Abstract
Artemisinin resistance is rapidly spreading in Southeast Asia. The efficacy of artemisinin-combination therapy (ACT) continues to be excellent across Africa. We performed parasite transcriptional profiling and genotyping on samples from an antimalarial treatment trial in Uganda. We used qRT-PCR and genotyping to characterize residual circulating parasite populations after treatment with either ACT or ACT-primaquine. Transcripts suggestive of circulating ring stage parasites were present after treatment at a prevalence of >25% until at least 14 days post initiation of treatment. Greater than 98% of all ring stage parasites were cleared within the first 3 days, but subsequently persisted at low concentrations until day 14 after treatment. Genotyping demonstrated a significant decrease in multiplicity of infection within the first 2 days in both ACT and ACT-primaquine arms. However, multiple clone infections persisted until day 14 post treatment. Our data suggest the presence of genetically diverse persisting parasite populations after ACT treatment. Although we did not demonstrate clinical treatment failures after ACT and the viability and transmissibility of persisting ring stage parasites remain to be shown, these findings are of relevance for the interpretation of parasite clearance transmission dynamics and for monitoring drug effects in Plasmodium falciparum parasites.
Malaria remains a leading cause of global morbidity and mortality with approximately 584,000 deaths and 198 million clinical cases of malaria in 20131. Prompt administration of effective antimalarial treatment remains a critical strategy for reducing the burden of malaria2,3. Artemisinin combination therapy (ACT) is universally recommended for uncomplicated P. falciparum malaria, and continued efficacy is crucial for successful elimination and eradication efforts. The high potency of artemisinins results in a rapid initial reduction of the biomass of asexual malaria parasites, while the more slowly eliminated partner drug contributes to parasite clearance and prevents subsequent recrudescent infections4,5. Despite the emergence and spread of parasites with a slow clearing phenotype after artesunate monotherapy across Southeast Asia, parasite clearance remains rapid in Africa with <5% of patients being parasite positive by microscopy on day 3 after initiation of ACT treatment6,7. However, at least one third of patients may remain parasite positive by DNA-based molecular detection methods8,9. If this DNA persistence reflects persistence of parasites following treatment, this may have implications for the likelihood of onward malaria transmission to mosquitoes, the feasibility of elimination in low transmission settings, and for the evolution of drug resistant parasites.
The significance of DNA-based detection of residual parasitemia after ACT treatment is currently unknown. While residual submicroscopic parasitemia has been associated with subsequent recrudescence of infections8, a PCR signal may also be explained by residual circulating DNA molecules, or by the persistence of non-replicating mature gametocytes that can persist at low concentrations for several weeks after treatment8. The persistence of replicating or dormant asexual stage parasites after ACT treatment would have important implications for understanding mechanisms of drug action and the ability of parasites to tolerate drug treatment10,11. Here, we use a recently developed transcript-based qRT-PCR assay12 to sensitively detect and quantify asexual and gametocyte populations at submicroscopic levels after treatment with the ACT artemether-lumefantrine alone (AL) or in combination with the gametocytocidal drug primaquine (AL−PQ).
