SUMMARY
While transcriptional controls over the size and relative position of cortical areas have been identified, less is known about regulators that direct acquisition of area-specific characteristics. Here, we report that the transcription factor Ctip1 functions in primary sensory areas to repress motor and activate sensory gene expression programs, enabling establishment of sharp molecular boundaries defining functional areas. In Ctip1 mutants, abnormal gene expression leads to aberrantly motorized corticocortical and corticofugal output connectivity. Ctip1 critically regulates differentiation of layer IV neurons, and selective loss of Ctip1 in cortex deprives thalamocortical axons of their receptive “sensory field” in layer IV, which normally provides a tangentially and radially defined compartment of dedicated synaptic territory. Therefore, although thalamocortical axons invade appropriate cortical regions, they are unable to organize into properly configured sensory maps. Together, these data identify Ctip1 as a critical control over sensory area development.
INTRODUCTION
The neocortex is responsible for processing and integrating all modalities of sensory information, generating precise motor output, and orchestrating higher-order cognitive tasks, a broad range of functions made possible by its tangential organization into specialized areas with distinct cytoarchitecture, connectivity, and patterns of gene expression (Rakic, 1988). In rodents, the neocortex is organized into four primary areas: motor (M1), somatosensory (S1), visual (V1), and auditory (A1). Area identity begins to be specified early in development by morphogens and signaling molecules secreted from patterning centers (O’Leary et al., 2007), which induce graded ventricular zone expression of several key transcription factors, including Emx1/Emx2, Couptf1, Pax6, Sp8, and Tbr2 (O’Leary et al., 2007; Woodworth et al., 2012; Elsen et al., 2013). These regulators appear to determine the final relative size and general position of cortical areas (Hamasaki et al., 2004; Armentano et al., 2007; Zembrzycki et al., 2013). Recent work has demonstrated that these early specification decisions are not made exclusively at the progenitor level, and that postmitotic neurons, while partially programmed, are sufficiently plastic to fully change their area identity (Alfano et al., 2014).
Substantially less is known about molecular controls that drive differentiation of cortical neurons toward particular area identities, including expression of area-specific genes, extension of axonal projections to area-specific targets, and interactions with thalamocortical input that enable its organization into modality-specific topographic maps. Recently, two transcription factors, Bhlhb5 and Lmo4, have been shown to influence acquisition of molecular area identity (Joshi et al., 2008; Sun et al., 2005). However, neither control is critical for establishment of cardinal features of area-specific connectivity, or for organization of sensory maps (Ross et al., 2012; Huang et al., 2009; Cederquist et al., 2013). A third transcription factor, Tbr1, is expressed by postmitotic neurons and regulates early gene expression gradients, but Tbr1 null mice die soon after birth, complicating investigation of later functions (Bedogni et al., 2010). Taken together, these studies begin to define the molecular mechanisms that direct area-specific differentiation in the cerebral cortex, but many important questions remain to be addressed.
Here, we identify that the transcription factor Ctip1 acts postmitotically to regulate acquisition of sensory area identity. Ctip1 was first described as an interacting partner of COUP transcription factors (COUP-TFs), which recruit Ctip1 to potentiate transcriptional repression (Avram et al., 2000), but it can also regulate gene expression independently of COUP-TFs by binding specific DNA sequence motifs through its zinc finger domains (Avram et al., 2002). Ctip1 has been most extensively studied in the hematopoietic system, where it controls specification of B-cells (Liu et al., 2003), and regulates the developmental switch from γ-globin to β-globin in red blood cells (Sankaran et al., 2008; Xu et al., 2011; Bauer et al., 2013). In the nervous system, Ctip1 regulates dorsal interneuron morphogenesis and sensory circuit formation in the spinal cord (John et al., 2012), and in vitro studies also support a role in hippocampal neuron dendrite outgrowth and axon branching (Kuo et al., 2010). Ctip1 also directs cortical projection neuron migration by regulating expression of Sema3c (Wiegreffe et al., 2015).
We report that Ctip1 is a critical postmitotic control over sensory area identity acquisition in the neocortex, including the establishment of sensory-specific gene expression and output connectivity, as well as the organization of thalamocortical input into sensory maps. Expression of CTIP1 in the cortical plate initially follows a high-caudomedial to low-rostrolateral gradient, and is further refined postnatally to be expressed most highly in primary sensory areas. In the absence of Ctip1 function, there is a striking failure of molecular differentiation in primary sensory areas, as indicated by absent or reduced expression of sensory genes, and by ectopic expression of motor genes. These molecular abnormalities result in abnormal area-specific connectivity of callosal, subcerebral, and corticothalamic projection neurons. In addition, Ctip1 regulates differentiation of layer IV neurons, and is, therefore, critical for the establishment of a receptive “sensory field” for thalamocortical input. Cortex-specific deletion of Ctip1 deprives thalamocortical afferents of this dedicated synaptic territory, resulting in apoptosis of thalamocortical neurons in sensory thalamic nuclei and severely disrupted organization of sensory maps in cortex. In an accompanying manuscript, we report that Ctip1 functions as a critical control over projection neuron subtype specification in deep layers (Woodworth et al., submitted to Cell Reports), thereby linking sensory area identity acquisition to the generation of area-appropriate proportions of neuronal subtypes (Ramón y Cajal, 1899; Brodmann, 1909).
RESULTS
Ctip1 is highly expressed in primary sensory areas during development
CTIP1 can first be detected by immunocytochemistry in the cortical plate at approximately E12.5, and, by E15.5, it is also evident in the postmitotic migratory neurons of the intermediate zone (Figure 1A–1B and Woodworth et al., submitted to Cell Reports). Notably, expression of CTIP1 initially follows a high-caudomedial to low-rostrolateral gradient in both the intermediate zone and the cortical plate, with an approximately 1.5-fold difference in mRNA expression along both axes (p<0.01; Figure 1A–1B; Figure S1A–S1B). Over the course of development, CTIP1 expression undergoes progressive refinement to primary sensory areas. By P7, CTIP1 expression is quite low in motor cortex, and staining becomes sparser across all layers, although areal differences between motor and sensory areas are most striking in layers VI, IV, and deeper II/III (Figure 1C–1D). In tangential sections through layer IV, CTIP1 distinctly labels primary somatosensory, visual, and auditory cortex (Figure 1E–1F). The areal boundaries of CTIP1 expression are similar to those of BHLHB5, with strongest expression of both transcription factors in primary sensory areas (Figure S1E–S1H), and are complementary to those of LMO4, which is expressed in motor cortex and higher-order visual areas (Figure S1I–S1L).
Figure 1. Neocortical expression of CTIP1 becomes areally patterned as the neocortex matures.

(A–B) At E15.5, CTIP1 is expressed by newly postmitotic projection neurons in a low-rostrolateral to high-caudomedial gradient across the cortex. In coronal sections (A), CTIP1 is expressed by postmitotic projection neurons at higher levels medially (A’) and lower levels laterally (A”), while in sagittal sections (B), CTIP1 is expressed at higher levels caudally (B’) and lower levels rostrally (B”).
(C–F) By P7, CTIP1 is highly expressed in primary sensory areas. In coronal sections, a distinct boundary of CTIP1 expression exists between primary somatosensory and primary motor cortex (C), with sparser expression of CTIP1 in primary motor cortex (D’) compared to primary sensory cortex (D”), especially in layer VI and deep layer II/III. In tangential sections of flattened cortex (E, F), CTIP1 expression clearly delineates primary sensory areas (V1, A1, and S1), and is absent from primary motor cortex (M1) and higher-order sensory areas.
A1, primary auditory cortex; M1, primary motor cortex; S1, primary somatosensory cortex; V1, primary visual cortex.
Scale bars: 500µm (D, F), 250µm (A, B), 25µm (A’–A”, B’–B” and D’–D”)
Acquisition of sensory molecular area identity is disrupted in the absence of Ctip1
The initial gradient and progressive tangential refinement of CTIP1 expression motivated us to investigate potential functions of Ctip1 in acquisition of area identity. Because Ctip1−/− mice die at P0 (Liu et al., 2003), but cortical arealization continues to unfold over the first week of postnatal development, we pursued loss-of-function studies using Ctip1fl/fl;Emx1-Cre cortex-specific conditional null mice, which survive to adulthood. As a first step toward interrogating area development in the absence of Ctip1 function in these cortex-specific mutants, we performed in situ hybridization (ISH) for genes with areally-restricted expression patterns.
