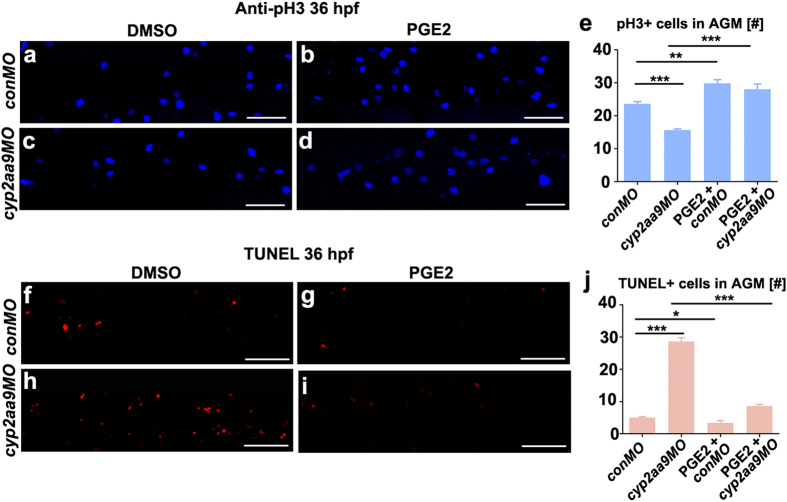
Canonical Wnt/β-catenin signalling and PGE2 are involved in the HSC specification and maintenance in vertebrates17. To examine whether Cyp2aa9 regulates the Wnt activity in vivo, we applied the Tg(TOP:dGFP) β-catenin responsive reporter line30. Decreases in the GFP expression in the VDA region of the cyp2aa9 morphants were observed at 36 hpf, which could be rescued by the administration of PGE2 (Fig. 3a–e, brackets). PGE2 could also rescue the down-regulated cmyb expression and decreases in the number of HSCs caused by cyp2aa9MO (Fig. 3f–n). Cyp2aa9MO led to reduced number of mitotic active cells and increased number of apoptotic cells in the AGM region at 36 hpf, both of which could be efficiently rescued by PGE2 (Fig. 4a–j). These data indicate that defects in HSC development and Wnt activation in the cyp2aa9 morphants can be rescued by PGE2.
Figure 3. PGE2 rescues defective HSC formation and reduced Wnt/β-catenin activity caused by cyp2aa9MO.
(a–d) Down-regulated Wnt/β-catenin activity in the VDA (brackets) caused by cyp2aa9MO was rescued by PGE2 as shown by WISH of GFP in the Tg(TOP:dGFP) Wnt-reporter line at 36 hpf. (e) Quantifications of total TOP-positive cells in the major trunk vessels (n = 6, mean ± SD). (f–i) Defective HSC formation in the cyp2aa9 morphants was rescued by PGE2 as shown by cmyb expression. (j–m) Decrease in the number of HSCs in the cyp2aa9 morphants was rescued by PGE2 as shown by the cmyb:GFP transgene. Scale bar, 50 μm. (n) Quantifications of the cmyb:GFP-positive cells in the AGM (n = 8, mean ± SD). *p < 0.05, ***p < 0.001, Student’s t-test. All the views of embryos are lateral, anterior left, dorsal top.
Figure 4. Defective proliferation and apoptosis caused by cyp2aa9MO were rescued by PGE2.
(a–d) cyp2aa9MO led to reduced number of mitotic active cells at 36 hpf in the AGM as shown by pH3 antibody staining, which could be rescued by PGE2. (e) Quantification of pH3-positive cells in the AGM (n = 6, mean ± SD). (f–i) cyp2aa9MO led to increases in the number of apoptotic cells at 36 hpf in the AGM as shown by TUNEL assay, which could be rescued by PGE2. (j) Quantification of the number of apoptotic cells in the AGM (n = 6, mean ± SD). *p < 0.05, **p < 0.01, ***p < 0.001, Student’s t-test. All the views are exactly the AGM area shown in lateral, anterior left, dorsal top. Scale bar, 50 μm.