Results
Persistence of RNA transcripts suggestive of circulating ring and mature gametocyte stages
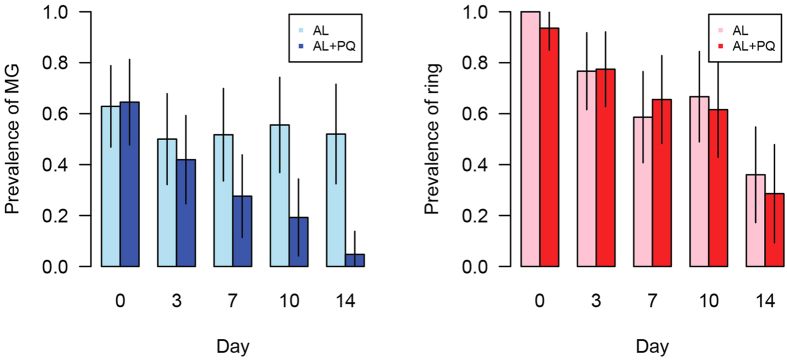
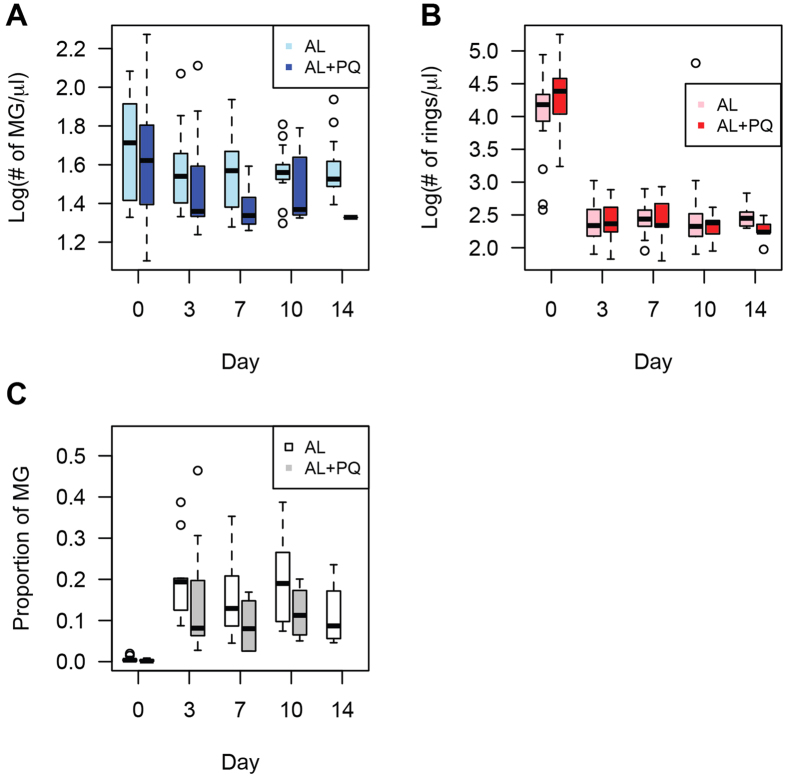
In the original analysis of samples analyzed in the AL or AL−PQ treatment arms of the study, total parasitemia and presence of gametocytes were determined by microscopy, and Pfs25 QT-NASBA was used for mature gametocyte detection. This analysis demonstrated complete clearance of patent parasitemia at day 3 in both treatment arms, and clearance of mature gametocyte transcripts in 94% of patients by day 14 upon primaquine treatment13. Using a recently developed and highly sensitive qRT-PCR assay for stage-specific detection of P. falciparum parasites12, we were able to confirm the persistence of mature gametocytes upon AL treatment and efficient clearance with the addition of 0.75 mg/kg primaquine (Fig. 1A and S2). Surprisingly, however, we also observed the presence of RNA transcripts suggestive of ring stage parasites at a prevalence of >25% until day 14 in both treatment arms (Fig. 1B). Estimates of parasite numbers using regression analysis based on data points with both microscopic and qRT-PCR detection of parasite stages (Fig. S1A) demonstrated reduction of the concentration of ring stages by >98% within the first 3 days of treatment but persistence at low levels until day 14 (Fig. 2B). Differential reduction in ring versus mature gametocyte loads upon treatment (Fig. 2A,B) resulted in an increase in the fraction of all parasites that were mature gametocytes. This fraction rose from <0.4% on day 0 to 20% on day 3 in the AL arm, and from 0.1 to 8.1% in the same time frame in the AL-PQ arm (Fig. 2C). We have previously demonstrated that the mature gametocyte marker is not expressed in ring stage parasites12, and although the ring stage marker is not exclusively expressed in the ring stage, we performed cellular sensitivity analysis across the parasite cycle for the ring stage marker, and showed that the observed signal of this marker could not originate from mature gametocytes in the patient samples (Fig. S1B). This hypothesis is supported by the lack of any significant correlation between the prevalence of ring stage marker and either PF3D7_1438800 or Pfs25 (from the QT-NASBA data) across patient samples and treatment arms. In contrast the two different gametocyte markers are highly correlated in the AL group (p-value = 2.2 × 10−14) and in the AL-PQ group (p-value = 3.2 × 10−5), and in the subset of persisting parasites in both groups (p-value = 1.1 × 10−18), using a partial correlation test controlling for the time of sampling. Together these data suggest the existence of low-level persistent ring stage parasites upon AL treatment, alone or in combination with primaquine, among African malaria patients from Uganda.
Figure 1. Changes in asexual and sexual stage parasite prevalence over time.
(A) The prevalence of mature gametocytes (MG, PF3D7_1438800) decreases more rapidly in the AL-PQ group (the slope of simple linear regression = −0.0045 (95% CI = [−0.014, 0.0047]) for the AL group and −0.040 [95% CI = [−0.049, −0.032] for the AL + PQ group). (B) The prevalence of rings (PF3D7_0501300) through time is similar in the AL group and the AL + PQ group (the slope of simple linear regression = −0.040 (95% CI = [−0.057, −0.023]) for the AL group and −0.042 [95% CI = [−0.054, −0.029] for the AL + PQ group).