In Ctip1fl/fl;Emx1-Cre brains, expression of motor-specific genes expands into sensory areas, but sensory-specific genes do not undergo complementary caudal shifts, though they are expressed at severely reduced levels. For example, expression of Epha7, which is normally present in motor cortex and higher order visual areas, but excluded from somatosensory cortex (Figure 2A), expands to fill this “gap” (Figure 2B). Conversely, expression of Efna5, normally quite high in somatosensory cortex, is strikingly and specifically reduced (Figure 2C–2D). Expression of the cell adhesion molecule Cdh6 expands caudally into higher order visual areas in layer I I/III, while expression of the transcription factor Id2 is lost in layer II/III in the same territory (Figure 2E–2H). Cyp26b1 and Bhlhe40, both of which are normally restricted to motor cortex, undergo striking caudal expansions in their expression domains (Figure 2I–2J, 2M–2N). In contrast, expression of Mdga1 and Cux1 in somatosensory cortex, is severely reduced, but does not appear to be shifted to more caudal positions (Figure 2K–2L, 2O–2P). Overall, primary sensory areas are partially motorized, with motor-specific genes expanding caudally and sensory areas failing to express sensory-specific genes at appropriately high levels.
Figure 2. Establishment of precise gene expression programs delineating motor and sensory areas is severely disrupted in Ctip1fl/fl;Emx1-Cre cortex.

(A–P) In the absence of Ctip1 function, molecular area identity in somatosensory cortex is severely disrupted. EphA7, largely excluded from wild-type somatosensory cortex (A), is extensively expressed in Ctip1fl/fl;Emx1-Cre somatosensory cortex (B). The sharp caudal boundaries of wild-type Efna5, Cdh6, Cyp26b1, and Bhlhe40 expression (C, E, I, M) are replaced by gradual gradients that aberrantly invade occipital cortex in Ctip1fl/fl;Emx1-Cre brains (D, F, J, N). Superficial-layer expression of Id2 in wild-type somatosensory and visual cortex (G) is lost in Ctip1fl/fl;Emx1-Cre cortex (H), while strong expression of Mdga1 and Cux1 in wild-type somatosensory cortex (K, O) is lost or greatly reduced in Ctip1fl/fl;Emx1-Cre cortex (L, P). (Q–T) Expression domains of Bhlhb5 and Lmo4 are strikingly abnormal in the absence of Ctip1 function. At P7, when area identity refinement is normally complete, distinct primary and higher-order visual areas in wild-type cortex (Q) are not discernible in Ctip1fl/fl;Emx1-Cre cortex (R) by Lmo4-driven LacZ expression. Bhlhb5 expression is almost absent from Ctip1fl/fl;Emx1-Cre cortex (T), compared with sharply-delineated sensory area expression in wild-type cortex (S). (U–V) Ctip1fl/fl;Emx1-Cre parietal cortex expresses genes characteristic of wild-type motor cortex, but not somatosensory cortex. We compared gene expression between wild-type frontal (M1) and parietal (S1) cortex at P4 by RNA-seq, designating genes enriched in one area as M1 or S1 genes, respectively (U). When wild-type and Ctip1fl/fl;Emx1-Cre parietal cortex are compared, 298 of 329 differentially expressed M1 genes are increased in expression in Ctip1 conditional mutants, and 210 of 238 differentially expressed S1 genes are decreased in expression (V).
A1, primary auditory cortex; M1, primary motor cortex; S1, primary somatosensory cortex; V1, primary visual cortex.
Scale bars: 500µm, all panels.
To monitor acquisition of molecular area identity globally throughout cortex, we performed whole-mount β-galactosidase staining on brains carrying LacZ reporter alleles for either Bhlhb5 or Lmo4 (Feng et al., 2006; Deng et al., 2010). In wild-type mice at P7, Bhlhb5 is expressed only in primary sensory areas (Figure 2S), while Lmo4 is highly expressed rostrally in motor cortex, medially in cingulate cortex, and caudally in higher-order visual areas, but is excluded from primary sensory areas, with sharp boundaries between these regions (Figure 2Q). Strikingly, in Ctip1 conditional null mice, expression of Bhlhb5 becomes faint and diffuse, such that primary somatosensory, visual, and auditory cortex are no longer discernible (Figure 2T). The boundary between sensory and motor cortex is still present, and its position is not shifted either rostrally or caudally. In contrast, expression of Lmo4 in motor and cingulate cortex expands into somatosensory territory, such that the boundary between them shifts caudally (Figure 2R). Importantly, establishment of molecular boundaries is comparatively preserved in the absence of either Lmo4 or Bhlhb5 function, using the same reporter alleles in Lmo4fl/fl;Emx1-Cre and Bhlhb5fl/fl;Emx1-Cre mice (Figure S2Q–S2V). Taken together, our data indicate that Ctip1 acts after the initial positions of cortical areas have been at least partially established, and plays a critical role repressing motor identity and driving sensory differentiation.
To more rigorously examine gene expression changes, we performed RNA-seq on microdissected cortical areas. First, we compared gene expression between microdissected frontal (motor) and parietal (somatosensory) cortex in wild-type mice at P4, to prospectively identify genes more highly expressed in each area, and designated these differentially expressed transcripts M1 genes and S1 genes, respectively (Figure 2U; Table S1). We then compared gene expression between microdissected wild-type parietal cortex and Ctip1fl/fl;Emx1-Cre parietal cortex, and specifically examined how loss of Ctip1 function affects expression of M1 genes and S1 genes (Figure 2V; Table S2). Of 329 M1 genes abnormally expressed in parietal cortex of Ctip1 conditional nulls, 298 are upregulated and 31 are downregulated. Conversely, of 238 S1 genes abnormally expressed in parietal cortex of Ctip1 conditional nulls, 210 are downregulated and 28 are upregulated. These data strongly reinforce the conclusion that Ctip1 acts in somatosensory cortex to repress expression of motor genes and activate expression of sensory genes.
Ctip1 functions cell-autonomously to establish sensory-area-specific callosal connectivity
Callosal projection neurons (CPN) extend axons to mirror-image locations in corresponding cortical areas of the contralateral hemisphere, enabling highly organized inter-hemispheric transfer and integration of motor, somatosensory, visual, and auditory information (Richards et. al., 2004). Because Ctip1 is highly expressed by CPN, and because CPN molecular area identity is severely disrupted in Ctip1 mutants, we hypothesized that Ctip1 might be important for establishment of areally precise connectivity by sensory cortex CPN. We first investigated the effects of global loss of Ctip1 function throughout cortex by focally injecting adeno-associated virus expressing EGFP (AAV-EGFP) into somatosensory cortex of Ctip1fl/fl;Emx1-Cre and control mice (Figure S3). In Ctip1fl/fl;Emx1-Cre brains, CPN axons from S1 continue to project to approximately correct coordinates. However, the distinct clusters of innervation to the forelimb (S1FL) and upper lip (S1ULp) regions seen in wild-type mice are no longer discernible and innervation becomes more uniform across the target field. This lack of precision in targeting might result from a corresponding lack of definition in the expression gradients of axon guidance molecules, such as Epha7, Efna5, and Cdh6 (Figure 2A–2F).
Because any gradients controlling homotypic connectivity would change symmetrically in the absence of Ctip1 function across the cortex, affecting both CPN and their contralateral targets, CPN targeting is less sharply defined, but relatively intact, as we observed in the experiments described above. Therefore, in a second set of experiments, we disrupted Ctip1 expression unilaterally, to investigate whether this might create an areal mismatch between the two hemispheres, resulting in more aberrant re-routing of CPN axons. We electroporated Cre and floxed(STOP)-EGFP expression constructs into the ventricular zone of wild-type and Ctip1fl/fl embryos at E14.5, and collected brains with matched S1 electroporations at P7 (Figure 3A). In control experiments, Cre-electroporated wild-type CPN project almost exclusively homotypically to S1, with distinct clusters of innervation in S1FL and S1ULp (Figure 3B). In striking contrast, Cre-electroporated Ctip1fl/fl CPN project across the entire medio-lateral extent of the contralateral hemisphere, without distinct clusters at S1FL and S1ULp (Figure 3C). Innervation of S1FL and S1ULp by S1 CPN lacking Ctip1 is significantly reduced, and their axons are misrouted to M1/2 and InsCx (p<0.05 in each region; Figure 3D–3F). Taken together with our gene expression data, these results indicate that Ctip1 is critical for proper areal specification of somatosensory cortex CPN, enabling precise targeting and refinement of connectivity between homotypic locations in the two cerebral hemispheres.
Figure 3. In the absence of Ctip1 function, callosal projection neurons fail to project to homotypic locations in the contralateral hemisphere.

(A) Schematic of experimental approach. Embryos were electroporated at E14.5 with CAG-Cre and CAG-floxed(STOP)-EGFP plasmids, and brains were collected at P7. For quantification, images of electroporated and contralateral hemispheres of cortex were divided into 200 bins from the M1/S1FL boundary medially to the Ins. Ctx./Pir. Ctx. boundary laterally. Fluorescence intensity was quantified in each bin using ImageJ.
(B–C) While axons of Cre-electroporated wild-type CPN cluster in S1FL and S1ULp (B), axons of Cre-electroporated Ctip1fl/fl CPN exhibit little areal specificity (C).
