Abstract
A protective effect of statins on primary liver cancer (PLC) risk has been suggested. However, issues about the dose–response relationship, the protective effect of individual statins, and PLC risk reduction among at-risk populations remain unsolved. Therefore, a meta-analysis was conducted. PubMed and EMBASE were searched for studies providing the risk ratio (RR) on statins and PLC risk. Summary RRs were calculated using a random-effects model. Twenty-five studies were identified. Stain use was significantly associated with a reduced risk of PLC (RR = 0.60, 95% confidence interval (CI) = 0.53–0.69). The summary RR for every additional 50 cumulative defined daily doses per year was 0.87 (95% CI = 0.83–0.91). Evidence of a non-linear dose–response relationship between statins and PLC risk was found (Pnon-linearity < 0.01). All individual statins significantly reduced PLC risk, and the risk reduction was more evident with rosuvastatin. The inverse association between statins and PLC risk remained among populations with common risk factors. Subgroup analyses revealed more significant reduction in PLC risk by statins in high- versus non-high-risk populations (Pinteraction = 0.02). Overall, these findings add to our understanding of the association between statins and PLC risk. Whether statin use is causally associated with a reduced risk of PLC should be further studied.
Primary liver cancer (PLC) is the sixth most frequently diagnosed cancer worldwide1, representing approximately 6% of all new cancer cases2. Epidemiological studies show that the five-year relative survival rate of PLC in the USA was approximately 18%3, and the age standardized 5-year relative survival in China was approximately 10%4. These data reveal the poor prognosis of PLC, and thus PLC becomes the second leading cause of cancer-related death1. Statins are among the most widely prescribed medications globally because of their well-established efficacy and safety in the prevention and management of cardiovascular diseases5. In addition to the cardiovascular benefits, mounting evidence has suggested the chemopreventive potential of statins in the oncological field6.
Recently, statin use has been found to be associated with decreased risks of some cancers, including colorectal cancer7, ovarian cancer8, esophageal cancer9, gastric cancer10 and PLC11. However, as for statin use and PLC risk, there are several unsolved issues. First, whether there is a dose–response relationship between statin use and PLC risk is unclear. Some studies found that statin use was associated with a reduced risk of PLC in a dose–dependent manner12,13,14,15,16, whereas others failed to find a dose–response relationship between statin use and PLC risk17,18,19. Moreover, the shape of the dose–response relationship on this topic has not been described. Second, inverse19,20 and null15,18,21,22,23 associations with PLC risk have been reported for pravastatin, a commonly prescribed statin in clinical practice. This fact raises an issue of whether individual statin use is still associated with a reduced risk of PLC. Furthermore, which statin has the most pronounced protective effect on PLC risk is largely unknown. Third, previous meta-analyses11,24,25 did not investigate the association between statin use and PLC risk in subjects with common risk factors, including diabetes, hepatitis B virus (HBV) infection and hepatitis C virus (HCV) infection. It is possible that at-risk populations enjoy more benefits from statin chemoprevention than general populations26,27. In addition to these unsolved issues, previous meta-analyses11,24,25 are limited by the inclusion of limited studies, resulting in incompleteness and low power of their subgroup analyses. In fact, a recent meta-analysis (involving a total of 12 studies)11 included around half of studies currently available. In light of these considerations, we conducted an overall and dose–response meta-analysis of published studies to clarify the aforementioned unsolved issues and address limitations in previous studies.
Methods
Search strategy and study selection
We performed this meta-analysis and reported its results according to the statement of the Meta-analysis Of Observational Studies in Epidemiology28. A comprehensive electronic search of PubMed and EMBASE databases was conducted from their inception to March 2016, without any restrictions. The search strategy is shown in detail in the Supplementary List S1. The reference lists of included studies and relevant reviews were checked manually for identifying additional citations. If necessary, the original authors were contacted to obtain extra information through e-mails.
Studies were considered for inclusion if they were observational studies (cohort, nested case-control or case-control studies) or post hoc analyses of randomized controlled trials (RCTs) where risk estimate on the association of statin use with PLC risk was available. Two investigators (K.W. and F.B.H.) employed a two-stage method to conduct study screening independently. At the first stage, titles and abstracts were scrutinized for excluding obviously ineligible studies. At the second stage, the full text was read carefully for further excluding ineligible studies. Any disagreements on the eligibility of studies were resolved by discussion.
Data extraction and quality assessment
Data extraction was performed by one investigator (K.W.), and was then checked independently for accuracy by another investigator (F.B.H.). The following information was extracted: first author, publication year, study location, study period, study design, study population, participants’ age, sample size, data source, definition of non-user, follow-up duration and statin dose where available, maximally adjusted risk estimate with corresponding 95% confidence interval (CI), and adjustment factors. Crude risk estimate with 95% CI was extracted when adjusted risk estimate was not available.
Two investigators (K.W. and F.B.H.) assessed the quality of included studies independently using the Newcastle-Ottawa quality assessment scale. Each study could be awarded to a maximum of 9 stars after evaluating its 3 aspects (selection, comparability, and outcome). A study with 7 or more stars was considered to be of high quality. Post hoc analysis of RCTs was treated as cohort studies to achieve quality assessment. Any disagreements on the results of data extraction and quality assessment were resolved by discussion.
Statistical analysis
A random-effects model was used to pool the risk estimate from each study. Risk ratio (RR) was employed as a common measure of the association between statin use and PLC risk. Both hazard ratio and odds ratio were directly regarded as equivalent to RR. When only risk estimates stratified by type of statins21 or sex29 were available, we pooled these stratum data to yield an average risk estimate using a random-effects model. The Cochrane’s Q statistic (Pheterogeneity < 0.10 suggesting statistically significance) and the I2 statistic (I2 > 75.0% representing substantial heterogeneity, 50.0% ≤ I2 ≤ 75.0% representing moderate heterogeneity, I2 < 50% representing low heterogeneity) were adopted to qualitatively and quantitatively evaluate heterogeneity across studies, respectively.
