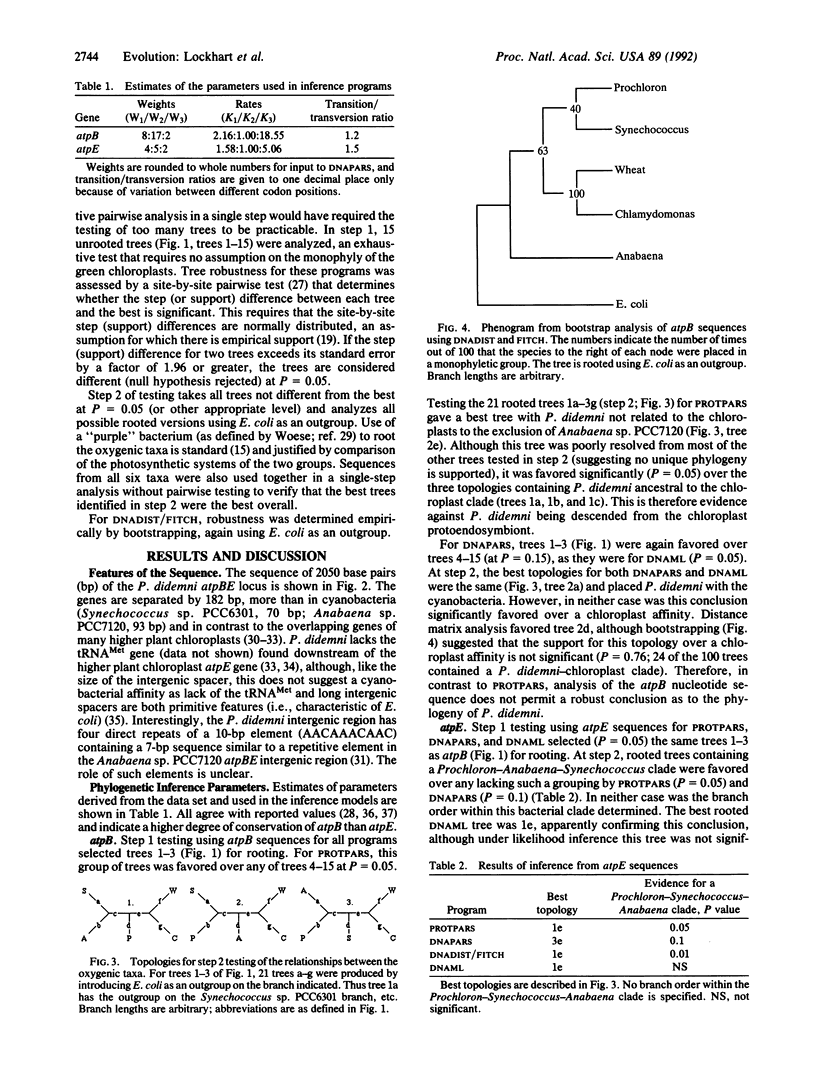
Abstract
The prochlorophytes, oxygenic photosynthetic prokaryotes containing chlorophylls a and b, have been put forward as descended from the organisms that gave rise to chloroplasts of green plants and algae by endosymbiosis, although this has always been controversial. To assess the phylogenetic position of the prochlorophyte Prochloron didemni, we have cloned and sequenced its atpBE genes. Phylogenetic inference under a range of models gives moderate to strong support for a cyanobacterial grouping rather than a chloroplast one. Possible systematic errors in this and previous analyses of prochlorophyte sequences are discussed.