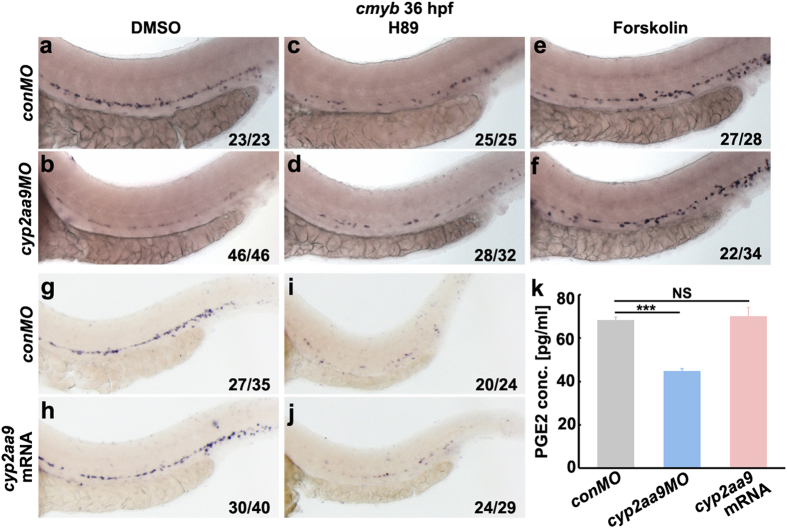
We then investigated whether Cyp2aa9 carried out its HSC regulatory function in parallel to PGE2 signalling or via PGE2. PGE2 has been demonstrated to regulate HSC formation and Wnt activity through cAMP and its downstream effector kinase PKA17. Reduced expression of cmyb in the VDA of cyp2aa9 morphants was rescued by the treatment of Forskolin, a cAMP activator (Fig. 5a,b,e,f). Treatment of H89, a PKA inhibitor, resulted in defective HSC development, which could not be rescued by the injection of cyp2aa9 mRNA (Fig. 5g–j), but did not exacerbate the HSC phenotype in the cyp2aa9 morphants (Fig. 5c,d). To analyse whether Cyp2aa9 participates in the synthesis of PGE2 in vivo, we examined the PGE2 concentration using ELISA. Although cyp2aa9 mRNA had no impact on the PGE2 synthesis, cyp2aa9MO led to decreases in the endogenous PGE2 concentration at 36 hpf (Fig. 5k), which became the direct evidence that Cyp2aa9 regulates PGE2 synthesis and acts upstream of PGE2 in vivo. These results demonstrate that Cyp2aa9 is required for PGE2 synthesis and regulates definitive HSC development through the PGE2/cAMP/PKA/Wnt cascade.
Figure 5. Cyp2aa9 regulate HSC development through the PGE2/cAMP/PKA signalling.
(a–f) Inhibition of PKA by H89 resulted in reduced cmyb expression in control, but did not exacerbate the HSC phenotype in cyp2aa9 morphants. Enhancement of cAMP by Forskolin increased the cmyb expression in control, and rescued the defective HSC development in cyp2aa9 morphants. (g–j) The inhibitory effects of H89 on HSC formation could not be rescued by the injection of cyp2aa9 mRNA. (k) In vivo PGE2 concentration measured by ELISA assay. n = 3 tubes of lysates, mean ± SD, ***p < 0.001, NS, not significant, Student’s t-test. All the views of embryos are lateral, anterior left, dorsal top.