On the basis of specific statin dose, distribution of cases and person years or controls, and adjusted RRs with 95% CIs, a two-stage dose–response meta-analysis was conducted to determine whether higher statin dose was associated with a lower risk of PLC. First, we used the generalized least square regression proposed by Orsini et al.30 to obtain study-specific slopes (linear trends) and 95% CIs for every 50 cumulative defined daily doses (cDDDs) per year increment in statin dose within each study from the natural logs of adjusted RRs and CIs across categories of statin dose. Then, we pooled them using a random-effects model to obtain an overall risk estimate. The defined daily dose (DDD) is a dose unit for statins, and refers to “the assumed average maintenance dose per day for a drug used for its main indication in adults”; the cDDD refers to the sum of dispensed DDDs of any statins during exposure period. Since all included studies provided statin dose as range, we designated the midpoint of each range as the assigned dose. If the highest range was open-ended, we assumed that it shared the same width as the preceding range. If the lowest range was open-ended, we assumed that the lower limit was equal to zero. For one study12 whose authors provided a cumulative dose of statins, we transformed it into cDDD under assumptions that the average DDD for statins was 25 mg, and the duration of statin use was the length of study period. To examine the robustness of the results from the two-stage dose–response meta-analysis, we performed a random-effects meta-analysis comparing the highest versus the lowest category of statin dose, which additionally included studies being not eligible for the two-stage dose–response meta-analysis. Restricted cubic spline function with 4 knots at the 5th, 35th, 65th and 95th percentiles was employed to identify any potential non-linear dose–response association between statin use and PLC risk. A Pnon-linearity was obtained by testing the null hypothesis that the regression coefficients of both the second and the third spline are equal to zero31.
To check the stability of the pooled results and to identify the possible sources of heterogeneity, diverse sensitivity analyses were conducted using the following methods: ignoring a single study in turn, repeating meta-analysis through a fixed-effects model and using unadjusted risk estimates, and applying various eligibility criteria. To identify potential effect modifiers, prespecified subgroup analyses were conducted stratified by study design, source of subjects, sex, individual age, sample size, study location, risk level of developing PLC, study quality, adjustment for diabetes, HCV, or HBV, and level of adjustment for confounders. A Pinteraction for difference between subgroups was calculated using meta-regression. Sensitivity and subgroup analyses were carried out based on the overall meta-analysis concerning statin use and PLC risk.
Begg’s test and Egger’s test were used to test publication bias when there were 10 or more included studies. We conducted all statistical analyses using STATA software (version 12.0, StataCorp, College Station, TX). Statistical significance level was set at P < 0.05 under two-sided test unless otherwise specified.
Results
Literature search
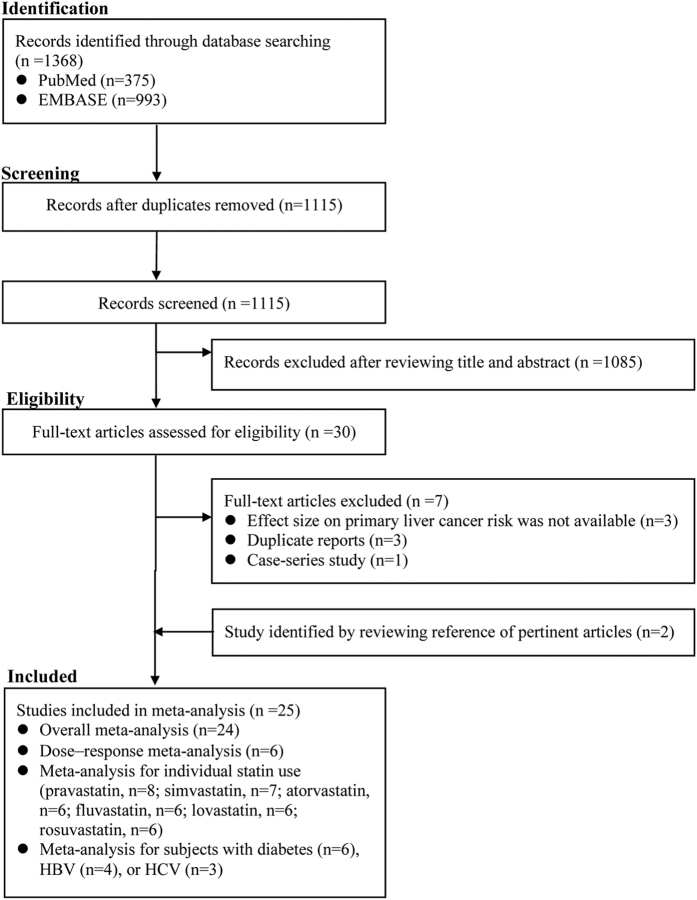
Our electronic search initially identified 375 citations and 993 citations from PubMed and EMBASE, respectively. A total of 30 citations were thought to be potentially relevant after reviewing titles and abstracts. Seven citations were further excluded after reading carefully the full text. Two studies32,33 were found to be eligible for inclusion in the process of handsearch. Two studies16,34 originating from the same cohort were included, because the data they provided could be used to do different analyses (one for overall meta-analysis34, the other for dose–response analysis16). Thus, 25 studies were included in this meta-analysis (Fig. 1).
Figure 1. The flowchart of identifying relevant studies.