(D–F) Quantification of data in B–C. Although Cre electroporations into wild-type and Ctip1fl/flbrains were similar in size and location (D), axons of electroporated wild-type neurons clearly target specific areas of contralateral cortex preferentially, while axons of electroporated Ctip1fl/flneurons distribute evenly across the medio-lateral extent of cortex (E). Significantly more axons from Cre-electroporated Ctip1fl/fl neurons aberrantly target motor cortex and insular cortex, and significantly fewer target forelimb and upper lip regions of primary somatosensory cortex (F). *, p<0.05.
(G–P) High magnification images of axons from B–C. Wild-type axons preferentially innervate S1FL and S1ULp (I, M), and avoid M1/M2, S1J, and Ins. Ctx. (G, K, O), while Cre-electoroporated Ctip1fl/fl axons innervate all areas approximately equally (H, J, L, N, P). M1, primary motor cortex; M2, secondary motor cortex; S1FL, primary somatosensory cortex, forelimb region; S1J, primary somatosensory cortex, jaw region; S1ULp, primary somatosensory cortex, upper lip region; S2, secondary somatosensory cortex; Ins. Ctx., insular cortex; Pir. Ctx., piriform cortex; CL, contralateral.
Scale bars: 100µm (B, C), 10µm (G–P). Data are represented as mean ± SEM.
Ctip1 represses motor identity in sensory area corticothalamic projection neurons
The strong differential expression of CTIP1 between motor and sensory cortex in layer VI prompted us to investigate whether Ctip1 might also direct acquisition of sensory area identity by corticothalamic projection neurons (CThPN), instructing them to establish connections with sensory thalamic nuclei. To investigate this connectivity, we anterogradely labeled CThPN axons from M1 and S1 of Ctip1fl/fl;Emx1-Cre and wild-type pups at P1 (Figure 4A) and examined thalamic sections at P7. In wild-type mice, motor CThPN project to the ventral lateral nucleus (VL), while somatosensory CThPN project to the ventral posterior nucleus (VP), with little overlap between the axons of these two populations (Figure 4B–4C). In Ctip1fl/fl;Emx1-Cre mice, motor CThPN project normally to VA/VL (Figure 4D–4E), consistent with the lack of significant molecular abnormalities in motor cortex. In contrast, although a subset of somatosensory CThPN still project to VP in the absence of Ctip1 function, many aberrantly innervate VL, leading to a striking increase in the overlap of somatosensory and motor cortical input in motor thalamus (p<0.01; Figure 4F), consistent with Ctip1 functioning to repress motor identity in sensory areas. To directly investigate acquisition of molecular area identity by CThPN, we examined genes specifically expressed in either S1 or M1 of layer VI. We find that, in the absence of Ctip1 function, there is a striking expansion in the expression of motor-specific CThPN genes (Figure S4A–F), with a reciprocal reduction in expression of sensory CThPN genes (Figure S4G–S4H). Taken together, these data indicate that Ctip1 is required to repress motor identity in sensory CThPN.
Figure 4. Ctip1 represses motor identity in sensory cortex subcerebral and corticothalamic projection neurons.
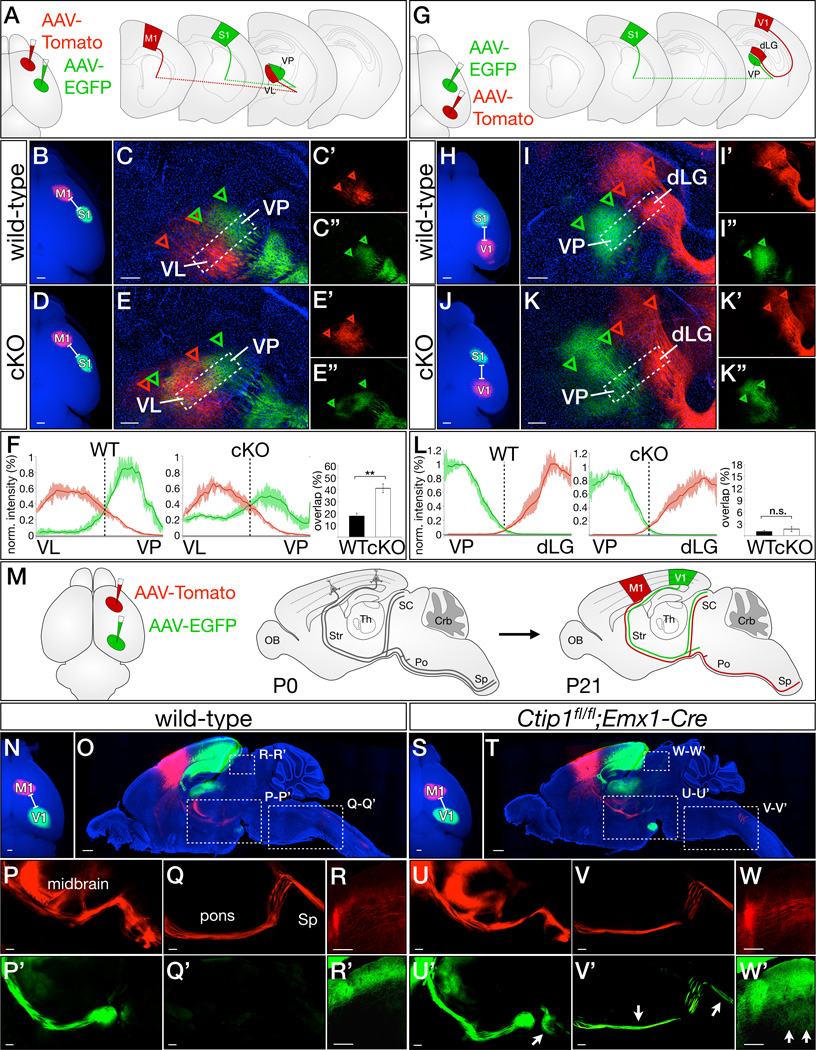
(A–F) Ctip1 is necessary for corticothalamic axons from somatosensory cortex to repress motor identity and project specifically to sensory thalamic nuclei. Schematic of AAV labeling approach (A). In wild-type mice, axons anterogradely labeled by AAV injection into motor or somatosensory cortex sort into VL (motor; red) or VP (sensory, green) (B, C), but axons from Ctip1fl/fl;Emx1-Cre somatosensory cortex project to both VL and VP (D, E). Extent of AAV-tdTomato and AAV-EGFP labeling is indicated by red and green arrowheads, respectively.
Quantification of normalized fluorescence intensity, demonstrating significantly more overlap between motor and somatosensory injections in Ctip1fl/fl;Emx1-Cre mutants (F). **, p<0.01 (G–L) Ctip1 is not required for corticothalamic axons from distinct sensory areas to sort into appropriate sensory thalamic nuclei. Schematic of AAV labeling approach (G). In wild-type mice, axons anterogradely labeled by AAV injection into somatosensory and visual cortex project to distinct thalamic nuclei in both wild-type (H–I) and Ctip1fl/fl;Emx1-Cre (J–K) mice. Visual cortex CThPN (red) project to dLG, while somatosensory cortex CThPN (green) project to VP. Extent of AAV-tdTomato and AAV-EGFP labeling is indicated by red and green arrowheads, respectively. Quantification of normalized fluorescence intensity, demonstrating minimal overlap between somatosensory and visual injections in both wild-type mice and Ctip1fl/fl;Emx1-Cre mutants (L).
(M–W) Ctip1 is required for repression of motor identity by visual subcerebral projection neurons. Schematic of labeling approach (M). P1 injection of AAV-tdTomato into motor cortex and AAV-EGFP into visual cortex labels descending projections in P21 wild-type (N–O) and Ctip1fl/fl;Emx1-Cre (S–T) cortex. Motor cortex SCPN from both wild-type and Ctip1fl/fl;Emx1-Cre brains project to the cerebral peduncle in the midbrain (P, U), and continue into the pons and spinal cord (Q, V). In wild-type brain, visual cortex SCPN do not project beyond the midbrain (arrow in P’, Q’). However, in Ctip1fl/fl;Emx1-Cre brains, visual cortex SCPN aberrantly project past the midbrain to enter the pons (arrow in U’), then continue into the spinal cord (V’). Axons from motor cortex SCPN project to deep layers of the superior colliculus in wild-type and Ctip1fl/fl;Emx1-Cre brains (R and W). Axons from visual cortex SCPN project exclusively to superficial layers of the superior colliculus in wild-type brains (R’). However, while visual cortex SCPN still project to superficial layers of the superior colliculus in Ctip1fl/fl;Emx1-Cre brains, they also aberrantly innervate deep layers (arrows in W’).
dLG, dorsal lateral geniculate nucleus of thalamus; M1, primary motor cortex; S1, primary somatosensory cortex; SC, superior colliculus; Sp, spinal cord; V1, primary visual cortex; VL, ventral lateral nucleus of thalamus; VP, ventral posterior nucleus of thalamus
Scale bars: 500µm (B, H), 200µm (D–F, I–K). Data are represented as mean ± SEM.