Cyp2aa9 functions at the step of PGH2-to-PGE2 conversion
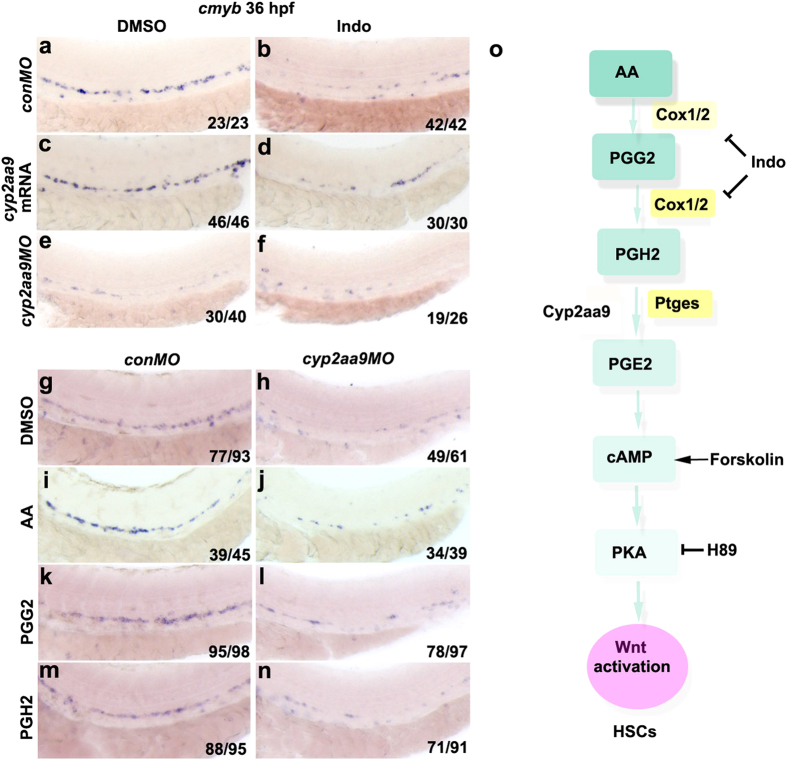
Steps of PGE2 synthesis include conversion of AA to PGG2 and in turn to PGH2 by Cox-1/2. Then, PGE2 is synthesized from PGH2 by prostaglandin E synthase (Ptges) (Fig. 6o). We investigated at which step Cyp2aa9 contributed to the prostaglandin metabolism. Impaired HSC development caused by the incubation with indomethacin (Indo), a non-selective cyclooxygenases inhibitor, could not be rescued by the injection of cyp2aa9 mRNA (Fig. 6a–d), but did not exacerbate the HSC phenotype in the cyp2aa9 morphants (Fig. 6e,f). Defective HSC development in the cyp2aa9 morphants could be rescued by PGE2 (Fig. 3f–i), but not by any of its precursors AA, PGG2, or PGH2 (Fig. 6g–n), indicating that Cyp2aa9 is non-functional without production of PGH2. All these results suggest that Cyp2aa9 carries out its HSC regulatory functions at the step PGH2-to-PGE2 conversion (Fig. 6o).
Figure 6. Roles of Cyp2aa9 in HSC development act at the step of PGH2-to-PGE2 conversion.
(a–f) Down-regulation of cmyb expression caused by Indomethacin (Indo) could not be rescued by cyp2aa9 mRNA, and could not be exacerbated by cyp2aa9MO. (g–n) The PGE2 precursors AA, PGG2 or PGH2 could not rescue the defective HSC formation in cyp2aa9 morphants. (o) Illustration of the prostaglandin synthesis and PGE2/cAMP/PKA pathway involved in HSC development in zebrafish. Note that Cyp2aa9 acts at the step of PGH2-to-PGE2 conversion. Indo, non-selective cyclooxygenases inhibitor. Forskolin, cAMP activator. H89, PKA inhibitor. All the views of embryos are lateral, anterior left, dorsal top.
Discussion
This study reveals roles of a cytochrome P450 (CYP) member Cyp2aa9 in HSC development through promotion of PGE synthesis in zebrafish. Previous studies have shown that activation of Wnt signalling is required for HSC development31,32,33, and PGE2 interact with the Wnt pathways to regulate survival and proliferation of HSCs17. Our work found reduced Wnt activity and PGE2 synthesis in the cyp2aa9 morphants, suggesting a model that Cyp2aa9 acts upstream of PGE2 signalling to mediate the full effects of Wnt activation in HSC development.