Study characteristics
The results for study characteristics are shown in Supplementary Tables S1–S4. Twelve studies were conducted in Asia13,14,15,18,19,20,21,22,23,26,35,36, and remaining studies were conducted in western countries12,16,17,29,32,33,34,37,38,39,40,41,42. Among included studies, 12 were cohort studies13,14,15,16,26,29,33,34,35,36,38,42, 6 were case-control studies18,19,20,32,37,41, 4 were nested case-control studies12,17,21,40 and remaining 3 were post hoc analyses of RCTs22,23,39 of statin use and cardiovascular diseases. Our study involved 2,176,213 individuals, consisting of 1,330,795 men (61.2%) and 845,418 women (38.8%). Eighteen studies recruited subjects from population registers and remaining 7 were hospital-based21,22,23,26,32,39,40. Most studies were conducted in the general population, whereas 4 studies13,15,26,35 were conducted in population with HBV infection, 4 14,16,34,41 in those with HCV infection, and 2 21,40 in those with diabetes. Most studies defined non-user as subjects with no statin prescription, whereas 10 studies12,13,14,15,16,34,35,36,37,42 defined non-users as subjects with low cumulative dose of statins during study period. Adjusted risk estimates were available for all studies except one post hoc analysis of RCTs39 and one case-control study32. As for quality assessment, 18 studies were found to be of high quality, indicating the quality of included studies was generally good.
Overall meta-analysis
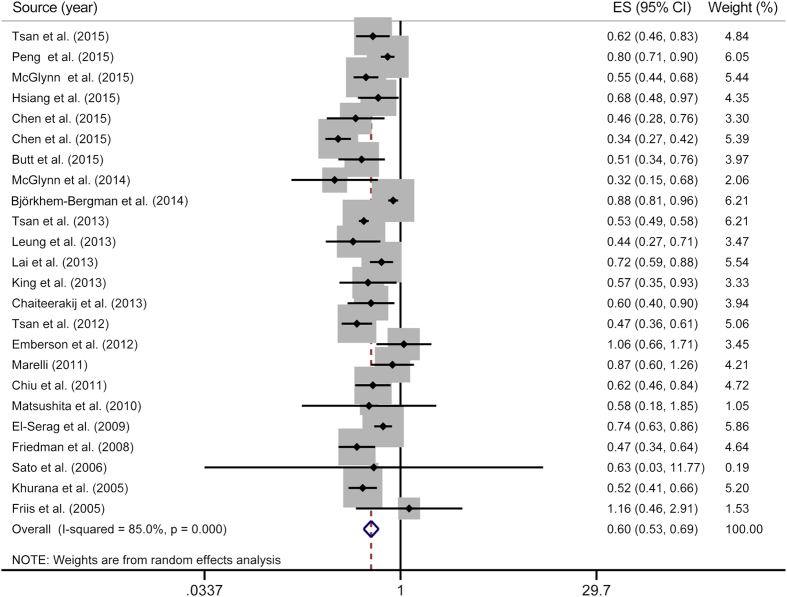
On the basis of 24 studies, compared with statin non-users, statin users experienced a significantly decreased risk for developing PLC (RR = 0.60, 95% CI = 0.53–0.69), with substantial heterogeneity (Pheterogeneity < 0.01, I2 = 85.0%) (Fig. 2).
Figure 2. Overall meta-analysis on statin use and primary liver cancer risk.
The squares represent the risk estimate for each individual study, with the area reflecting the weight assigned to the study. The horizontal line across each square represents the 95% confidence interval. The diamond represents the summary risk estimate, with width representing 95% confidence interval. CI, confidence interval; ES, effect size.
Dose–response meta-analyses
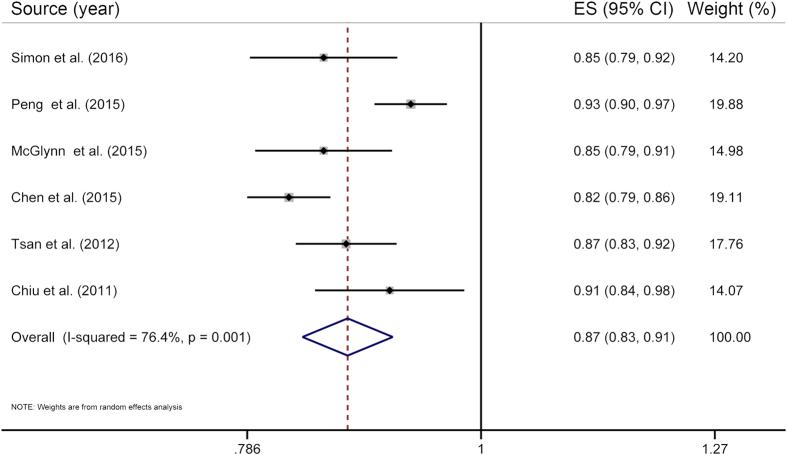
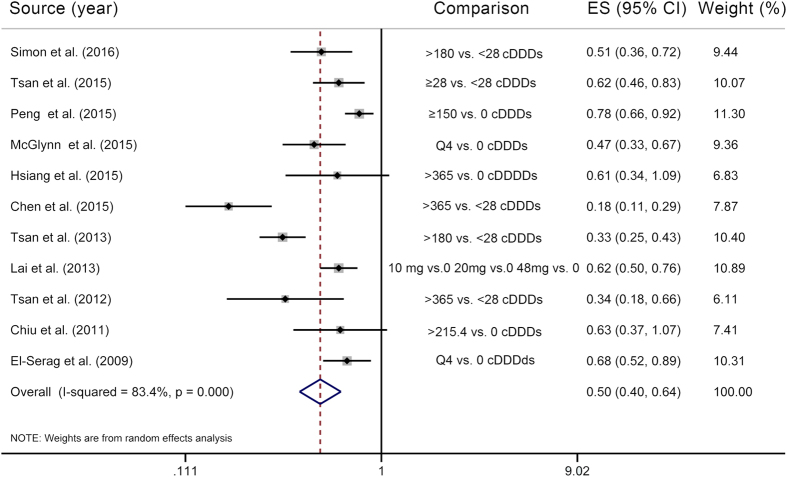
Six studies12,13,15,16,19,20 were included in the two-stage dose–response meta-analysis on statin use and PLC risk, involving 8,530 PLC cases and 118,961 subjects, and corresponding results showed that for every 50 cDDDs per year increment in statin dose, the PLC risk significantly decreased by 13% (RR = 0.87, 95% CI = 0.83–0.91), with substantial heterogeneity (Pheterogeneity < 0.01, I2 = 76.4%) (Fig. 3). Highest versus lowest meta-analysis included a total of 11 studies12,13,14,15,16,18,19,20,26,35,40, involving 45,335 PLC cases and 548,518 subjects, and found that the combined RR for PLC was 0.50 (95% CI = 0.40–0.64), with substantial heterogeneity (Pheterogeneity < 0.01, I2 = 83.4%) (Fig. 4). Using restricted cubic spline function, we found a potential non-linear dose–response association between statin use and PLC risk (Pnon-linearity < 0.01) (Fig. 5). The non-linear curve showed that there was a dose–response association between statin dose and decreased risk of PLC below approximately 100 cDDDs per year or above approximately 200 cDDDs per year, whereas the PLC risk did not decrease further between 100 and 200 cDDDs per year. Compared with statin non-use, the estimated RRs for PLC were 0.77 (95% CI = 0.71–0.84) for 25 cDDDs per year, 0.65 (95% CI = 0.57–0.73) for 55 cDDDs per year, 0.60 (95% CI = 0.54–0.67) for 200 cDDDs per year, 0.46 (95% CI = 0.39–0.53) for 320 cDDDs per year, and 0.22 (95% CI = 0.15–0.32) for 500 cDDDs per year.