Figure 2. Changes in ring stage and mature gametocyte stages through time.
(A) The number of mature gametocytes after day 3 is significantly smaller in the AL + PQ group (p-value = 0.0057). (B) The number of rings decreases between day 0 and day 3, and the level of the decrease is similar in the AL group and the AL + PQ group (p-value = 0.18). (C) The proportion of the total parasite population that represents mature gametocytes increases during follow-up (Mann-Whitney test, p-values = 7.92 × 10−10 [AL] and 1.01 × 10−7 [AL + PQ]).
Genotypic analysis of persisting ring stage parasites
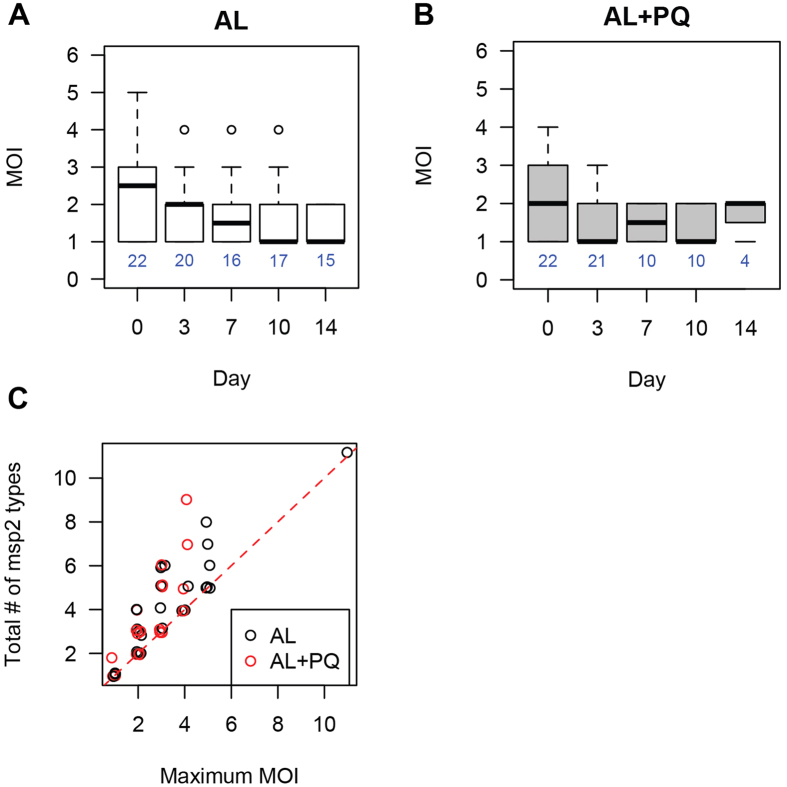
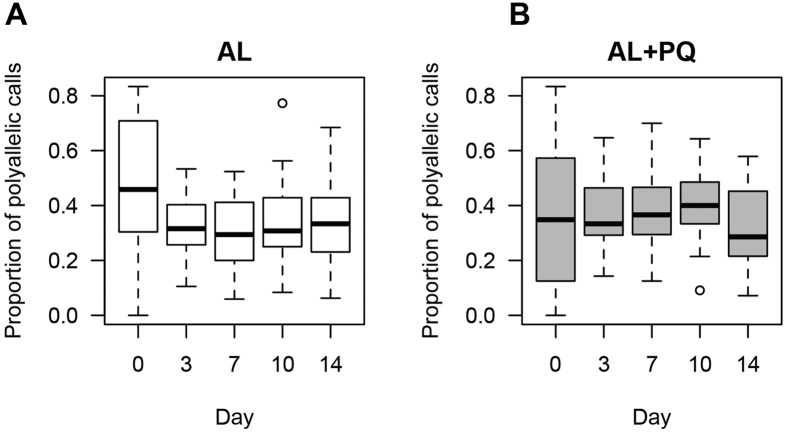
The observed presence of a low level reservoir of circulating ring stage parasites at day 14 demonstrated that these parasites persisted after a fully observed curative dose of AL. To determine possible selection of parasite subpopulations upon treatment we performed msp2 genotyping (Fig. 3). We found that multiplicity of infection (MOI) decreased significantly in both the AL and the AL-PQ arms between day 0 and day 3 (Mann-Whitney Test, p-value = 0.05 and 0.02, respectively), however many of them remained >1 (Fig. 3A,B). Interestingly, a subset of msp2 genotypes was significantly enriched in the persistent parasite population (Table S1). We also found that in several children the total number of msp2 genotypes detected during follow-up was greater than the maximum MOI for any given day (Fig. 3C), suggesting some dynamics in the composition of the parasite population at later days post treatment. In 88.2% (16/18) of patients with successful msp2 genotyping of persisting parasites on day 14 and all preceding days, the parasite genotypes on day 14 were also detected on preceding days. This indicates that re-infections were uncommon during the first 14 days of follow-up14 and the vast majority of parasites detected on day 14 were already present at enrollment. Similar analyses using a recently developed molecular barcode tool15 found a high proportion of polyallelic calls in the barcode (Fig. 4A,B) and that the major barcode alleles changed across the time of infection, suggesting that multiple clones are circulating in the patients and that the dominant genotype can fluctuate over time.