The regulatory role of Cyp2aa9 in PGE2 synthesis and HSC development requires the prostaglandin precursor PGH2. However, mechanisms underlying regulation of PGE2 synthesis by Cyp2aa9 remain unclear. Currently, there is no metabolic study characterizing the activity of Cyp2aa9 with potential substrates and metabolites34,35. The predicted Cyp2aa9 protein structure contains two transmembrane domains. According to the crystal structure of Cyp2aa1, which has been constructed based on crystal structures of its closely related mammalian CYP2s family, the most variable region within the Cyp2aa cluster occurs close to the substrate access channels35. These structural information suggests that different Cyp2aa members indeed obtain differences in substrate specificity, and these differences are possibly controlled by the variable residues near the entrances of substrate channels. Although we cannot conclude that PGH2 is the endogenous, direct substrate of Cyp2aa9, this could be a possible mechanism through which Cyp2aa9 regulates PGE2 synthesis. Another enzyme Ptges has been demonstrated to convert PGH2 to PGE2, and the relationship between Cyp2aa9 and Ptges remains unclear. The second possible mechanism is that Cyp2aa9 plays roles in the prostaglandin metabolism in an indirect manner, for example acting as the co-enzyme of Ptges. These hypotheses and detailed working mechanisms of Cyp2aa9 need further investigations.
In zebrafish, the Cyp2aa family consists of 10 genes that are tandemly duplicated in a locus on chromosome 23. However, this genomic locus share poor synteny with mammalian CYP2 genes34,35, and the different Cyp2aa members may have distinct substrate specificities32. Previous studies have demonstrated that the Cyp2aas transform a broad range of xenobiotic substrates35,36, whereas little is known regarding their physiological functions. Our study has identified physiological roles of Cyp2aa9 in HSCs formation during embryonic development.
Cox1/2 are involved in the conversion of AA to PGH2, which is a common substrate for other prostanoids/prostaglandins (PGD2, PGE2, PGI2 and TXA2)37. Alterations in the Cox1/2 activity have an impact on endothelial-derived HSCs16. Similar to the expression pattern of cox2 (ref. 16), cyp2aa9 is also expressed in the mesoderm and tail region at 20 hpf and 36 hpf (See Supplementary Fig. S3, arrowheads). This expression pattern is spatially and temporally correlated with the budding of recognizable HSCs from VDA. The cyp2aa9 morphants exhibit reduced numbers of the runx1-positive cells and budding HSCs, in accordance with the regulatory roles of Cox2 in HSC development. Although HSC proliferation and survival are defective in the cyp2aa9 morphant (Fig. 4a–j), the budding process of residual HSCs still occur (Fig. 1i–m, and see Supplementary Videos 1 to 2). Our study has demonstrated the regulatory roles of Cyp2aa9 in HSC formation through regulation of PGE2 synthesis, providing further insights into the physiological functions of Cyp2aa family members and HSC development.
Materials and Methods
Ethics statement
All experimental protocols were approved by the School of Life Sciences, Southwest University (Chongqing, China), and the methods were carried out in accordance with the approved guidelines. The zebrafish facility and study were approved by the Institutional Review Board of Southwest University (Chongqing, China). Zebrafish were maintained in accordance with the Guidelines of Experimental Animal Welfare from Ministry of Science and Technology of People’s Republic of China (2006) and the Institutional Animal Care and Use Committee protocols from Southwest University (2007).
Fish lines
The zebrafish transgenic lines Tg(cmyb:GFP)11, Tg(TOP:dGFP)24, Tg(kdrl:GFP)38, Tg(gata1:DsRed)39 and Tg(CD41:GFP)40, Tg(kdrl:mCherryRas)41 were previously described. Embryos were treated with 0.003% 1-phenyl-2-thiourea (PTU, Sigma) from 24 hpf to inhibit pigmentation.