Figure 3. The two-stage dose–response meta-analysis on statin use and the primary liver cancer risk.
The squares represent the risk estimate for each individual study, with the area reflecting the weight assigned to the study. The horizontal line across each square represents the 95% confidence interval. The diamond represents the summary risk estimate, with width representing 95% confidence interval. CI, confidence interval; ES, effect size.
Figure 4. Highest versus lowest meta-analysis on statin use and the primary liver cancer risk.
The squares represent the risk estimate for each individual study, with the area reflecting the weight assigned to the study. The horizontal line across each square represents the 95% confidence interval. The diamond represents the summary risk estimate, with width representing 95% confidence interval. CI, confidence interval; cDDDs, cumulative defined daily doses; ES, effect size.
Figure 5. Non-linear dose–response analysis on statin use and the primary liver cancer risk.
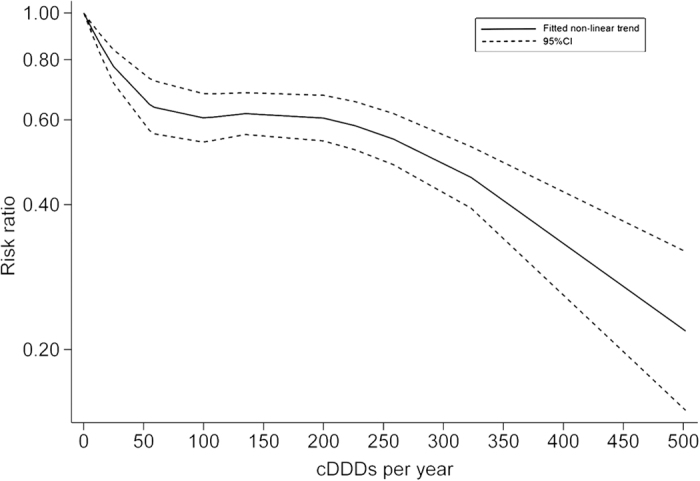
CI, confidence interval; cDDDs, cumulative defined daily doses.
Meta-analyses for individual statins
The results of meta-analyses for individual statins are summarized in Supplementary Table S5. All statins studied were found to be significantly associated with a reduced risk of PLC. Specifically, the pooled RRs for PLC were 0.71 (95% CI = 0.58–0.88) for pravastatin, 0.61 (95% CI = 0.54–0.69) for simvastatin, 0.53 (95% CI = 0.42–0.67) for atorvastatin, 0.65 (95% CI = 0.52–0.81) for fluvastatin, 0.49 (95% CI = 0.34–0.69) for rosuvastatin, and 0.65 (95% CI = 0.56–0.74) for lovastatin.
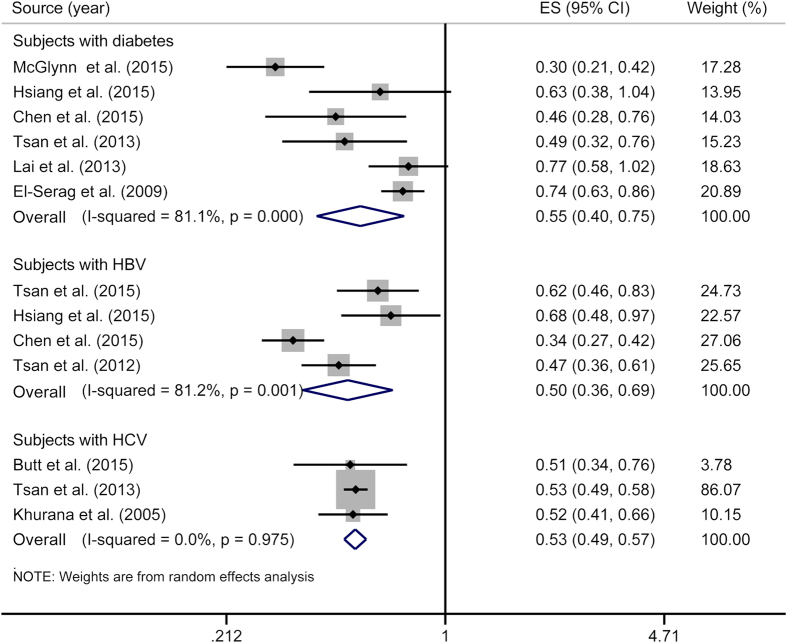
Meta-analyses for individuals with diabetes, HBV or HCV
The overall RRs for PLC were 0.55 (95% CI = 0.40–0.75; Pheterogeneity < 0.01, I2 = 81.1%) for individuals with diabetes, 0.50 (95% CI = 0.36–0.69; Pheterogeneity < 0.01, I2 = 81.2%) for those with HBV infection, and 0.53 (95% CI = 0.49–0.57; Pheterogeneity = 0.98, I2 = 0.0%) for those with HCV infection (Fig. 6).