Figure 3. MSP2 genotyping to determine multiplicity of infection.
(A,B) MOI decreases after treatment in both groups (Mann-Whitney test, p-values = 0.0016 [AL] and 0.0081 [AL + PQ]). Instead of having one dominant genotype, multiple genotypes are circulating on later days in some samples. One patient sample has MOI = 11 on day 0 in the AL group and is not shown in the figure. (C) Total number of msp2 types is greater than maximum MOI in some patients, indicating that new genotypes emerge later in the patients.
Figure 4. Molecular barcode analysis to determine multiplicity of infection.
(A,B) Highly polymorphic molecular barcodes suggest that multiple genotypes are circulating both before and after treatment.
Residual ring stage parasitemia by day 3 was not significantly associated with patient age (medians are 4 [cleared] and 4.5 [residual], Mann-Whitney Test p-value = 0.70), enrollment parasite density (medians are 23,950 [cleared] and 26,670 [residual], Mann-Whitney Test p-value = 0.45) or any other patient or infection characteristics (Table 1). Similarly, residual ring stage parasitemia by day 14 was not associated with these patient or infection characteristics (Table S2). The proportion of individuals with gametocytes by microscopy, QT-NASBA or multiplexed qRT-PCR, at day 0 was not associated with residual ring stage parasitemia (Fisher’s Exact Test, p-values are 0.70 [microscopy], 0.61 [QT-NASBA], and 0.44 [qRT-PCR]).
Table 1. Characteristics of cleared and residual parasitemia*.
| Cleared parasitemia | Residual parasitemia | p-value | |
|---|---|---|---|
| Number of patients (day 3) | 9 | 61 | |
| Number of patients (day 14) | 55 | 15 | |
| Age | 4 | 4.5 | 0.70 |
| Gender (male:female) | 5:4 | 27:34 | 0.72 |
| Asexual parasite densityϕ by microscopy at enrollment | 23950 | 26670 | 0.45 |
| Gametocyte prevalence by microscopy at enrollment | 50% | 37% | 0.70 |
| Gametocyte prevalence by microscopy at day 7 | 0 | 7.84% | 1 |
| Treatment, % AL + PQ treated | 44% | 49% | 1 |
| MOI at enrollment | 1.5 | 2 | 0.38 |
ϕUnit of parasite density is the number of parasites per μl.
*Medians are shown.
Discussion
Using a multiplexed qRT-PCR assay for stage-specific detection of P. falciparum mRNA transcripts, we provide evidence of persisting submicroscopic densities of ring stage parasites after successful treatment with either AL alone or plus primaquine. While parasite densities and the estimated number of clones in infections were reduced in the first 3 days after treatment9, more than 25% of the patients harbored low-density parasite populations based on stage-specific transcript quantification, that were frequently clonally complex and persisted for at least 14 days after initiation of treatment. Transcripts of our ring marker disappear after radical cure of experimental infections in human volunteers; when subcurative doses are administered and recrudescent infection occurs, ring marker transcripts reappear in the blood coincidentally with recrudescence (James McCarthy, personal communication). Although the dynamics of transcript clearance may be different in naturally exposed malaria-infected individuals who have clonally complex infections, non-synchronous infections and pre-exiting immunity, our findings suggest the persistence of very low concentrations of ring-stage parasites after apparently successful ACT treatment.
