Abstract
Rhodococcus equi is the causative agent of rhodococcosis in horses, resulting in significant morbidity and mortality in foals. This bacterium has also been isolated from a variety of animals and is being increasingly reported as a cause of infection in humans, mainly in immunosuppressed individuals. Laboratory diagnostics of R. equi infections based only on conventional microbiological methods shows low accuracy and can lead to misidentification. The objective of the study was to develop and evaluate a real-time PCR assay for direct detection of R. equi in various clinical specimens, including tissue samples. The species-specific region of the gene encoding R. equi cholesterol oxidase, choE, was used as a qPCR-target. The diagnostic applicability of the assay was confirmed by testing various tissue specimens obtained from horses with clinical signs of rhodoccocal infection and swine submaxillary lymph nodes. The rate of R. equi detection in clinical specimens by the developed assay was higher in comparison to the culture method (90% vs. 60.0% of positive samples) and conventional PCR (90.0% vs. 20.0% of positive samples). In case of 13 samples that were negative in the culture-based method, R. equi was detected by the developed assay. Only in one case, it gave negative result for culture-positive sample. The assay may provide a simple and rapid tool to complement the classical methods of R. equi detection based on culture and phenotypic identification of isolates, as the performed evaluation indicated a high specificity and accuracy of the results.
Keywords: choE, detection, diagnostics, real-time PCR, Rhodococcus equi
Rhodococcus equi is an important opportunistic pathogen of animals and humans. In foals up to 6 months of life, it causes rhodococcosis, most often manifested itself with serious purulent bronchopneumonia, and in pigs and cattle, a variety of suppurative infections, mainly extrapulmonary [4, 7]. R. equi infections in other domestic animals, such as goats or sheep, and wild animals, have been reported occasionally [7, 23, 32]. They are also rare in humans and generally occur mainly in host with immunosuppression [19, 30, 31, 33]. Moreover, R. equi may be found in the gastrointestinal tract, lymph nodes and tonsils of asymptomatic animal hosts [7, 13].
R. equi is a Gram-positive, irregular, non-motile, non-spore-forming, capsule-forming rod. It is a facultative intracellular pathogen that is able to survive and multiply inside the macrophages [13]. Virulent isolates harbor plasmids carrying genes encoding surface virulence-associated proteins (Vaps) [2, 13]. R. equi pathogenicity and host specificity are related to a type of Vap [11, 35]. The majority of equine isolates contain VapA, porcine and human isolates mainly VapB, whereas bovine isolates often contain VapN [34]. The VapA-positive R. equi strains are classified as virulent and the VapB-positive ones as intermediately virulent based on virulence in mice [27]. Strains that do not carry virulence-associated plasmids (VAP), mostly environmental isolates, are considered to be avirulent [28]. Additionally, some chromosomally encoded factors, including cholesterol oxidase (ChoE), hydroxamate siderophore rhequichelin, fibronectin-binding proteins, NADPH-dependent glutamate synthases and others, are required for R. equi virulence [14, 35].
Laboratory detection of R. equi in clinical specimens is currently based mainly on conventional bacteriological methods including isolation of bacteria and their phenotypic identification [16]. However, in some cases, these methods are insufficient to detect the pathogen or may lead to its misidentification. Many authors have reported problems with discrimination between R. equi and Dietzia spp. only on the basis of their morphological and biochemical properties [17, 18]. Moreover, isolates that had been previously phenotypically identified as R. equi, were finally reclassified with a support of molecular methods, not only as other closely related actinomycetes but also as more taxonomically distant species, such as Staphylococcus epidermidis, Exiguobacterium acetylicum, Ochrobactrum oryzae or Sphingomonas dokdonensis [9, 10, 17]. The 16S rDNA sequence and pyrolysis mass spectrometric analyses showed that R. equi strains form a heterogeneous taxonomic group [12]. Thus, the use of diagnostic methods based on PCR has greatly increased in recent years. Nucleic acid amplification techniques allow rapid, accurate and sensitive detection and quantification of many pathogens and have become a valuable tool in routine diagnostics of infectious diseases. Molecular assays have also been developed for R. equi detection, but they are mainly focused on recognition of equine isolates [5, 6, 10, 20, 21, 24, 26, 29, 36].