Figure 6. Meta-analyses for individuals with diabetes, HBV or HCV.
The squares represent the risk estimate for each individual study, with the area reflecting the weight assigned to the study. The horizontal line across each square represents the 95% confidence interval. The diamond represents the summary risk estimate, with width representing 95% confidence interval. HBV, hepatitis B virus; HCV, hepatitis C virus. CI, confidence interval; ES, effect size.
Subgroup analyses
The results of subgroup analyses are summarized in Supplementary Table S6. Overall, the significantly inverse association between statin use and PLC risk remained in all subgroups, except in post hoc analyses of RCTs, or in studies with unadjusted risk estimates. A significant difference between subgroups stratified by risk level of developing PLC was detected (Pinteraction = 0.02).
Sensitivity analyses
The results of sensitivity analyses are present in Supplementary Table S7 and Supplementary Fig. S1. Ignoring a single study in turn did not significantly alter the initial relationship of statin use with PLC risk, with the pooled RR ranging from 0.59 (95% CI = 0.52–0.66)37 to 0.62 (95% CI = 0.55–0.70)13. Repeating meta-analysis through a fixed-effects model and using crude risk estimates produced a RR of 0.65 (95% CI = 0.63–0.68) and 0.61 (95% CI = 0.52–0.72), respectively. Restricting meta-analysis to studies defining statin non-user as subjects with no statin prescription yielded a similar risk estimate (RR = 0.68, 95% CI = 0.60–0.76), with the I2 statistic dropping from 85.0% to 50.1%.
Publication bias
There was no evidence of publication bias for any association as revealed by Begg’s test and Egger’s test (all P > 0.05).
Discussion
Findings from the overall meta-analysis supported an inverse association between statin use and PLC risk, which are consistent with previous meta-analyses11,24,25. Our two-stage dose–response analysis indicated that higher statin dose was associated with a lower risk of PLC. We also found evidence of a potential non-linear dose–response association between statin use and PLC risk. Our subgroup analyses revealed more significant reduction in PLC risk by statins in high- versus non-high-risk populations (high-risk population refers to subjects with HBV infection or HCV infection). Interestingly, as shown by meta-analyses for individual statins, the protective effect of rosuvastatin on PLC risk was more pronounced compared with other studied statins. In addition, we found that the inverse association between statin use and PLC risk persisted in patients with diabetes, HBV infection, or HCV infection.
The statin class is comprised of some heterogeneous medications varying in properties. Considering this fact, individual statins may have different chemopreventive effects. In the present study, we found that all studied statins reduced the risk of PLC, and the risk reduction was more evident with rosuvastatin, a hydrophilic statin. The latter finding is of particular interest, and is inconsistent with the perspective that the chemopreventive effect of lipophilic statins against cancer may be greater than that of hydrophilic statins43. The underlying mechanisms for the pronounced risk reduction by rosuvastatin may be related to its unique chemical properties, which make it have the highest affinity for 3-hydroxy-3-methylglutaryl coenzyme A reductase among commonly used statins44. In fact, in addition to the chemopreventive effect observed in our study, several studies have consistently suggested that rosuvastatin presents greater efficacy in reducing low-density lipoprotein cholesterol concentrations compared with all other available statins45,46,47. Rosuvastatin is the most prescribed brand name drug in the USA currently, and findings from the present study possibly encourage physicians continuing to prescribe this drug.
Our comprehensive search did not identify any RCTs of statins where the incidence of PLC was a primary outcome. In fact, considering huge costs and ethical concerns, conducting such a study is almost impossible. Nevertheless, the present meta-analysis identified 3 post hoc analyses of RCTs of statin use and cardiovascular diseases22,23,39. We observed that pooled risk estimates on statin use and PLC risk reached statistical significance for observational studies, but not for post hoc analyses of RCTs, which was not unexpected given weaknesses of post hoc analyses of RCTs. A major weakness is the insufficient follow-up duration, with the longest follow-up duration of only 5.1 years among these 3 studies22. Consequently, they observed few incident PLC cases, and thus had limited power to examine any effects of statin use on PLC risk. Indeed, only a total of 81 PLC cases were observed during their entire study period. Generally, for many types of cancer, the ideal follow-up duration of a trial in relation to cancer prevention should be equal to or more than 10 years, which can produce an ample number of cancer cases48. When it comes to PLC, however, it may be longer considering the low incidence of PLC in the general population. Another weakness is that the incidence of PLC was a secondary outcome. As a result, data on statin use and PLC risk from these 3 post hoc analyses of RCTs were not systematically collected, and thus might be driven by ascertainment bias. The difference of risk estimates between observational studies and post hoc analyses of RCTs has also been observed in other studies10,49.
In the present study, substantial heterogeneity was observed in the overall meta-analysis. Of included studies, definitions of statin non-users presented high diversity, which might explain the observed heterogeneity to some extent. Indeed, as shown by our sensitivity analysis, moderate heterogeneity was observed when restricting our analysis to studies where statin non-users referred to individuals with no statin prescription. In addition, our subgroup analysis suggested that the risk level of developing PLC was another possible source of heterogeneity. Specifically, there was more significant reduction in PLC risk by statins in high- versus non-high-risk populations. The precise mechanism behind this phenomenon remains unknown, which possibly involves interactions between statins and hepatitis viruses. Indeed, in vitro studies have found that statins are capable of inhibiting HBV and HCV replication50,51. The exact mechanism of the antiviral effect of statins is still unclear, which may be associated with the inhibition of geranylgeranylation of cellular proteins52. On the basis of the above benefits as well as the proven efficacy and safety of statins in patients with chronic liver disease53, healthcare practitioners should discontinue withholding statins from those with chronic liver disease.