Morpholino and mRNA injections
The ATG-morpholino cyp2aa9MO (5′-CTTCAGGAGAGCCGTAAACATGATG-3′) and conMO (5′-CTaCAGGtGAGCgGTAtACAaGcTG-3′, lowercase letters denote mismatched bases) were synthesized (Gene-Tools, LLC). To generate the cyp2aa9MO-resistant mRNA, five nucleotides within the MO target site were changed without altering the encoding amino acids. The morpholino-resistant cyp2aa9 mRNA were synthesized from the SacII-linearized pCS2(+) constructs using the Message mMachine kit (Ambion) as previously described42. 5 ng of cyp2aa9MO and conMO, and 70 pg of cyp2aa9 mRNA were injected as previously described42.
Whole mount in situ hybridizations
The plasmids for probe synthesis were amplified and sub-cloned into the pGEMT-easy vector (Promega). Digoxigenin-labelled probes were generated and in situ hybridization was performed as previously described24,42,43. Images were captured using a SteREO Discovery 20 microscope equipped with Axio Vision Rel 4.8.2 software (Carl Zeiss).
TUNEL assay and antibody staining
TUNEL assay was carried out using the In Situ Cell Death Detection TMR red (Roche) according to the manufacturer’s instructions. The whole-mount antibody staining was performed as previously described44. For the permeability of the whole mount embryos, embryos were treated with Proteinase K (10 μg/ml) at room temperature (RT) for 15 minutes and then with acetone at −20 °C for 30 minutes. Embryos were incubated with antibodies against phospho-histone 3 (pH3, 1:500; Millipore), then incubated with Alexa fluorescent-conjugated secondary antibodies (1:500; Invitrogen) diluted in the blocking solution at 4 °C overnight. After washed with the PBST, embryos were preceded for mounting and imaging. Images were captured by ZEN2010 software equipped on an LSM780 confocal microscope (Carl Zeiss).
Treatment of embryos with chemicals
All analyses were performed at 36 hpf. Embryos were exposed to the following compounds in fish facility water from 10 hpf to 36 hpf17,45: 10 μM PGE2 (Sigma), 10 μM Indomethacin (Sigma); 0.5 μM Forskolin (Abcam, ab120058), 0.5 μM H89 (Abcam, ab120341), 20 μM Arachidonic acid (Sigma, A9673). DMSO carrier content was at 0.1%. Equivalent amount of DMSO was added to the media as control. For PGG2 (0.1 mg/ml, Cayman, 17010) and PGH2 (0.1 mg/ml, Santa Cruz, sc201266), embryos were injected with these chemicals in DMSO at the bud stage46.
Fluorescent microscopy and in vivo time-lapse imaging
Antibody stained or live embryos were mounted in 1.2% low melting point agarose and imaged using ZEN2010 software equipped on an LSM780 confocal microscope (Carl Zeiss). For time-lapse live imaging, zebrafish embryos were raised in the presence of 0.003% PTU to avoid pigmentation. Time-lapse images were captured using a 20× water immersion objective mounted on the LSM780 confocal microscope equipped with heating stage to maintain 28.5 °C. Z image stacks were collected every 10 minutes, and three-dimensional data sets were compiled using ZEN2010 software (Carl Zeiss).
ELISA measurement of PGE2
The embryonic lysates (50 embryos at 36 hpf in a tube) were prepared using the PGE2 ELISA kit (Invitrogen, KHL1701) according to the manufacturer’s instruction. The total PGE2 concentration was measured at a wavelength of 412 nm in the SPECTRA MAX 190 (Molecular Devices).
Additional Information
How to cite this article: Chen, J. et al. Cyp2aa9 regulates haematopoietic stem cell development in zebrafish. Sci. Rep. 6, 26608; doi: 10.1038/srep26608 (2016).