The role of persisting submicroscopic infections in determining treatment outcome, transmission dynamics and possibly drug resistance remains to be established. Previous studies on continued PCR positivity after ACT treatment were unable to reliably differentiate between residual circulating DNA molecules or gametocytes8,9,16,17. Our analysis based on stage specific mRNA transcripts, overcomes the uncertainties of DNA-based assays. Although our findings fall short of conclusively showing that RNA transcripts represent viable parasites, they may shed new light on parasite clearance dynamics after ACT treatment. Parasite carriage has been reported to persist for several months in a fraction of chloroquine-treated patients18,19. This chronic carriage is predominantly submicrosopic, only rarely being detectable by microscopy, and associated with continued gametocyte production18,19. It is conceivable that also after AL a fraction of patients may continue to harbor viable parasites that persist as chronic infections. In our study, the apparent persistence of ring stage parasites was not associated with gametocyte carriage or later recurrent parasite carriage, as recrudescence was only detected in 3.7% of patients13. We therefore did not observe direct consequences of residual ring stage parasitemia in terms of clinical response or transmission potential during the 28 days of follow-up. This also means that we did not provide conclusive evidence on the viability of the parasite populations that we detect based on ring-stage RNA. Studies that involve monitoring parasite populations for a longer period of follow-up or studies that attempt to culture very low parasite densities that persist after treatment may provide more definitive evidence for the viability and relevance of the parasite populations we detected. Our findings may also reflect a normal but previously unknown process of slow clearing, submicroscopic parasite populations that is specific to ACT treatment.
We hypothesized that increasing age, being an indicator of higher cumulative malaria exposure and thus immunity, would be associated with faster clearance of infections20. However, we observed persistence of rings among all ages (1 to 10 years), and there was no significant difference in age between patients with cleared and residual parasitemia. We observed that the number of rings at day 0 decreases with age, while neither the number of mature gametocytes nor MOI at day 0 change with age (Fig. S3). These observations suggest that age and therefore increasing exposure protects from high (asexual) parasite burden but not from gametocyte burden. Hence, the proportion of the total parasite population that represents mature gametocytes increases with age (irrespective of treatment), consistent with previous studies using microscopy or QT-NASBA data21,22.
The estimated ring stage parasite concentrations were very low beyond day 3 after initiation of treatment, with values below 2% of the original parasite density8. The precision of individual parasite densities is limited at these low concentrations, illustrated by our mean estimated densities of ~200 parasites/μl at day 3, which, although not detected by microscopy in our study, should be detectable by an expert microscopist. A better quantification of low parasite densities requires model optimization that would benefit from a larger number of natural low-density infections with asexual parasite quantification by qRT-PCR and microscopy to take into account possible variation in transcript numbers between parasite strains.
The reason for the persistence of parasite transcripts that is suggestive of ring-stage persistence, after apparently successful treatment is unknown. Low levels of persisting parasites might originate from dormant parasite populations or a sequestered reservoir, might represent remaining parasites during therapy due to therapeutic pharmacokinetic properties, or might be associated with resistance to drugs. We observed no evidence of an association with drug resistance, parasitological and clinical cure rates by microscopy were excellent13, as is reported elsewhere in Uganda23,24. If the persistence would be due to drug resistance, only drug-resistant lineages could persist and we expect to observe low MOI and similar parasites that are persisting. However, although MOI decreased after treatment, many MOIs were greater than one (Fig. 3A,B), the major barcode changed through time, and persisting parasites have very diverse msp2 types (although two msp2 types are enriched among persisting parasite populations). The detection of msp2 genotypes at individual time points is imperfect and influenced by parasite densities, sequestration patterns and differences in amplification efficiency of smaller and larger msp2 alleles25. Although this may have influenced the accuracy of MOI estimates at individual time points, it is unlikely to have affected comparisons between treatment arms and a decline in MOI over time, or persistence of certain genotypes. Overall, our findings suggest that ring persistence is not caused by drug resistance selection on a particular genetic background. In vitro studies have revealed that parasites may be able to tolerate artemisinin treatment by entering a temporarily growth-arrested state of dormancy10 from which a fraction may recover. If the persisting ring stage parasites in our population reflect such dormant parasite populations, re-treatment of individuals with the same drug may be equally efficacious26 and ring stage parasite persistence should not be seen as an indicator of parasite resistance or clinically relevant altered clearance dynamics. Persisting ring stage parasites may also originate from a sequestered reservoir that is partially protected from drug treatment. Recent identification of the human bone marrow as a reservoir for parasite sequestration and gametocyte development27 supports this hypothesis and would explain our finding of newly appearing genotypes during follow up; this may also be associated with the imperfect detectability of individual parasite clones28(Fig. 2A). We observed that the majority of these new clones appeared in the first week after initiation of treatment, reinforcing previous observations that re-infections are unlikely within the first 14 days after AL14, and suggesting that they are an unlikely explanation for the observed long persistence of ring stage parasites in our study.