The protective effect of statins on PLC risk involves several potential mechanisms. It is well-known that statins can competitively inhibit the activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a rate-limiting enzyme in the mevalonate synthesis pathway. Consequently, the synthesis of not only cholesterol but also isoprenoids is inhibited. The isoprenoids, mainly including farnesyl pyrophosphate and geranylgeranyl pyrophosphate, are required for prenylation of Ras and Rho oncoproteins54 that play a pivotal role in cell proliferation and survival55. Thus, the inactivation of Ras and Rho oncoproteins resulting from the inhibited synthesis of isoprenoids possibly contributes to cell apoptosis. Of note, it is also suggested that statins may exert their proapoptotic effects through altering the levels of Bcl-2 family members56. Statins can modulate both activity and expression of cell cycle regulating proteins, including cyclins, as well as cyclin-dependent kinases and their inhibitors, finally resulting in inhibition of cell proliferation54. In addition to the aforementioned mechanisms, in hepatocellular cancer cell lines, it is observed that atorvastatin can block both Myc (an oncogene closely related to hepatocarcinogenesis) phosphorylation and activation, and thus suppress tumor initiation and growth57.
Several limitations of this study should be acknowledged. First, the common side effects of statins include hepatotoxicity, myopathy, myoglobinuria as well as acute renal failure58. Many healthcare workers avoid prescribing statins to patients with chronic liver disease for fear of the occurrence of potential hepatotoxicity. This fact raises a concern that the observed inverse association of statin use with PLC risk might be explained by the confounding by indication. Nonetheless, some individual studies have found that statins reduce the risk of PLC in patients with and without chronic liver disease12,18, which possibly attenuates the concern to some degree. In addition, considering most included cohort studies employed a time-fixed analysis to quantify the association of statin use with PLC risk, the observed protective effect of statins on PLC risk might attribute to immortal time bias59. Nevertheless, Hsiang and colleagues26 observed that statin use was still associated with a reduced risk of hepatocellular carcinoma after eliminating this bias with the landmark analysis method, indicating that immortal time bias did not play a major role in the association between statin use and PLC risk. Second, many included studies attempted to adjust various potential confounders, but the possibility of residual confounding cannot be ruled out, considering that our findings are derived predominantly from observational studies where residual confounding always exists. Third, in the present study, publication bias was tested with formal statistical tests only in the case of ≥10 included studies. Therefore, we cannot exclude the possibility that the results from meta-analyses involving <10 studies are driven by publication bias. Finally, we observed substantial heterogeneity for the present meta-analysis. Such heterogeneity raises some concerns on the reliability of our pooled results. Nevertheless, clinical and methodological heterogeneity exist for all meta-analyses, especially meta-analysis of observational studies. Moreover, we have identified the sources of the observed heterogeneity through sensitivity and subgroup analyses.
In summary, our study suggests a protective effect of statins on PLC risk, most notably of rosuvastatin. This protective effect is more pronounced in high-risk populations, presents a dose–dependent manner, and remains in patients with diabetes, HBV infection, or HCV infection. These findings appear to support that satins can serve as chemopreventive agents of PLC to reduce the public health burden of this malignancy. However, whether the observed inverse association of statin use with PLC risk is causal or is spurious due to potential biases remains uncertain. Prospective cohort studies with sufficient follow-up duration and large sample size are therefore warranted to address this issue before formally recommending statins for preventing PLC.
Additional Information
How to cite this article: Zhong, G.-C. et al. Meta-analysis of studies using statins as a reducer for primary liver cancer risk. Sci. Rep. 6, 26256; doi: 10.1038/srep26256 (2016).
Supplementary Material
Acknowledgments
No funding or financial support was received for this study. The authors thank Dr. Szu-Yuan Wu (Department of Radiation Oncology, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan) for providing original data.
Footnotes
Author Contributions G.-C.Z. and J.-P.G. conceived the study idea. K.W. and F.-B.H. performed literature search, study selection, and data extraction. Y.L. performed statistical analyses and interpretation of corresponding results. G.-C.Z. drafted the initial manuscript. Y.-Y.Y. proposed valuable suggestions for revising the manuscript and carefully edited the entire manuscript. J.-P.G. had primarily responsibility for the final content.
References
- Ferlay J. et al. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer. GLOBOCAN 2012 v1.0. (2013) Available at: http://globocan.iarc.fr. (Accessed: 20th January 2014).