In contrast, anterograde labeling of S1 and V1 (Figure 4G) reveals proper segregation of somatosensory and visual axons in both wild-type and Ctip1fl/fl;Emx1-Cre brains, with somatosensory CThPN projecting to VP and visual CThPN projecting to the dorsolateral geniculate nucleus (Figure 4H–4K) and no significant difference in the overlap of somatosensory and visual input (Figure 4L). These results are consistent with the equivalently high levels of CTIP1 expression by CThPN in S1 and V1, which point toward a common function across all primary sensory areas. Expression of CTIP1 is necessary to prevent somatosensory CThPN from establishing connections with motor thalamic nuclei, but not for somatosensory and visual CThPN to establish connections with appropriate modality-specific sensory thalamic nuclei. Taken together, these data support the interpretation that Ctip1 is specifically required for the broad distinction between CThPN specialized for motor output and those specialized for sensory output, but is not required for CThPN to become specialized for distinct sensory modalities.
Ctip1 represses motor identity in sensory area subcerebral projection neurons
Initially, subcerebral projection neurons (SCPN) in all areas of cortex send axons to the spinal cord, establishing collaterals to midbrain, pontine, and cerebellar nuclei along the way. This connectivity undergoes extensive pruning in the early postnatal period (P1-P21), such that SCPN in motor cortex maintain projections to the spinal cord and to the deep layers of the superior colliculus, while SCPN in visual cortex prune their projections to the spinal cord, and maintain projections to the superficial layers of the superior colliculus (Stanfield et al., 1982). Interestingly, while CTIP1 is progressively excluded from SCPN in motor cortex (Woodworth et al., submitted to Cell Reports), it continues to be expressed at low levels by SCPN in somatosensory and visual cortex, indicating that it might function in area-specific SCPN collateral pruning. At P4, only 6±2% of SCPN in motor cortex express CTIP1, while 73±3% of SCPN in sensory cortex express CTIP1 (Figure S4I–S4K).
To investigate the hypothesis that Ctip1 might repress motor identity in sensory SCPN, we anterogradely labeled M1 and V1 SCPN in wild-type and Ctip1fl/fl;Emx1-Cre mice (Figure 4M). In wild-type mice, motor cortex SCPN project to the spinal cord and to the deep layers of the superior colliculus (Figure 4N–4R), and this precise area-specific connectivity is also evident in Ctip1fl/fl;Emx1-Cre mice (Figure 4S–4W). Visual cortex SCPN rarely extend axons beyond the midbrain in wild-type mice (Figure 4P ’-4Q’). In striking contrast, they aberrantly project into pons, through the medulla, and enter the dorsal corticospinal tract in Ctip1fl/fl;Emx1-Cre mice (Figure 4U’–4V’). We quantified this phenotype by retrogradely labeling from the dorsal corticospinal tract at P21: an equal number of neurons in motor cortex maintain spinal projections in the absence of Ctip1 function (Figure S4L–S4N), but there is a five-fold increase in somatosensory cortex (p<0.05), and a 20-fold increase in visual cortex (p<0.01; Figure S4O–S4T). These results clearly indicate that output connectivity of somatosensory and visual cortex SCPN becomes partially motorized in the absence of Ctip1 function. Further, expression of genes specific to SCPN in motor areas increases significantly in Ctip1fl/fl;Emx1-Cre sensory areas compared with wild-type (p<0.01; Figure S4U–Z). These data indicate that SCPN in sensory cortex become motorized in the absence of Ctip1 function, and that this change in their transcriptionally specified area identity causes corresponding changes in connectivity.
Postnatal overexpression of Ctip1 is sufficient to repress motor identity in subcerebral projection neurons
We next investigated whether Ctip1 is sufficient to repress motor identity in postmitotic SCPN, performing gain-of-function in utero electroporation experiments. Because CAG-promoter-driven overexpression of Ctip1 starting in progenitors drastically reduces SCPN specification (Woodworth et al., submitted to Cell Reports), we used a tamoxifen-inducible form of Cre recombinase (ERT2-Cre-ERT2) to initiate expression of EGFP and/or Ctip1 at P2, after subtype identity has already been established (Figure S5A–S5C). We find that postnatal misexpression of Ctip1 in motor cortex dramatically changes the pruning decisions of SCPN in this area, which normally do not express Ctip1 during early postnatal development. While most SCPN electroporated with EGFP maintain their spinal projections at P21 (Figure 5D–5G), remarkably few EGFP/Ctip1-electroporated SCPN maintain their spinal projections at P21 (Figure 5H–5K). This four-fold reduction in projections (p<0.01; Figure 5L) indicates that downregulation of Ctip1 by SCPN in motor cortex during normal development is necessary to maintain a spinal projection. Together, our loss- and gain-of-function data demonstrate that Ctip1 represses motor identity in sensory area SCPN, instructing appropriate gene expression and final patterns of connectivity.
Figure 5. Postnatal overexpression of Ctip1 causes motor area SCPN to prune their spinal axons by P21.

(A–C) Schematic of experimental approach (A). Control wild-type embryos were electroporated at E12.5 with CAG-ERT2CreERT2, CAG-FLEX-FlpO, and CAG-FRT-STOP-FRT-EGFP, while experimental embryos were electroporated with these same plasmids, as well as CAG-FRT-STOP-FRT-Ctip1. Electroporated pups were injected with tamoxifen at P2, enabling ERT2-CreERT2 to invert the orientation of FlpO, activating FlpO expression (B). FlpO then activates expression of both CTIP1 and EGFP in turn (C). Mice were perfused at P21.
(D–L) In control animals, motor cortex electroporations (D–E) result in many axons projecting through the cerebral peduncle (F), and continuing into cervical spinal cord (G). In experimental animals, motor cortex electroporations (H and I) similarly result in many axons projecting through the cerebral peduncle (J). However, very few axons continue into the cervical spinal cord (K), indicating that most Ctip1-overexpressing motor cortex SCPN have pruned their spinal collaterals. Quantification (L). n.s., not significant; **, p<0.01
Scale bars: 500µm (D, H), 200µm (E–G, I–K). Data are represented as mean ± SEM.
Thalamocortical input fails to organize into sensory maps in the absence of cortical Ctip1 function
Because sensory areas undergo striking motorization of output connectivity in the absence of Ctip1 function, we next examined whether organization of sensory thalamocortical input is also affected. Using serotonin (5HT) immunohistochemistry, we stained thalamocortical afferents, revealing sensory maps on tangential sections through layer IV of cortex. In wild-type brains, the triangular visual map is positioned in occipital cortex, and parietal cortex contains the somatotopic representations of the hindlimb, forelimb, and lower jaw, as well as the field of vibrissal barrels, arranged precisely to reflect the positions of whiskers on the snout (Figure 6A). In Ctip1fl/fl;Emx1-Cre mice, the entire sensory map is strikingly disorganized (Figure 6B). Blurry and imprecise representations of the hindlimb, forelimb, and lower jaw are still recognizable, but there are no similarly identifiable remnants of the visual map or the posteromedial barrel subfield. In spite of this severe disorganization, the overall size of the sensory and motor domains does not change (Figure 6C). Importantly, areal refinement of Rorb and Cdh8 expression, which depends on thalamocortical input (Chou et al., 2013; Vue et al., 2013), continues to reflect the overall configuration of sensory maps in Ctip1fl/fl;Emx1-Cre mice (Figure S6A–S6E). These data demonstrate that cortex-specific deletion of Ctip1 is sufficient to dramatically disrupt thalamocortical organization into sensory maps, but does not affect the relative size of the specified sensory and motor domains, or the ability of cortical neurons to modify their gene expression in response to signals from thalamocortical axons.
Figure 6. Cortical Ctip1 function is required for organization of thalamocortical input into sensory maps.

(A–C) In wild-type P7 cortex, thalamocortical afferents cluster into highly organized maps in somatosensory, visual, and auditory cortex (A), as revealed by serotonin (5HT) immunostaining in flattened cortices. In the absence of cortical Ctip1 function, however, almost no discernible organization exists (B). Despite the extreme disorganization of thalamocortical input to Ctip1fl/fl;Emx1-Cre cortex, the relative size of the motor and sensory domains does not change (C).
(D–I) Sensory maps in thalamic and brainstem nuclei that convey information from whiskers develop in the absence of cortical Ctip1 function, but later attenuate. Cytochrome oxidase-stained barrelettes in brainstem (schematic, D) are well-organized in both wild-type and Ctip1fl/fl;Emx1-Cre mice (E, F). Barreloids in the ventral posterior nucleus of thalamus (schematic, G) are smaller in Ctip1fl/fl;Emx1-Cre (I) compared with wild-type (H), potentially as a result of “top-down” plasticity, though clear organization is still evident.