In summary, we present preliminary evidence for persisting submicroscopic parasite populations that comprise ring stage parasites and gametocytes after treatment with AL or AL-PQ, treatment combinations that were efficacious in preventing recurrent parasite carriage by microscopy for at least 28 days. While the viability of these parasite populations remains to be established and we observed no evidence that parasite populations have no immediate clinical consequences for the treated patients, our findings are relevant for the interpretation of parasite clearance and transmission dynamics. Future in vivo studies are needed to confirm that the persistence of transcripts of ring stage parasites indicate viable parasite populations. These studies should be designed to determine whether the phenomenon of ring stage persistence is a characteristic of ACT treatment and allow for the assessment of late recrudescent infections and/or de novo generation of gametocytes from low levels of persisting parasite populations.
Methods
Ethics statement
All experiments were performed in accordance with relevant guidelines and regulations. Specifically samples for this study were derived from a previously published randomized, double blind, placebo-controlled trial with four treatment arms13. This study was approved by Makerere University School of Medicine (2011–210), the Uganda National Council of Science and Technology (HS1056), and the London School of Hygiene and Tropical Medicine (5987) and was registered at ClinicalTrials.gov (NCT01365598). Informed consent was obtained from all subjects, and all samples were made anonymous for analysis.
Study cohort
Children aged 1–10 years with uncomplicated P. falciparum mono-infection with a parasite density lower than 500,000 parasites per μL, normal G6PD enzyme function and hemoglobin concentration ≥ 80 g/L were randomized to treatment with AL alone (AL group) or in combination with 0.1, 0.4 or 0.75 mg/kg primaquine (AL-PQ group). For the current analysis, we selected 37 children from the AL arm and 33 children from the AL-PQ arm with a dose of 0.75 mg/kg primaquine, using random tables generated in Stata (version 12.0). Children were followed for 28 days for safety parameters, recrudescence or re-infection. Blood samples were obtained by finger prick on days 0, 3, 7, 10 and 14 after initiation of treatment, and immediately transferred to L6 buffer (Severn Biotech Limited, Kidderminster, UK) and stored at −80 °C until extraction. Nucleic acid material was extracted from 50 μL aliquots using Total Nucleic Acid Isolation Kits–High Performance (Roche Applied Science, Mannheim, Germany) and a MagNA Pure LC automated extractor (Roche Applied Science).
Molecular detection of parasite stages
The patient’s parasite burden was quantified using microscopy, gametocyte detection by Pfs25 mRNA quantitative nucleic acid sequence based amplification13 and with a highly sensitive stage-specific qRT-PCR assay, as described previously12. The latter assay combines stage-specific markers to quantify the relative abundance of asexual and sexual P. falciparum parasites from patient samples using probe-based qRT-PCR. Reverse transcription was performed from extracted nuclear acid samples using SuperScript III First-Strand Synthesis System (Life Technologies, Carlsbad, CA). Custom primers (Integrated DNA Technologies, Coralville, IA) and Taqman probes (Life Technologies) were used at 900 nM and 250 nM final concentrations, respectively, to measure the transcript levels of PF3D7_0501300 (previous accession number PFE0065w; for ring stage parasites), PF3D7_1438800 (PF14_0367; for mature gametocyte parasites) and PF3D7_1120200 (PF11_0209; as a constitutive parasite marker) on a Viia7 qRT-PCR machine (Life Technologies).
A linear regression model was developed to estimate the density of circulating ring and mature gametocyte parasites. We used the patient’s patent parasitemia (i.e., density of ring and mature gametocyte stages) obtained using Giemsa stained microscopy slide to define the relationship between parasite density and CT values from qRT-PCR (ring and mature gametocyte marker, respectively) by regression analysis. The regression between microscopy and qRT-PCR was then used to infer parasite density in the remainder of the data points with sub-patent parasite levels (Fig. S1A). Multiple linear regressions were used to test the difference in parasite density in the AL and the AL + PQ groups while controlling for the day on which the samples were collected.