- Zimmermann E., Berentzen T. L., Gamborg M., Sorensen T. I. & Baker J. L. Sex-specific associations between birth weight and adult primary liver cancer in a large cohort of Danish children. Int. J. Cancer 138, 1410–1415, doi: 10.1002/ijc.29900 (2016). [DOI] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D. & Jemal A. Cancer statistics, 2016. CA: Cancer J. Clin. 66, 7–30, doi: 10.3322/caac.21332 (2016). [DOI] [PubMed] [Google Scholar]
- Zeng H. et al. Cancer survival in China, 2003–2005: a population-based study. Int. J. Cancer 136, 1921–1930, doi: 10.1002/ijc.29227 (2015). [DOI] [PubMed] [Google Scholar]
- Pisanti S., Picardi P., Ciaglia E., D’Alessandro A. & Bifulco M. Novel prospects of statins as therapeutic agents in cancer. Pharmacol. Res. 88, 84–98, doi: 10.1016/j.phrs.2014.06.013 (2014). [DOI] [PubMed] [Google Scholar]
- Stryjkowska-Gora A., Karczmarek-Borowska B., Gora T. & Krawczak K. Statins and cancers. Contemp. Oncol. 19, 167–175, doi: 10.5114/wo.2014.44294 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. et al. Association between statin use and colorectal cancer risk: a meta-analysis of 42 studies. Cancer Causes Control 25, 237–249, doi: 10.1007/s10552-013-0326-6 (2014). [DOI] [PubMed] [Google Scholar]
- Liu Y., Qin A., Li T., Qin X. & Li S. Effect of statin on risk of gynecologic cancers: a meta-analysis of observational studies and randomized controlled trials. Gynecol. Oncol. 133, 647–655, doi: 10.1016/j.ygyno.2014.04.007 (2014). [DOI] [PubMed] [Google Scholar]
- Singh S., Singh A. G., Singh P. P., Murad M. H. & Iyer P. G. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 11, 620–629, doi: 10.1016/j.cgh.2012.12.036 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P. P. & Singh S. Statins are associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Ann. Oncol. 24, 1721–1730, doi: 10.1093/annonc/mdt150 (2013). [DOI] [PubMed] [Google Scholar]
- Shi M., Zheng H., Nie B., Gong W. & Cui X. Statin use and risk of liver cancer: an update meta-analysis. BMJ Open 4, e005399, doi: 10.1136/bmjopen-2014-005399 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn K. A. et al. Statin use and risk of primary liver cancer in the Clinical Practice Research Datalink. J. Natl. Cancer Inst. 107, doi: 10.1093/jnci/djv009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. I. et al. Cancer risk in HBV patients with statin and metformin use: a population-based cohort study. Medicine 94, e462, doi: 10.1097/md.0000000000000462 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan Y. T. et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J. Clin. Oncol. 31, 1514–1521, doi: 10.1200/jco.2012.44.6831 (2013). [DOI] [PubMed] [Google Scholar]
- Tsan Y. T., Lee C. H., Wang J. D. & Chen P. C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J. Clin. Oncol. 30, 623–630, doi: 10.1200/jco.2011.36.0917 (2012). [DOI] [PubMed] [Google Scholar]
- Simon T. G., Bonilla H., Yan P., Chung R. T. & Butt A. A. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and HCC, among patients with HCV Results from ERCHIVES. Hepatology, doi: 10.1002/hep.28506 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn K. A. et al. Statin use and risk of hepatocellular carcinoma in a U.S. population. Cancer Epidemiol. 38, 523–527, doi: 10.1016/j.canep.2014.06.009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S. W. et al. Statin use and risk of hepatocellular carcinoma. Eur. J. Epidemiol. 28, 485–492, doi: 10.1007/s10654-013-9806-y (2013). [DOI] [PubMed] [Google Scholar]
- Chiu H. F., Ho S. C., Chen C. C. & Yang C. Y. Statin use and the risk of liver cancer: A population-based case-control study. Am. J.Gastroenterol. 106, 894–898, doi: 10.1038/ajg.2010.475 (2011). [DOI] [PubMed] [Google Scholar]
- Peng Y. C. et al. Statins are associated with a reduced risk of cholangiocarcinoma: A population-based case-control study. Br. J. Clin. Pharmacol. 80, 755–761, doi: 10.1111/bcp.12641 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. H. et al. Combination Therapy of Metformin and Statin May Decrease Hepatocellular Carcinoma Among Diabetic Patients in Asia. Medicine 94, e1013, doi: 10.1097/md.0000000000001013 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita Y. et al. Pravastatin use and cancer risk: A meta-analysis of individual patient data from long-term prospective controlled trials in Japan. Pharmacoepidemiol. Drug Saf. 19, 196–202, doi: 10.1002/pds.1870 (2010). [DOI] [PubMed] [Google Scholar]
- Sato S., Ajiki W., Kobayashi T. & Awata N. Pravastatin use and the five-year incidence of cancer in coronary heart disease patients: from the prevention of coronary sclerosis study. J. Epidemiol. 16, 201–206 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Singh P. P., Singh A. G., Murad M. H. & Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: A systematic review and meta-analysis. Gastroenterology 144, 323–332, doi: 10.1053/j.gastro.2012.10.005 (2013). [DOI] [PubMed] [Google Scholar]
- Pradelli D. et al. Statins and primary liver cancer: a meta-analysis of observational studies. Eur. J. Cancer Prev. 22, 229–234, doi: 10.1097/CEJ.0b013e328358761a (2013). [DOI] [PubMed] [Google Scholar]
- Hsiang J. C. et al. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: A propensity score landmark analysis. J. Hepatol. 63, 1190–1197, doi: 10.1016/j.jhep.2015.07.009 (2015). [DOI] [PubMed] [Google Scholar]
- Lytras T., Nikolopoulos G. & Bonovas S. Statins and the risk of colorectal cancer: an updated systematic review and meta-analysis of 40 studies. World J. Gastroenterol. 20, 1858–1870, doi: 10.3748/wjg.v20.i7.1858 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012 (2000). [DOI] [PubMed] [Google Scholar]
- Friedman G. D. et al. Screening statins for possible carcinogenic risk: Up to 9 years of follow-up of 361 859 recipients. Pharmacoepidemiol. Drug Saf. 17, 27–36 (2008). [DOI] [PubMed] [Google Scholar]
- Orsini N., Bellocco R. & Greenland S. Generalized least squares for trend estimation of summarized dose–response data. The Stata Journal 6(1), 40–57 (2006). [Google Scholar]