(J–S) Thalamic barreloids atrophy in the absence of cortical Ctip1 function following initial appropriate development. At P0, VB and dLG are the same size in wild-type and Ctip1fl/fl;Emx1-Cre thalamus (outlines, J–K), but by P7, Ctip1fl/fl;Emx1-Cre VB and dLG have atrophied in comparison with wild-type VB and dLG (outlines, L–M). Quantification (N). This atrophy is due in part to apoptosis, which occurs at higher rates in Ctip1fl/fl;Emx1-Cre sensory thalamus than in wild-type (O–S). **, p<0.01.
A1, auditory cortex; CC3, cleaved caspase 3; dLG, dorsal lateral geniculate nucleus of thalamus; fl, forelimb; hl, hindlimb; ll, lower lip; lw, large whiskers; M, motor cortex; S, sensory cortex; SpV, spinal trigeminal nucleus; sw, small whiskers; V1, primary visual cortex; VP, ventral posterior nucleus of thalamus
Scale bars: 500µm (A and B), 200µm (E–I), 100µm (J–P). Data are represented as mean ± SEM.
We next investigated the possibility that abnormal subplate development in Ctip1fl/fl;Emx1-Cre mice might impair sensory map formation by disrupting navigation or topographic organization of thalamocortical axons en route to cortex, as successful invasion and preordering are both critical for subsequent map formation (Lokmane et al., 2013). First, we examined the earliest stages of thalamocortical development by genetically labeling sensory thalamic nuclei in E14.5 wild-type and Ctip1 null embryos using Gbx2-CreERT2. These anterograde labeling experiments demonstrate that thalamocortical axons project to cortex along a normal trajectory, and that a comparable number have reached the cortical plate at E14.5 in both wild-type and Ctip1 null mice (Figure S6F–S6J). We also directly visualized thalamocortical input at P1 on flattened cortices. In wild-type mice, diffuse 5HT staining roughly delineates presumptive V1, A1, and S1 (Figure S6K). Although organization of thalamocortical input is already less defined in Ctip1fl/fl;Emx1-Cre brains at P1, the boundary between the motor and sensory domains remains sharp (Figure S6L). These data indicate that thalamocortical axons successfully reach appropriate regions of cortex in the absence of cortical Ctip1 function, but are subsequently unable to refine into topographically-organized sensory maps.
To address the possibility that Ctip1 function in Emx1-expressing cells outside the cerebral cortex might cause or contribute to this phenotype, we investigated formation of sensory maps in the brainstem and thalamus. We find that brainstem and thalamic sensory maps are indistinguishable in wild-type and Ctip1fl/fl;Emx1-Cre brains (Figure 6D–6I), although the VP nucleus of thalamus is smaller in Ctip1fl/fl;Emx1-Cre brains, making fine details more difficult to discern. Thalamic nuclei are normally sized in Ctip1fl/fl;Emx1-Cre brains at P0, but shrink approximately 40% by P7 (Figure 6J–6N; p<0.01), possibly due to atrophy via top-down remodeling (Zembrzycki et al., 2013). Indeed, there is increased cell death by apoptosis in sensory, but not motor, thalamic nuclei (Figure 6O–6S and data not shown). Taken together, these data indicate that, despite reaching appropriate cortical areas, sensory thalamocortical axons are unable to organize into topographic maps in the absence of cortical Ctip1 function.
Ctip1 regulates differentiation of layer IV neurons, and is necessary for their proper integration into barrels
Thalamocortical axons interact extensively with layer IV neurons as they organize into precise topographic representations within a permissive sensory cortical field established under precise regulation by cortex-intrinsic transcriptional programs. We hypothesized that sensory maps might fail to form in Ctip1fl/fl;Emx1-Cre mice due to abnormal development of this permissive sensory field more broadly, and of layer IV neurons in particular, since nearly all layer IV granule neurons in barrel cortex express Ctip1 (83±3%; Figure S7A–S7C). In the absence of Ctip1 function, cytoarchitectural organization of layer IV neurons in barrel cortex is entirely lost (Figure 7A–7D). Although layer IV remains readily identifiable by Rorb expression in coronal sections, we find strikingly reduced or entirely absent expression of other layer IV-specific genes (Figure 7E–7L). Concomitantly, expression of several genes that are normally excluded from layer IV markedly increases (Figure S7D–S7I). To investigate whether these molecular abnormalities affect compartmentalization of input within cortex, we combined 5HT immunostaining, to label thalamocortical input, with contralateral injection of AAV-EGFP, to label callosal input (Figure 7M). In wild-type mice, thalamocortical input clusters into distinct barrels, while callosal input from contralateral S1 is clearly excluded from the barrel compartment (Figure 7N–7R). In Ctip1fl/fl;Emx1-Cre mice, thalamocortical input is still restricted to layer IV, though barrels are absent and staining is markedly reduced; even more strikingly, there is robust callosal input to layer IV (Figure 7P–7R). These data indicate that Ctip1 regulates expression of layer IV-specific genes, and is required for layer IV neurons to select specific sensory thalamocortical input over contralateral cortical input and to successfully establish the appropriate cytoarchitectural organization to receive this input.
Figure 7. Layer IV granule neuron differentiation is impaired in the absence of Ctip1 function, and cell-autonomous loss of Ctip1 function leads to their exclusion from barrels.

(A–D) At P7, wild-type layer IV granular neurons are oriented into barrel structures visible by DAPI staining (A) or Rorb expression (C) in tangential sections. In layer IV of Ctip1fl/fl;Emx1-Cre cortex, neurons do not organize into barrels (B) or express Rorb in periodic clusters (D). (E–L) Genes expressed in barrel cortex by wild-type layer IV granule neurons, including Rorb (E), Mdga1 (F), Grm4 (G), and Dbp (H), are expressed in Ctip1fl/fl;Emx1-Cre barrel cortex at reduced levels (I) or absent entirely (J–L).
(M–R) In the absence of Ctip1 function, axons of contralateral CPN compete with thalamocortical axons for synaptic partners in layer IV. Schematic of approach (M). CPN terminals are labeled with AAV-EGFP injected into the contralateral hemisphere, and thalamocortical axon terminals (ThCNVB) are labeled with 5HT immunostaining. Wild-type layer IV granule neurons are receptive to synaptic contact with thalamocortical axons (N) and avoid contact with contralaterally-projecting CPN (P). In contrast, Ctip1fl/fl;Emx1-Cre layer IV neurons attract few ThCNVB axon terminals (O), and layer IV is instead occupied by CPN axon terminals (Q). Quantification of 5HT and EGFP fluorescence as percentage of maximum fluorescence intensity (R).
(S–W) Mosaic loss of Ctip1 function causes layer IV neurons and their dendrites to be excluded from barrels. Wild-type layer IV neurons electroporated with Cre primarily populate barrels (T-T”, W-W”), but Ctip1fl/fl neurons electroporated with Cre are excluded from barrels (U-U”, X-X”). Schematic of experimental approach (S) and quantification (V, measuring across dashed boxes in W’, X’).
Scale bars: 200µm (A–D and T–U), 100µm (E–Q). Data are represented as mean ± SEM.
To gain further insight into the abnormal development of layer IV neurons in Ctip1fl/fl;Emx1-Cre mice, we investigated the effects of cell-autonomous loss of Ctip1 function. For these experiments, we electroporated CAG-Cre and CAG-floxed(STOP)-EGFP expression constructs into somatosensory cortex of Ctip1fl/fl embryos or wild-type controls at E14.5 to target layer IV neurons (Figure 7S). Cre-electroporated neurons in wild-type brains adopt an unbiased distribution across barrel cortex, although more electroporated neurons are present in cell-dense barrel walls (Figure 7T). Remarkably, Cre-electroporated neurons in Ctip1fl/fl brains completely avoid barrels, and instead position themselves exclusively between barrels, within the septae (Figure 7U). In addition, while most wild-type layer IV neurons orient their dendrites toward the center of each barrel to synapse with thalamocortical axons (Figure 7W, 7V), the dendritic trees of Ctip1 null neurons accumulate in septae, completely avoiding barrels (Figure 7X, 7V). We also performed electroporations at E13.5 to evaluate radial positioning of electroporated neurons across layers. While Cre-electroporated neurons in wild-type brains found at high density in layer IV, Cre-electroporated neurons in Ctip1fl/fl brains primarily found deep or superficial to layer IV, with the few neurons in layer IV residing primarily between barrels, within the septae (Figure S7O–S7Q). Importantly, mosaic loss of Ctip1 function in a subset of layer IV neurons does not interfere with the ability of thalamocortical axons to organize into properly configured sensory maps (Figure S7J–S7N). These experiments indicate that Ctip1 function is necessary for the integration of layer IV neurons into barrels, and for their assembly into the circuits that receive sensory information from thalamocortical afferents.