To determine the sensitivity of our ring marker detection by qRT-PCR assay, we performed a cellular dilution experiment under controlled in vitro conditions with stage-specific populations of P. falciparum strain 3D7 (Fig. S1B). Parasites were cultured in human type O+ red blood cells (Research Blood Components, Boston, MA) diluted to 4% hematocrit in RPMI-1640 medium (Sigma-Aldrich, St. Louis MO) supplemented with 10% human serum (Interstate Blood Bank, Memphis, TN), 25 mM HEPES (EMD Biosciences), sodium bicarbonate (Sigma), and hypoxanthine (Sigma), as described previously29. Parasite cultures were maintained at 37 °C in an atmosphere of 1% O2 and 5% CO, 94% N2. The parasites were synchronized with 5% D-sorbitol, and time points were collected for pure stage-specific populations at 8, 16, 24, 32, 40 hours post invasion for asexual parasite stages, and days 2, 4, and 7 post gametocyte induction using partially spent (“conditioned”) medium for sexual parasite stages30,31. Serial dilutions of purified red blood cells (RBC) infected synchronized populations of the above parasite stages were made into uninfected RBCs, and RNA was extracted using Trizol (Life Technologies) and RNeasy (Qiagen, Valencia, CA) for subsequent cDNA synthesis and qRT-PCR as described above.
Multiplicity of infection and genotyping analysis
DNA from all time points was tested for clonal complexity based on genotyping for the polymorphic marker gene merozoite surface protein 2 (msp2) using capillary electrophoresis for fragment sizing32 following chelex-saponin extraction from filter papers (Whatman 3 MM, Maidstone, UK). Results were analyzed with Peak Scanner version 1.0 (Applied Biosystems) with discrete peaks being interpreted as unique clones, after adjustment for background levels per experiment. Nucleic acids from the whole blood samples in L6 buffer that were extracted by MagNA Pure LC automated extractor, as described above, were further analyzed using previously described molecular barcode methods15. Briefly, 1 μL of each extracted sample was preamplified33 in 20 μL reactions using the TaqMan Preamp Master Mix for 14 cycles according to manufacturer’s directions (Applied Biosystems) using 0.2× pooled TaqMan barcoding assays. Samples were diluted 1:20 and 5 μL each preamplified product used for the molecular barcode. TaqMan barcoding assays were run on a ViiA system (Applied Biosystems) and genotypes called using the pre-installed analysis software.
Additional Information
How to cite this article: Chang, H.-H. et al. Persistence of plasmodium falciparum parasitemia after artemisinin combination therapy: evidence from a randomized trial in Uganda. Sci. Rep. 6, 26330; doi: 10.1038/srep26330 (2016).
Supplementary Material
Acknowledgments
This work was supported by Bill and Melinda Gates Foundation grants OPP1053604 (E.M., J.Z.., R.D., E.C.M., D.F.W., S.K.V. and M.M.) and OPP1034789 (A.C.E., F.T., L.G., C.D. and T.B.). T.B. was also supported by a fellowship from the European Research Council (ERC-2014-StG 639776), and C.D. by the Wellcome Trust (090558). H.H.C. and C.B. were supported by the National Institute Of General Medical Sciences (U54GM088558). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author Contributions H.H.C. and C.B. performed statistical data analysis. E.M., J.Z. and E.C.M. performed all qRT-PCR assays under the direction of M.M. R.D. performed molecular barcode genotyping under the direction of S.K.V. and D.F.W. F.T. and L.G. performed MSP2 genotyping under the direction of C.D. and T.B. A.C.E. and R.J. provided input in study design and analysis. T.B. and M.M. designed the study with input from C.B., D.F.W., S.K.V. and C.D. H.H.C., T.B. and M.M. wrote the manuscript with input from all co-authors.
References
- Organization W. H. World Malaria Report 2014 (2014).