Supplementary Material
Acknowledgments
We thank Weijun Pan for discussions and technical assistance. This work was supported by the National Key Basic Research Program of China (2015CB942800), National Natural Science Foundation of China (91539201, 31330051, 31130038, 31371473, 31571508), and the 111 Program (B14037) (to L.L.).
Footnotes
Author Contributions L.L., D.Y. and J.C. designed the experimental strategy, analysed data, and wrote the manuscript. J.H. performed the chemical treatment experiment. L.L. provided the Tg(cmyb:GFP; kdrl:mCherryRas) transgenic line. J.C. performed all the other experiments in the study.
References
- Gering M. & Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev. Cell 8, 389–400 (2005). [DOI] [PubMed] [Google Scholar]
- Chen A. T. & Zon L. I. Zebrafish blood stem cells. J. Cell Biochem. 108, 35–42 (2009). [DOI] [PubMed] [Google Scholar]
- Müller A. M., Medvinsky A., Strouboulis J., Grosveld F. & Dzierzakt E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1, 291–301 (1994). [DOI] [PubMed] [Google Scholar]
- Boisset J. C. et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116–120 (2010). [DOI] [PubMed] [Google Scholar]
- Bahary N. & Zon L. I. Use of the Zebrafish (Danio rerio) to Define Hematopoiesis. STEM CELLS 16, 89–98 (1998). [DOI] [PubMed] [Google Scholar]
- Kissa K. & Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115 (2010). [DOI] [PubMed] [Google Scholar]
- Lam E. Y., Hall C. J., Crosier P. S., Crosier K. E. & Flores M. V. Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood 116, 909–914 (2010). [DOI] [PubMed] [Google Scholar]
- Tamplin O. J. et al. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 160, 241–252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan-Bogdan M. & Zon L. I. Hematopoiesis. Development 140, 2463–2467 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P. & Zon L. I. Stem cell migration: a zebrafish model. Methods Mol. Biol. 750, 157–168 (2011). [DOI] [PubMed] [Google Scholar]
- Murayama E. et al. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 25, 963–975 (2006). [DOI] [PubMed] [Google Scholar]
- Jin H., Xu J. & Wen Z. Migratory path of definitive hematopoietic stem/progenitor cells during zebrafish development. Blood 109, 5208–5214 (2007). [DOI] [PubMed] [Google Scholar]
- Lord A. M., North T. E. & Zon L. I. Prostaglandin E2: making more of your marrow. Cell Cycle 6, 3054–3057 (2007). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J. Pharmacol. Exp. Ther. 314, 522–532 (2005). [DOI] [PubMed] [Google Scholar]
- Madanayake T. W., Fidler T. P., Fresquez T. M., Bajaj N. & Rowland A. M. Cytochrome P450 2S1 depletion enhances cell proliferation and migration in bronchial epithelial cells, in part, through modulation of prostaglandin E(2) synthesis. Drug Metab. Dispos. 40, 2119–2125 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- North T. E. et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007–1011 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W. et al. Genetic Interaction of PGE2 and Wnt Signaling Regulates Developmental Specification of Stem Cells and Regeneration. Cell 136, 1136–1147 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M. F. et al. Biomechanical forces promote blood development through prostaglandin E2 and the cAMP-PKA signaling axis. J. Exp. Med. 212, 665–680 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. G. et al. Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res. 67, 6665–6674 (2007). [DOI] [PubMed] [Google Scholar]
- Alderton W. et al. Accumulation and metabolism of drugs and CYP probe substrates in zebrafish larvae. Xenobiotica. 40, 547–557 (2010). [DOI] [PubMed] [Google Scholar]