Ctip1 functions in layer IV to establish a permissive cortical field for sensory map formation
To determine whether Ctip1 acts directly downstream of transcriptional programs that promote specification of sensory area identity, we investigated its genetic interactions with Couptf1 (Armentano et al., 2007; Alfano et al. 2014). Most CTIP1-positive neurons in sensory areas also express COUPTF1 (Figure S8A–S8E; 71±4%), suggesting that Couptf1, which is expressed in progenitors, might activate expression of Ctip1. To test this hypothesis, we analyzed CTIP1 immunostaining in Couptf1fl/fl;Emx1-Cre mice, and find that it is reduced in areas that have been respecified to acquire motor identity, but preserved in the caudally displaced sensory domain (Figure S8F–S8G). These data indicate that Ctip1 expression is responsive to changes in area identity specification, but is expressed within the sensory domain independently of Couptf1 function. Conversely, in Ctip1fl/fl;Emx1-Cre mice, COUPTF1 expression is clearly preserved, though at somewhat reduced levels (Figure S8H–S8I). These data indicate that regulation of sensory area identity acquisition by Ctip1 increases Couptf1 expression levels.
Since neither transcription factor is required for expression of the other, we investigated their epistatic relationship by generating double conditional null mice (Ctip1fl/fl;Couptf1fl/fl;Emx1-Cre). We focused on sensory map formation, because the single conditional mutants have largely non-overlapping phenotypes, with caudal displacement of thalamocortical input in Couptf1fl/fl;Emx1-Cre mice (Figure 8B, 8F, 8G; Armentano et al., 2007; Alfano et al. 2014), but not in Ctip1fl/fl;Emx1-Cre mice (Figure 6B), and a dramatic failure in organization of topographic maps in Ctip1fl/fl;Emx1-Cre mice, but not in Couptf1fl/fl;Emx1-Cre mice. Interestingly, combined loss of cortical Ctip1 and Couptf1 function results in a largely additive phenotype, with thalamocortical input both caudally displaced and unable to organize into topographic maps (Figure 8C, 8H, 8I). Taken together, these data suggest that Couptf1 and Ctip1 have largely independent functions, with Couptf1 determining the size and position of the sensory domain, and Ctip1 regulating subsequent differentiation within that domain.
Figure 8. Ctip1 functions independently of Couptf1, and restoring expression of Ctip1 in layer IV of Ctip1fl/fl;Emx1-Cre cortex is sufficient to rescue sensory map formation.

(A–I) CTIP1 and COUPTF1 act sequentially to regulate the position and topography of sensory maps. In wild-type somatosensory cortex, thalamocortical afferents and clusters of Rorb-expressing neurons form barrels (A, D–E). In Couptf1fl/fl;Emx1-Cre cortex, sensory representations are miniaturized, and shifted to the caudal extreme of cortex, but maintain their periodic organization (B, F–G). In double mutant Ctip1fl/fl;Couptf1fl/fl;Emx1-Cre cortex, sensory representations are shifted caudally, as in Couptf1fl/fl;Emx1-Cre cortex, but are also completely disorganized, as in Ctip1fl/fl;Emx1-Cre cortex (C, H–I).
(J–P) Mosaic Ctip1 misexpression partially compensates for cortex-wide loss of Ctip1 function. Schematic of approach (J). CAG-floxed(STOP)-Egfp and CAG-floxed(STOP)-Ctip1 are co-electroporated into E14.5 Ctip1fl/fl;Emx1-Cre cortex, and electroporated brains are recovered and flatmounted at P7. Ctip1fl/fl;Emx1-Cre barrel cortex electroporated with EGFP alone contains neither clustering of electroporated neurons nor discernible periodic organization of thalamocortical afferents (K-M, insets in L and M), but Ctip1fl/fl;Emx1-Cre barrel cortex electroporated with both EGFP and Ctip1 contains clustered electroporated neurons and rudimentary barrel-like structures in barrel cortex (N-P, insets in O and P).
(Q) Model. Ctip1 is expressed in sensory cortical areas, and controls sensory area gene expression, output connectivity, and establishment of receptive fields for sensory input connectivity.
Scale bars: 500µm (A–C) and 200µm (D–I).
To investigate whether the requirement for Ctip1 function in sensory map formation is primarily related to its function in layer IV neuron differentiation, we performed in utero electroporation surgeries to restore Ctip1 expression exclusively in superficial layers of Ctip1fl/fl;Emx1-Cre cortex (Figure 8J). In control Egfp-only experiments, thalamocortical afferents established substantially disorganized maps, with rudimentary somatotopic representations, but no recognizable barrel field, as expected (Figure 8K–8M). Remarkably, however, Ctip1 expression in superficial layers was sufficient to rescue organization of thalamocortical afferents into precisely arranged rows of distinctly formed barrels in the most densely electroporated portion of somatosensory cortex (Figure 8N–8P). In combination with our earlier data on layer IV neuron differentiation, these experiments demonstrate that Ctip1 acts cell-autonomously to establish a receptive field of dedicated synaptic territory in layer IV that enables thalamocortical afferents to organize into properly configured sensory maps.
DISCUSSION
Elucidating transcriptional programs that direct specialization of cortical areas is critical for understanding the emergence of area-specific connectivity, cytoarchitecture, and function, as well as the diversification of neuronal subtypes and their precise differentiation during cortical development. We report here that Ctip1 is highly expressed in primary sensory areas, and that it is a critical control over acquisition of sensory molecular area identity (Figure 8Q). By repressing motor programs, Ctip1 prevents projection neurons in sensory cortical areas from establishing aberrant connections to motor-specific targets. In addition, Ctip1 regulates differentiation of layer IV neurons and their integration into thalamorecepient circuits, which is critical, in turn, for organization of thalamocortical afferents into appropriately-configured topographic maps. Taken together, our findings indicate that Ctip1 acts across multiple neuronal subtypes to coordinately regulate sensory-specific gene expression, thus linking output connectivity, cytoarchitectural organization, and sensory map formation during development.
Postmitotic controls intersectionally regulate acquisition of molecular area identity
Genes belonging to diverse functional categories are expressed in an area-specific fashion, including transcriptional regulators (e.g., Id2 and Rorb), cell adhesion molecules (e.g., Cdh6 and Cdh8), intracellular signaling molecules (e.g., Plcb1), and axon guidance molecules (e.g., Epha7 and Efna5) (Greig et al., 2013). Although they are useful readouts of area identity, the complex expression patterns of these genes represent the final outcome of intersectional regulation by multiple transcriptional programs acting in specific cortical layers and areas, rather than directly illuminating the basic organizational principles governing cortical arealization. The identification of transcriptional controls over postmitotic acquisition of area identity will enable deconstructing this complex regulatory network into individual “developmental vectors” acting within defined tangential and laminar domains to intersectionally establish the more complex expression patterns of downstream genes.
We show here that Ctip1 is critical for the establishment of molecular boundaries between motor and sensory areas, which are comparatively preserved in the absence of either Lmo4 or Bhlhb5 function (Figure S2). Broadly speaking, our RNA-seq data demonstrate that Ctip1 acts within the sensory domain to repress expression of motor genes and to activate expression of sensory genes (Figure 2U–2V). However, individual genes demonstrate complex patterns of misexpression in the absence of Ctip1. For example, Lmo4 expands from motor and cingulate cortex into somatosensory territory, and recedes from higher-order visual areas into a narrow caudal band (Figure 2Q–2R). These symmetrically opposite changes suggest that Lmo4 expression in motor and cingulate cortex might be established by mechanisms independent from those acting in higher-order visual areas, with Ctip1 acting intersectionally with other regulators to activate or repress Lmo4 expression, depending on context. Moreover, Ctip1 functions in a number of distinct subtype-specific cellular contexts, potentially cooperating and competing with distinct sets of transcription factors in each neuronal subtype. Some of the changes in area-specific gene expression we have identified are consistent across subtypes (Figure 2A–2D), while others affect one specific neuronal subtype, while spare other neuronal subtypes, (Figure 2E–2H). These data suggest that Ctip1 controls area identity both by regulating a common set of genes across multiple neuronal subtypes (acting autonomously or interacting with broadly expressed transcriptional regulators), and by regulating other sets of genes in a subtype-specific fashion (interacting with subtype-specific transcriptional regulators).
Ctip1 and Bhlhb5 act in parallel to regulate sensory area development
The similar temporal and areal expression patterns of Ctip1 and Bhlhb5 suggest that they act downstream of a common set of regulators (Figure S1E–S1H). However, the two transcription factors have largely non-overlapping functions. First, molecular abnormalities in Bhlhb5 null mice are much more limited (Joshi et al., 2008; Figure S2Q–S2V), and do not indicate overall motorization of the sensory domain that we identify here in Ctip1fl/fl;Emx1-Cre mice (Figure 2A–2T). Second, while most SCPN axons fail to reach the pyramidal decussation in Bhlhb5 nulls (Joshi et al., 2008; Ross et al., 2012), loss of Ctip1 function has the opposite effect, and more neurons project to the spinal cord (Figure S4L–S4T). These findings reinforce that Bhlhb5 plays a broad role promoting SCPN axon outgrowth, especially in caudal motor cortex, while Ctip1 instructs SCPN to acquire sensory area identity. One potentially shared function of Bhlhb5 and Ctip1 is barrel development, since cytoarchitectural organization in layer IV is disrupted in both Bhlhb5 null and Ctip1fl/fl;Emx1-Cre mice (Joshi et al., 2008; Figure 7A–7D). However, these abnormalities are considerably more severe in Ctip1fl/fl;Emx1-Cre mice, with abnormal layer IV neuron differentiation also preventing thalamocortical axons from clustering into barrels (Figure 6A–6B). Overall, Ctip1 regulates multiple critical aspects of postmitotic acquisition of sensory area identity independently of Bhlhb5, though both are involved in cytoarchitectural organization of layer IV.