Full text
PDF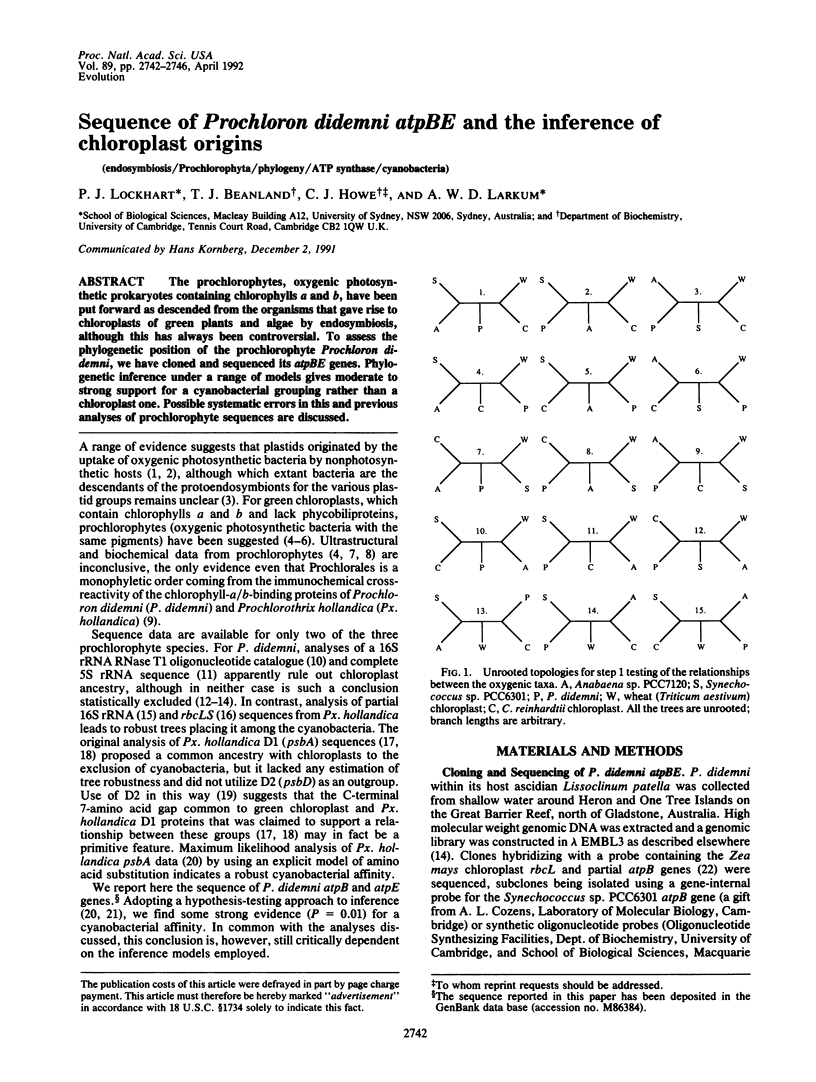



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beanland T. J. Evolutionary relationships between "Q-type" photosynthetic reaction centres: hypothesis-testing using parsimony. J Theor Biol. 1990 Aug 23;145(4):535–545. doi: 10.1016/s0022-5193(05)80487-4. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Coen D. M., Beaton A. R., Bogorad L., Rich A. Location of the single gene for the large subunit of ribulosebisphosphate carboxylase on the maize chloroplast chromosome. J Biol Chem. 1979 Feb 10;254(3):905–910. [PubMed] [Google Scholar]
- Bullerjahn G. S., Jensen T. C., Sherman D. M., Sherman L. A. Immunological characterization of the Prochlorothrix hollandica and Prochloron sp. chlorophyll a/b antenna proteins. FEMS Microbiol Lett. 1990 Jan 15;55(1-2):99–105. doi: 10.1016/0378-1097(90)90176-q. [DOI] [PubMed] [Google Scholar]
- Cozens A. L., Walker J. E. The organization and sequence of the genes for ATP synthase subunits in the cyanobacterium Synechococcus 6301. Support for an endosymbiotic origin of chloroplasts. J Mol Biol. 1987 Apr 5;194(3):359–383. doi: 10.1016/0022-2836(87)90667-x. [DOI] [PubMed] [Google Scholar]
- Curtis S. E. Genes encoding the beta and epsilon subunits of the proton-translocating ATPase from Anabaena sp. strain PCC 7120. J Bacteriol. 1987 Jan;169(1):80–86. doi: 10.1128/jb.169.1.80-86.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Gray M. W. The evolutionary origins of organelles. Trends Genet. 1989 Sep;5(9):294–299. doi: 10.1016/0168-9525(89)90111-x. [DOI] [PubMed] [Google Scholar]
- Kimura M. Estimation of evolutionary distances between homologous nucleotide sequences. Proc Natl Acad Sci U S A. 1981 Jan;78(1):454–458. doi: 10.1073/pnas.78.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino H., Hasegawa M. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in hominoidea. J Mol Evol. 1989 Aug;29(2):170–179. doi: 10.1007/BF02100115. [DOI] [PubMed] [Google Scholar]
- Lewin R. A. Prochloron--a status report. Phycologia. 1984;23(2):203–208. doi: 10.2216/i0031-8884-23-2-203.1. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Altschul S. F., Kececioglu J. D. A tool for multiple sequence alignment. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4412–4415. doi: 10.1073/pnas.86.12.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart P. J., Howe C. J., Bryant D. A., Beanland T. J., Larkum A. W. Substitutional bias confounds inference of cyanelle origins from sequence data. J Mol Evol. 1992 Feb;34(2):153–162. doi: 10.1007/BF00182392. [DOI] [PubMed] [Google Scholar]