- Goldstone J. V. et al. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics 11, 643 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J. Y. et al. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development 134, 4147–4156 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robu M. E. et al. p53 Activation by Knockdown Technologies. PLoS Genet. 3, e78 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements W. K. et al. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature 474, 220–224 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann J., Bakkers J. & Schulte-Merker S. Early Endocardial Morphogenesis Requires Scl/Tal1. PLoS Genet. 3, e140 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Gomez G. A., Zhang B. & Lin S. Scl isoforms act downstream of etsrp to specify angioblasts and definitive hematopoietic stem cells. Blood 115, 5338–5346 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S., Jorniak T. & Lin S. Identification of novel vascular endothelial-specific genes by the microarray analysis of the zebrafish cloche mutants. Blood 106, 534–541 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C. C., Peterson Q. P., Hong J. Y. & Peterson R. T. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr. Biol. 16, 1366–1372 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift M. R. & Weinstein B. M. Arterial-venous specification during development. Circ. Res. 104, 576–588 (2009). [DOI] [PubMed] [Google Scholar]
- Dorsky R. I., Sheldahl L. C. & Moon R. T. A Transgenic Lef1/β-Catenin-Dependent Reporter Is Expressed in Spatially Restricted Domains throughout Zebrafish Development. Developmental Biology 241, 229–237 (2002). [DOI] [PubMed] [Google Scholar]
- Reya T. et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414 (2003). [DOI] [PubMed] [Google Scholar]
- Scheller M. et al. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 7, 1037–1047 (2006). [DOI] [PubMed] [Google Scholar]
- Kirstetter P., Anderson K., Porse B. T., Jacobsen S. E. & Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat. Immunol. 7, 1048–1056 (2006). [DOI] [PubMed] [Google Scholar]
- Kubota A. et al. Cytochrome P450 CYP2 genes in the common cormorant: Evolutionary relationships with 130 diapsid CYP2 clan sequences and chemical effects on their expression. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology 153, 280–289 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota A., Bainy A. C., Woodin B. R., Goldstone J. V. & Stegeman J. J. The cytochrome P450 2AA gene cluster in zebrafish (Danio rerio): expression of CYP2AA1 and CYP2AA2 and response to phenobarbital-type inducers. Toxicol. Appl. Pharmacol. 272, 172–179 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota A. et al. Role of pregnane X receptor and aryl hydrocarbon receptor in transcriptional regulation of pxr, CYP2, and CYP3 genes in developing zebrafish. Toxicol. Sci. 143, 398–407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser T., Yusuff S., Cheskis E., Pack M. A. & FitzGerald G. A. Developmental expression of functional cyclooxygenases in zebrafish. Proc. Natl. Acad. Sci. USA 99, 8418–8423 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai S., Horiguchi M. & Weinstein B. M. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev. Biol. 230, 278–301 (2001). [DOI] [PubMed] [Google Scholar]
- Long Q. M. et al. GATA-1 expression pattern can be recapitulated in living transgenic zebrafish using GFP reporter gene. Development 124, 4105–4111 (1997). [DOI] [PubMed] [Google Scholar]
- Bertrand J. Y., Kim A. D., Teng S. & Traver D. CD41+ cmyb+ precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis. Development 135, 1853–1862 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi N. C. et al. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 22, 734–739 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Ma J., Yang Y., Shi W. & Luo L. EpCAM is an endoderm-specific Wnt derepressor that licenses hepatic development. Dev. Cell 24, 543–553 (2013). [DOI] [PubMed] [Google Scholar]
- Liu X. et al. NF-kappaB and Snail1a coordinate the cell cycle with gastrulation. J. Cell Biol. 184, 805–815 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Lu H., Zou Q. & Luo L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology 146, 789–800 e788 (2014). [DOI] [PubMed] [Google Scholar]
- Leu B. H. & Schmidt J. T. Arachidonic acid as a retrograde signal controlling growth and dynamics of retinotectal arbors. Dev. Neurobiol. 68, 18–30 (2008). [DOI] [PubMed] [Google Scholar]
- Lewis G. P., Westwick J. & Williams T. J. Microvascular responses produced by the prostaglandin endoperoxide PGG2 in vivo [proceedings]. Br. J. Pharmacol. 59, 442P (1977). [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.