Cortex-intrinsic regulation of layer IV neuron differentiation and sensory map formation
We report here that Ctip1 links earlier cortex-intrinsic regulators that specify area identity with later cortex-extrinsic signals that are required for refinement of area identity, by directing differentiation of layer IV neurons within the sensory domain. Deletion of Ctip1 from cortex results in a striking failure of sensory map formation, with no discernible organization of thalamocortical axons into barrels (Figure 6B). Layer IV neurons lack cytoarchitectural organization (Figure 7B) and fail to express layer IV-specific genes (Figure 7E–7L). In addition, axon terminals of contralateral callosal projection neurons invade layer IV of Ctip1fl/fl;Emx1-Cre cortex, infringing on synaptic territory normally reserved for thalamocortical input (Figure 7P–7R). Cell-autonomous loss of Ctip1 function in layer IV neurons completely disrupts their integration into barrels and assembly into thalamorecepient circuits (Figure 7S–7X). Taken together, these data indicate that Ctip1 is a cortex-intrinsic control acting within the sensory domain to regulate layer IV neuron differentiation, enabling appropriate spatial organization of their dendritic trees and interactions with thalamocortical input, which are in turn critical for subsequent stages of sensory area development.
Multiple lines of investigation have identified that thalamocortical axons provide information required both for sensory map formation, and for parallel changes in layer IV neuron cytoarchitectural organization and molecular area identity during the final stages of cortical arealization (Wu et al., 2011; Li and Crair, 2011). In barrelless (Adcy1 null) mice, thalamocortical axons and layer IV neurons fail to segregate into individual barrels (Van der Loos et. al., 1986; Abdel-Majid et al., 1998) due to the loss of adenylyl cyclase-dependent signals normally conveyed by thalamocortical axons (Iwasato et al., 2008). Genetically blocking neurotransmission at thalamocortical synapses disrupts barrel organization and molecular differentiation of layer IV neurons (Li et al., 2013). Additionally, genetic ablation of sensory thalamic nuclei disrupts refinement of gene expression (Chou et al., 2013; Pouchelon et al., 2014). Together, these data highlight the importance of extrinsic signals for proper postnatal differentiation of cortical neurons.
Cortex-intrinsic programs acting at early stages of arealization have also been extensively investigated. Thalamocortical axons are attracted to specific regions of cortex by areally-specified cortical cues, which can be shifted rostrally or caudally by altering expression of transcription factors that specify area identity, like Emx2 and Couptf1 (O’Leary et al., 2007; Armentano et al., 2007). Once thalamocortical axons reach the cortical plate, however, their organization into sensory maps proceeds largely normally, regardless of the size or position of the respecified sensory domain (Hamasaki et al., 2004; Armentano et al., 2007; Zembrzycki et al., 2013). Loss of transcriptional controls over acquisition of sensory area identity has been associated with only subtle defects in cortical barrel cytoarchitecture and clustering of thalamocortical axons (Joshi et al., 2008). It has therefore remained unclear whether cortex-intrinsic programs direct differentiation of neurons within the sensory domain in order to enable the complex interactions with thalamocortical axons that are required for sensory map formation and further refinement of gene expression. We show here that Ctip1 is a critical control over precisely this process in layer IV of sensory areas.
The complex interplay of cortex-intrinsic and cortex-extrinsic mechanisms in the specification of projection neuron area identity has important implications for a variety of neurodevelopmental disorders, but the precise molecular controls underlying these interactions remain poorly understood. Identification of Ctip1 as a central regulator of layer IV neuron differentiation represents an important first step toward defining specific molecules that direct layer IV dendritic development, cytoarchitectural organization, and circuit integration. It will be of particular interest, in future studies, to investigate potential interactions between cortex-intrinsic transcription factors that control neuronal subtype and area identity, and transcription factors operating under the influence of thalamocortical activity. Further elucidation of the transcriptional mechanisms and molecular programs by which Ctip1 regulates cortex-intrinsic aspects of arealization will offer important insights into the evolutionary and functional compartmentalization of cortex into distinct areas, and will also identify potential nodes of intersection with recently delineated maturational processes driven by thalamocortical input.
EXPERIMENTAL PROCEDURES
Animals
All mouse studies were approved by the Harvard University IACUC, and were performed in accordance with institutional and federal guidelines. The date of vaginal plug detection was designated E0.5, and the day of birth as P0. Further details are listed in Supplemental Experimental Procedures.
Immunocytochemistry, histology, and in situ hybridization
Mice were transcardially perfused with 4% paraformaldehyde, and brains were dissected and post-fixed at 4°C overnight. Tissue was sectioned at 50µm on a vibrating microtome (Leica). Embryonic brains were fixed at 4°C overnight, dissected, cryoprotected in 30% sucrose, and sectioned at 20µm on a cryostat (Leica). Riboprobe synthesis and nonradioactive in situ hybridization were performed using standard methods (Tiveron et al., 1996). Antibodies used and probe sources/primer sequences are listed in Supplemental Experimental Procedures.
In utero electroporation
Surgeries were performed as previously described (Molyneaux et al., 2005). Further details about procedure and plasmids are provided in the Supplemental Experimental Procedures.
Anterograde labeling with AAV
All virus work was approved by the Harvard Committee on Microbiological Safety, and conducted according to institutional guidelines.. Further details on procedure and viral constructs are listed in Supplemental Experimental Procedures.
RNA sequencing
Frontal (motor) and parietal (somatosensory) cortex was dissected from P4 wild-type and Ctip1fl/fl;Emx1-Cre mice, mRNA was isolated using mRNA Direct beads (Invitrogen), and libraries were prepared using NEBNext RNA Library Prep Kit (New England Biolabs). Sequencing was performed on an Illumina HiSeq 2500 at the Harvard Bauer core facility. Data was analyzed using Tuxedo tools (Trapnell et al., 2012).
Supplementary Material
Acknowledgments
We thank L. Gan, E. Grove, S. Orkin, S. Ross, M. Studer, and H. Tucker for generous sharing of mice and reagents; B. Brandler, P. Davis, E. Gillis-Buck, and I. Florea for technical assistance; J. Flanagan, S. Dymecki, L. Goodrich, H. Padmanabhan, M.J. Galazo, and V. Sahni for scientific discussions; and members of the Macklis lab for helpful suggestions. This work was supported by grants to J.D.M. from the National Institutes of Health (NS045523, NS075672, and NS049553), with additional infrastructure support from NS041590, the Harvard Stem Cell Institute, the Massachusetts Spinal Cord Injury Trust Fund, the Jane and Lee Seidman Fund for CNS Research, and the Emily and Robert Pearlstein Fund for Nervous System Repair. L.C.G. was partially supported by the Harvard Medical Scientist Training Program, NIH individual predoctoral National Research Service Award NS080343, and the DEARS Foundation. M.B.W. was partially supported by NIH individual predoctoral National Research Service Award NS064730 and the DEARS Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
L.C.G. and J.D.M. conceived the project; L.C.G. and M.B.W. designed and performed all experiments; L.C.G., M.B.W., and J.D.M. analyzed and interpreted the data; and L.C.G. and M.B.W. made figures. C.G. performed embryonic in situ hybridization experiments. L.C.G., M.B.W., and J.D.M. synthesized and integrated the findings, and wrote and revised the paper. All authors read and approved the final manuscript.