- Desquilbet L. & Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 29, 1037–1057, doi: 10.1002/sim.3841 (2010). [DOI] [PubMed] [Google Scholar]
- Chaiteerakij R. et al. Risk factors for intrahepatic cholangiocarcinoma: association between metformin use and reduced cancer risk. Hepatology 57, 648–655, doi: 10.1002/hep.26092 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marelli C. et al. Statins and risk of cancer: a retrospective cohort analysis of 45,857 matched pairs from an electronic medical records database of 11 million adult Americans. J. Am. Coll. Cardiol. 58, 530–537, doi: 10.1016/j.jacc.2011.04.015 (2011). [DOI] [PubMed] [Google Scholar]
- Butt A. A. et al. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons: Results from ERCHIVES. Hepatology 62, 365–374, doi: 10.1002/hep.27835 (2015). [DOI] [PubMed] [Google Scholar]
- Tsan Y., Lin M., Ho W. & Chen P. Nucleoside analogues, statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. Value Health 18, A193 (2015). [Google Scholar]
- Leung H. W., Chan A. L., Lo D., Leung J. H. & Chen H. L. Common cancer risk and statins: A population-based case-control study in a Chinese population. Expert Opin. Drug Saf. 12, 19–27, doi: 10.1517/14740338.2013.744392 (2013). [DOI] [PubMed] [Google Scholar]
- Björkhem-Bergman L., Backheden M. & Söderberg Löfdal K. Statin treatment reduces the risk of hepatocellular carcinoma but not colon cancer-results from a nationwide case-control study in Sweden. Pharmacoepidemiol. Drug Saf. 23, 1101–1106, doi: 10.1002/pds.3685 (2014). [DOI] [PubMed] [Google Scholar]
- King L. Y., Khalili H., Huang E., Chung R. T. & Chan A. T. Statins are associated with a reduced risk of liver cancer: Data from a large U.S. Prospective cohort study. Hepatology 58, 1216A–1217A (2013). [Google Scholar]
- Emberson J. R. et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PloS one 7, e29849, doi: 10.1371/journal.pone.0029849 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag H. B., Johnson M. L., Hachem C. & Morgana R. O. Statins Are Associated With a Reduced Risk of Hepatocellular Carcinoma in a Large Cohort of Patients With Diabetes. Gastroenterology 136, 1601–1608, doi: 10.1053/j.gastro.2009.01.053 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana V., Saluja A., Caldito G., Fort C. & Schiff E. Gastroenterology A714–A714 (2005). [Google Scholar]
- Friis S. et al. Cancer risk among statin users: A population-based cohort study. Int. J. Cancer 114, 643–647 (2005). [DOI] [PubMed] [Google Scholar]
- Demierre M. F., Higgins P. D., Gruber S. B., Hawk E. & Lippman S. M. Statins and cancer prevention. Nat. Rev. Cancer 5, 930–942, doi: 10.1038/nrc1751 (2005). [DOI] [PubMed] [Google Scholar]
- McTaggart F. et al. Preclinical and clinical pharmacology of Rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am. J. Cardiol. 87, 28B–32B (2001). [DOI] [PubMed] [Google Scholar]
- Efthimiadis A. Rosuvastatin and cardiovascular disease: did the strongest statin hold the initial promises? Angiology 59, 62S–64S, doi: 10.1177/0003319708321668 (2008). [DOI] [PubMed] [Google Scholar]
- Jones P. H. et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am. J. Cardiol. 92, 152–160 (2003). [DOI] [PubMed] [Google Scholar]
- Paoletti R., Fahmy M., Mahla G., Mizan J. & Southworth H. Rosuvastatin demonstrates greater reduction of low-density lipoprotein cholesterol compared with pravastatin and simvastatin in hypercholesterolaemic patients: a randomized, double-blind study. J. Cardiovasc. Risk 8, 383–390 (2001). [DOI] [PubMed] [Google Scholar]
- Hennekens C. H. et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 334, 1145–1149, doi: 10.1056/nejm199605023341801 (1996). [DOI] [PubMed] [Google Scholar]
- Wang Z. J. et al. Drug-eluting stents versus bare-metal stents in patients with decreased GFR: a meta-analysis. Am. J. Kidney Dis. 62, 711–721, doi: 10.1053/j.ajkd.2013.04.014 (2013). [DOI] [PubMed] [Google Scholar]
- Bader T. & Korba B. Simvastatin potentiates the anti-hepatitis B virus activity of FDA-approved nucleoside analogue inhibitors in vitro. Antiviral Res. 86, 241–245, doi: 10.1016/j.antiviral.2010.02.325 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delang L. et al. Statins potentiate the in vitro anti-hepatitis C virus activity of selective hepatitis C virus inhibitors and delay or prevent resistance development. Hepatology 50, 6–16, doi: 10.1002/hep.22916 (2009). [DOI] [PubMed] [Google Scholar]
- Verpaalen B., Neyts J. & Delang L. Are statins a viable option for the treatment of infections with the hepatitis C virus? Antiviral Res. 105, 92–99, doi: 10.1016/j.antiviral.2014.02.020 (2014). [DOI] [PubMed] [Google Scholar]
- Lewis J. H. et al. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 46, 1453–1463, doi: 10.1002/hep.21848 (2007). [DOI] [PubMed] [Google Scholar]
- Matusewicz L., Meissner J., Toporkiewicz M. & Sikorski A. F. The effect of statins on cancer cells--review. Tumour Biol. 36, 4889–4904, doi: 10.1007/s13277-015-3551-7 (2015). [DOI] [PubMed] [Google Scholar]
- Jaffe A. B. & Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell. Dev. Biol. 21, 247–269, doi: 10.1146/annurev.cellbio.21.020604.150721 (2005). [DOI] [PubMed] [Google Scholar]
- Wood W. G., Igbavboa U., Muller W. E. & Eckert G. P. Statins, Bcl-2, and apoptosis: cell death or cell protection? Mol. Neurobiol. 48, 308–314, doi: 10.1007/s12035-013-8496-5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z. et al. MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 71, 2286–2297, doi: 10.1158/0008-5472.can-10-3367 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifulco M. Debate on adverse effects of statins. Eur. J. Intern. Med. 25, e95, doi: 10.1016/j.ejim.2014.08.006 (2014). [DOI] [PubMed] [Google Scholar]
- Levesque L. E., Hanley J. A., Kezouh A. & Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 340, b5087, doi: 10.1136/bmj.b5087 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.