- Moonen B. et al. Operational strategies to achieve and maintain malaria elimination. Lancet 376, 1592–1603, doi: 10.1016/S0140-6736(10)61269-X (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugs m. C. G. o. A research agenda for malaria eradication: drugs. PLoS medicine 8, e1000402, doi: 10.1371/journal.pmed.1000402 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N. J. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrobial agents and chemotherapy 41, 1413–1422 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjuik M. et al. Artesunate combinations for treatment of malaria: meta-analysis. Lancet 363, 9–17 (2004). [DOI] [PubMed] [Google Scholar]
- Ashley E. A. et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. The New England journal of medicine 371, 411–423, doi: 10.1056/NEJMoa1314981 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Four Artemisinin-Based Combinations Study, G. A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS medicine 8, e1001119, doi: 10.1371/journal.pmed.1001119 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beshir K. B. et al. Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infect Dis 208, 2017–2024, doi: 10.1093/infdis/jit431 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosi P. et al. Evaluation of parasite subpopulations and genetic diversity of the msp1, msp2 and glurp genes during and following artesunate monotherapy treatment of Plasmodium falciparum malaria in Western Cambodia. Malar J 12, 403, doi: 10.1186/1475-2875-12-403 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher F. et al. Artemisinin-induced dormancy in Plasmodium falciparum: duration, recovery rates, and implications in treatment failure. J Infect Dis 202, 1362–1368, doi: 10.1086/656476 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonis N. et al. Altered temporal response of malaria parasites determines differential sensitivity to artemisinin. Proceedings of the National Academy of Sciences of the United States of America 110, 5157–5162, doi: 10.1073/pnas.1217452110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joice R. et al. Inferring developmental stage composition from gene expression in human malaria. PLoS Comput Biol 9, e1003392, doi: 10.1371/journal.pcbi.1003392 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eziefula A. C. et al. Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. The Lancet. Infectious diseases 14, 130–139, doi: 10.1016/S1473-3099(13)70268-8 (2014). [DOI] [PubMed] [Google Scholar]
- Okell L. C. et al. Contrasting benefits of different artemisinin combination therapies as first-line malaria treatments using model-based cost-effectiveness analysis. Nat Commun 5, 5606, doi: 10.1038/ncomms6606 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R. et al. A general SNP-based molecular barcode for Plasmodium falciparum identification and tracking. Malar J 7, 223, doi: 1475-2875-7-223 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A. M. et al. Plasmodium falciparum population dynamics during the early phase of anti-malarial drug treatment in Tanzanian children with acute uncomplicated malaria. Malar J 10, 380, doi: 10.1186/1475-2875-10-380 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betson M. et al. Detection of persistent Plasmodium spp. infections in Ugandan children after artemether-lumefantrine treatment. Parasitology 141, 1880–1890, doi: 10.1017/S003118201400033X (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassir E. et al. Impact of genetic complexity on longevity and gametocytogenesis of Plasmodium falciparum during the dry and transmission-free season of eastern Sudan. International journal for parasitology 35, 49–55, doi: 10.1016/j.ijpara.2004.10.014 (2005). [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab A. et al. Dynamics of gametocytes among Plasmodium falciparum clones in natural infections in an area of highly seasonal transmission. J Infect Dis 185, 1838–1842, doi: JID011146 (2002). [DOI] [PubMed] [Google Scholar]
- White N. J. The parasite clearance curve. Malar J 10, 278, doi: 10.1186/1475-2875-10-278 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T. & Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clinical microbiology reviews 24, 377–410, doi: 10.1128/CMR.00051-10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouedraogo A. L. et al. The plasticity of Plasmodium falciparum gametocytaemia in relation to age in Burkina Faso. Malaria journal 9, 281, doi: 10.1186/1475-2875-9-281 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeka A. et al. Artesunate/Amodiaquine Versus Artemether/Lumefantrine for the Treatment of Uncomplicated Malaria in Uganda: A Randomized Trial. J Infect Dis 213, 1134–1142, doi: 10.1093/infdis/jiv551 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapisi J. et al. Efficacy and safety of artemether-lumefantrine for the treatment of uncomplicated malaria in the setting of three different chemopreventive regimens. Malar J 14, 53, doi: 10.1186/s12936-015-0583-9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. T. et al. Detectability of Plasmodium falciparum clones. Malar J 9, 234, doi: 10.1186/1475-2875-9-234 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries P. J. & Dien T. K. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs 52, 818–836 (1996). [DOI] [PubMed] [Google Scholar]
- Joice R. et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Science translational medicine 6, 244re245, doi: 10.1126/scitranslmed.3008882 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepfli C. et al. How much remains undetected? Probability of molecular detection of human Plasmodia in the field. PLoS One 6, e19010, doi: 10.1371/journal.pone.0019010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W. & Jensen J. B. Human malaria parasites in continuous culture. Science 193, 673–675 (1976). [DOI] [PubMed] [Google Scholar]
- Fivelman Q. L. et al. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol Biochem Parasitol 154, 119–123 (2007). [DOI] [PubMed] [Google Scholar]
- Lambros C. & Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. The Journal of parasitology 65, 418–420 (1979). [PubMed] [Google Scholar]
- Mueller I. et al. Force of infection is key to understanding the epidemiology of Plasmodium falciparum malaria in Papua New Guinean children. Proceedings of the National Academy of Sciences of the United States of America 109, 10030–10035, doi: 10.1073/pnas.1200841109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mharakurwa S. et al. Pre-amplification methods for tracking low-grade Plasmodium falciparum populations during scaled-up interventions in Southern Zambia. Malar J 13, 89, doi: 10.1186/1475-2875-13-89 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.