BIBLIOGRAPHY
- Alfano C, Magrinelli E, Harb K, Hevner RF, Studer M. Postmitotic control of sensory area specification during neocortical development. Nat Commun. 2014;5:5632. doi: 10.1038/ncomms6632. [DOI] [PubMed] [Google Scholar]
- Abdel-Majid RM, Leong WL, Schalkwyk LC, Smallman DS, Wong ST, Storm DR, Fine A, Dobson MJ, Guernsey DL, Neumann PE. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat Genet. 1998;19:289–291. doi: 10.1038/980. [DOI] [PubMed] [Google Scholar]
- Armentano M, Chou S-J, Tomassy GS, Leingärtner A, O’Leary DDM, Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- Avram D, Fields A, Pretty On Top K, Nevrivy DJ, Ishmael JE, Leid M. Isolation of a novel family of C(2)H(2) zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors. J Biol Chem. 2000;275:10315–10322. doi: 10.1074/jbc.275.14.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avram D, Fields A, Senawong T, Topark-Ngarm A, Leid M. COUP-TF (chicken ovalbumin upstream promoter transcription factor)-interacting protein 1 (CTIP1) is a sequence-specific DNA binding protein. Biochem J. 2002;368:555–563. doi: 10.1042/BJ20020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Kamran SC, Lessard S, Xu J, Fujiwara Y, Lin C, Shao Z, Canver MC, Smith EC, Pinello L, et al. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science. 2013;342:253–257. doi: 10.1126/science.1242088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni F, Hodge RD, Elsen GE, Nelson BR, Daza RAM, Beyer RP, Bammler TK, Rubenstein JLR, Hevner RF. Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. 2010;107:13129–13134. doi: 10.1073/pnas.1002285107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Brodmann’s Localisation in the Cerebral Cortex. Springer Verlag; 1909. translated by Garey 2006) [Google Scholar]
- Cederquist GY, Azim E, Shnider SJ, Padmanabhan H, Macklis JD. Lmo4 Establishes Rostral Motor Cortex Projection Neuron Subtype Diversity. J Neurosci. 2013;33:6321–6332. doi: 10.1523/JNEUROSCI.5140-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SJ, Babot Z, Leingärtner A, Studer M, Nakagawa Y, O’Leary DDM. Geniculocortical Input Drives Genetic Distinctions Between Primary and Higher-Order Visual Areas. Science. 2013;340:1239–1242. doi: 10.1126/science.1232806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Pan L, Xie X, Gan L. Requirement for Lmo4 in the vestibular morphogenesis of mouse inner ear. Dev Biol. 2010;338:38–49. doi: 10.1016/j.ydbio.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsen GE, Hodge RD, Bedogni F, Daza RAM, Nelson BR, Shiba N, et al. The protomap is propagated to cortical plate neurons through an Eomes-dependent intermediate map. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(10):4081–4086. doi: 10.1073/pnas.1209076110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Xie X, Joshi PS, Yang Z, Shibasaki K, Chow RL, Gan L. Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development. 2006;133:4815–4825. doi: 10.1242/dev.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci. 2013;14:755–769. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki T, Leingärtner A, Ringstedt T, O’Leary DDM. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Huang Z, Kawase-Koga Y, Zhang S, Visvader J, Toth M, Walsh CA, Sun T. Transcription factor Lmo4 defines the shape of functional areas in developing cortices and regulates sensorimotor control. Dev Biol. 2009;327:132–142. doi: 10.1016/j.ydbio.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Inan M, Kanki H, Erzurumlu RS, Itohara S, Crair MC. Cortical adenylyl cyclase 1 is required for thalamocortical synapse maturation and aspects of layer IV barrel development. J Neurosci. 2008;28:5931–5943. doi: 10.1523/JNEUROSCI.0815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John AA, Brylka HH, Wiegreffe CC, Simon RR, Liu P, Jüttner RR, Crenshaw EB, Luyten FPF, Jenkins NA, Copeland NG, et al. Bcl11a is required for neuronal morphogenesis and sensory circuit formation in dorsal spinal cord development. Development. 2012;139:1831–1841. doi: 10.1242/dev.072850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PS, Molyneaux BJ, Feng L, Xie X, Macklis JD, Gan L. Bhlhb5 regulates the postmitotic acquisition of area identities in layers II-V of the developing neocortex. Neuron. 2008;60:258–272. doi: 10.1016/j.neuron.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T-Y, Chen C-Y, Hsueh Y-P. Bcl11A/CTIP1 mediates the effect of the glutamate receptor on axon branching and dendrite outgrowth. J. Neurochem. 2010;114:1381–1392. doi: 10.1111/j.1471-4159.2010.06852.x. [DOI] [PubMed] [Google Scholar]
- Li H, Fertuzinhos S, Mohns E, Hnasko TS, Verhage M, Edwards R, Šestan N, Crair MC. Laminar and columnar development of barrel cortex relies on thalamocortical neurotransmission. Neuron. 2013;79:970–986. doi: 10.1016/j.neuron.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Crair MC. How do barrels form in somatosensory cortex? Ann. N. Y. Acad. Sci. 2011;1225:119–129. doi: 10.1111/j.1749-6632.2011.06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Keller JR, Ortiz M, Tessarollo L, Rachel RA, Nakamura T, Jenkins NA, Copeland NG. Bcl11a is essential for normal lymphoid development. Nat Immunol. 2003;4:525–532. doi: 10.1038/ni925. [DOI] [PubMed] [Google Scholar]
- Lokmane L, Proville R, Narboux-Nême N, Györy I, Keita M, Mailhes C, Léna C, Gaspar P, Grosschedl R, Garel S. Sensory map transfer to the neocortex relies on pretarget ordering of thalamic axons. Curr Biol. 2013;23:810–816. doi: 10.1016/j.cub.2013.03.062. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- O’Leary DDM, Chou S-J, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Pouchelon G, Gambino F, Bellone C, Telley L, Vitali I, Lüscher C, Holtmaat A, Jabaudon D. Modality-specific thalamocortical inputs instruct the identity of postsynaptic L4 neurons. Nature. 2014;511:471–474. doi: 10.1038/nature13390. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Ramóny Cajal S. Comparative Study of the Sensory Areas of the Human Cortex. 1899 [Google Scholar]
- Richards LJ, Plachez C, Ren T. Mechanisms regulating the development of the corpus callosum and its agenesis in mouse and human. Clin Genet. 2004;66:276–289. doi: 10.1111/j.1399-0004.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- Ross SE, McCord AE, Jung C, Atan D, Mok SI, Hemberg M, Kim T-K, Salogiannis J, Hu L, Cohen S, et al. Bhlhb5 and Prdm8 form a repressor complex involved in neuronal circuit assembly. Neuron. 2012;73:292–303. doi: 10.1016/j.neuron.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran VG, Menne TF, Xu J, Akie TE, Lettre G, Van Handel B, Mikkola HKA, Hirschhorn JN, Cantor AB, Orkin SH. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 2008;322:1839–1842. doi: 10.1126/science.1165409. [DOI] [PubMed] [Google Scholar]
- Stanfield BB, O’Leary DDM, Fricks C. Selective collateral elimination in early postnatal development restricts cortical distribution of rat pyramidal tract axons. Nature. 1982;298:371–373. doi: 10.1038/298371a0. [DOI] [PubMed] [Google Scholar]
- Sun T, Patoine C, Abu-Khalil A, Visvader J, Sum E, Cherry TJ, Orkin SH, Geschwind DH, Walsh CA. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308:1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Loos H, Welker E, Dörfl J, Rumo G. Selective breeding for variations in patterns of mystacial vibrissae of mice. Bilaterally symmetrical strains derived from ICR stock. J. Hered. 1986;77:66–82. doi: 10.1093/oxfordjournals.jhered.a110201. [DOI] [PubMed] [Google Scholar]
- Vue TY, Lee M, Tan YE, Werkhoven Z, Wang L, Nakagawa Y. Thalamic control of neocortical area formation in mice. J Neurosci. 2013;33:8442–8453. doi: 10.1523/JNEUROSCI.5786-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegreffe C, Simon R, Peschkes K, Kling C, Strehle M, Cheng J, Srivatsa S, Liu P, Jenkins NA, Copeland NG, Tarabykin V, Britsch S. Bcl11a (Ctip1) Controls Migration of Cortical Projection Neurons through Regulation of Sema3c. Neuron. 2015;87:311–325. doi: 10.1016/j.neuron.2015.06.023. [DOI] [PubMed] [Google Scholar]
- Woodworth MB, Custo Greig L, Ippolito GC, Tucker HO, Macklis JD. Ctip1 regulates the balance between specification of distinct projection neuron subtypes in deep cortical layers. Submitted to Cell Reports. 2016 doi: 10.1016/j.celrep.2016.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth MB, Custo Greig LF, Kriegstein AR, Macklis JD. SnapShot: Cortical Development. Cell. 2012;151:918–918. doi: 10.1016/j.cell.2012.10.004. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-S, Ballester Rosado CJ, Lu H-C. What can we get from “barrels”: the rodent barrel cortex as a model for studying the establishment of neural circuits. Eur J Neurosci. 2011;34:1663–1676. doi: 10.1111/j.1460-9568.2011.07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Peng C, Sankaran VG, Shao Z, Esrick EB, Chong BG, Ippolito GC, Fujiwara Y, Ebert BL, Tucker PW, et al. Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science. 2011;334:993–996. doi: 10.1126/science.1211053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembrzycki A, Chou S-J, Ashery-Padan RR, Stoykova A, O’Leary DDM. Sensory cortex limits cortical maps and drives top-down plasticity in thalamocortical circuits. Nat Neurosci. 2013;16:1060–1067. doi: 10.1038/nn.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