- MacKay R. M., Salgado D., Bonen L., Stackebrandt E., Doolittle W. F. The 5S ribosomal RNAs of Paracoccus denitrificans and Prochloron. Nucleic Acids Res. 1982 May 11;10(9):2963–2970. doi: 10.1093/nar/10.9.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morden C. W., Golden S. S. Sequence analysis and phylogenetic reconstruction of the genes encoding the large and small subunits of ribulose-1,5-bisphosphate carboxylase/oxygenase from the chlorophyll b-containing prokaryote Prochlorothrix hollandica. J Mol Evol. 1991 May;32(5):379–395. doi: 10.1007/BF02101278. [DOI] [PubMed] [Google Scholar]
- Morden C. W., Golden S. S. psbA genes indicate common ancestry of prochlorophytes and chloroplasts. Nature. 1989 Jan 26;337(6205):382–385. doi: 10.1038/337382a0. [DOI] [PubMed] [Google Scholar]
- Raven P. H. A multiple origin for plastids and mitochondria. Science. 1970 Aug 14;169(3946):641–646. doi: 10.1126/science.169.3946.641. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidow A., Wilson A. C. Compositional statistics: an improvement of evolutionary parsimony and its application to deep branches in the tree of life. J Mol Evol. 1990 Jul;31(1):51–68. doi: 10.1007/BF02101792. [DOI] [PubMed] [Google Scholar]
- Turner S., Burger-Wiersma T., Giovannoni S. J., Mur L. R., Pace N. R. The relationship of a prochlorophyte Prochlorothrix hollandica to green chloroplasts. Nature. 1989 Jan 26;337(6205):380–382. doi: 10.1038/337380a0. [DOI] [PubMed] [Google Scholar]
- Van den Eynde H., De Baere R., De Roeck E., Van de Peer Y., Vandenberghe A., Willekens P., De Wachter R. The 5S ribosomal RNA sequences of a red algal rhodoplast and a gymnosperm chloroplast. Implications for the evolution of plastids and cyanobacteria. J Mol Evol. 1988;27(2):126–132. doi: 10.1007/BF02138372. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Gay N. J. The unc operon. Nucleotide sequence, regulation and structure of ATP-synthase. Biochim Biophys Acta. 1984 Sep 6;768(2):164–200. doi: 10.1016/0304-4173(84)90003-x. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Structures of the genes for the beta and epsilon subunits of spinach chloroplast ATPase indicate a dicistronic mRNA and an overlapping translation stop/start signal. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6260–6264. doi: 10.1073/pnas.79.20.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]