The aim of this study was development, optimization and sensitivity evaluation of a quantitative real-time PCR assay for detection of R. equi in various clinical samples irrespective of their origin using TaqMan® chemistry.
MATERIALS AND METHODS
Primer design: Primers and a probe targeting a highly conserved region of R. equi gene encoding cholesterol oxidase (choE) (GeneBank: accession no. KF670817.1) were designed using LightCycler Probe Design 2 software (Roche Diagnostics Ltd., Rotkreuz, Switzerland) (Table 1). Sequence specificity of the primers and the probe was confirmed in silico using Basic Local Alignment Search Tool (BLAST) (www.ncbi.nlm.nih.gov/blast/). FAM was chosen as a reporter dye and BHQ-1 as a quencher. In order to control the efficiency of DNA extraction and the presence of PCR inhibitors in the reaction mixtures, an internal control (IC) was performed. The real-time PCR for IC was carried out in the same run, using the primers and the probes targeting the equine β-2-microglobulin gene (GeneBank: accession no. AY124664.1) and swine β-2-microglobulin gene (GeneBank: accession no. AF452448.1), labeled with ROX as a reporter dye and BHQ-2 as a quencher (Table 1). All primers and probes used in the described experiments were made by Metabion (Planegg-Martinsried, Germany).
Table 1. The sequences of primers and probes used in the real-time PCR assay for R. equi detection and for the internal control.
| Primer | Target gene | Sequence (5′–3′) | Amplicon size |
|---|---|---|---|
| RhodF | choE | TCGCTCGACAAGACCTAC | 120 bp |
| RhodR | TTCCATCGTCACGCTGT | ||
| RhodP | FAM-AGCTGACGATCACGACTCTGCA-BHQ1 | ||
| hB2M-F | equine β2-M | GACCTGTCTTTCAGCAAGGA | 113 bp |
| hB2M-R | GGGTCTTTGAGAGTAGAGTG | ||
| hB2M-P | ROX-TGGTGTGGATGAGTATAGTTGCCG-BHQ2 | ||
| sB2M-F | swine β2-M | GATCCTTAACCCACTGAGC | 122 bp |
| sB2M-R | AGAGTCAACTCAAAGTAGGTTTGTA | ||
| sB2M-P | ROX- GTTCCTAGTCGGATTCATTAACCACTGC-BHQ2 |
DNA extraction: Total genomic DNA from bacterial cultures or clinical specimens was isolated using GeneMATRIX Tissue & Bacterial DNA Purification Kit® (EURx, Gdansk, Poland), according to the manufacturer’s instructions with minor modification. Tissue samples (100 mg) were cut into pieces and completely homogenized with 1 ml of buffered saline. One hundred µl of the appropriate material (bacterial cultures, tissue homogenate or other clinical samples) was mixed with 300 µl of LyseT buffer, 40 µl of BL buffer and 10 µl of lysozyme (100 mg/ml), and the mixture was incubated for 30 min at 37°C. The next isolation steps were performed according to the instructions. Isolated DNA was resuspended in the final volume of 100 µl of elution buffer. The amount and quality of DNA were determined using The Thermo Scientific NanoDropTM 1000 Spectrophotometer (Thermo Scientific, Waltham, MA, U.S.A.).
Real-time PCR: All real-time PCR reactions were performed using the amplification mixture TaqMan Master Kit® (Roche Diagnostics GmbH, Mannheim, Germany). Besides chemicals supplied by the kit producer, each final reaction mixture contained 5 µl of DNA template, 1.75 µM of each primer and 150 nM of the probe, in the total volume of 20 µl. The best results of amplification were obtained with activation of thermostable hot-start DNA polymerase for 10 min at 95°C, followed by 40 cycles comprising: denaturation (10 sec at 95°C), primers annealing (15 sec at 62°C) and strand elongation (15 sec at 72°C). After the end of cycling, mixture was cooled down to 40°C for 60 sec. Fluorescence levels were read at wavelengths of 530 nm (FAM) for R. equi DNA and 610 nm (ROX) for IC detection. Each run included positive and negative control reactions. A positive result was defined as a Cp (crossing points) value less than 38.0 cycle. All experiments were performed using LightCycler 2.0 instrument (Roche Diagnostics Ltd.).
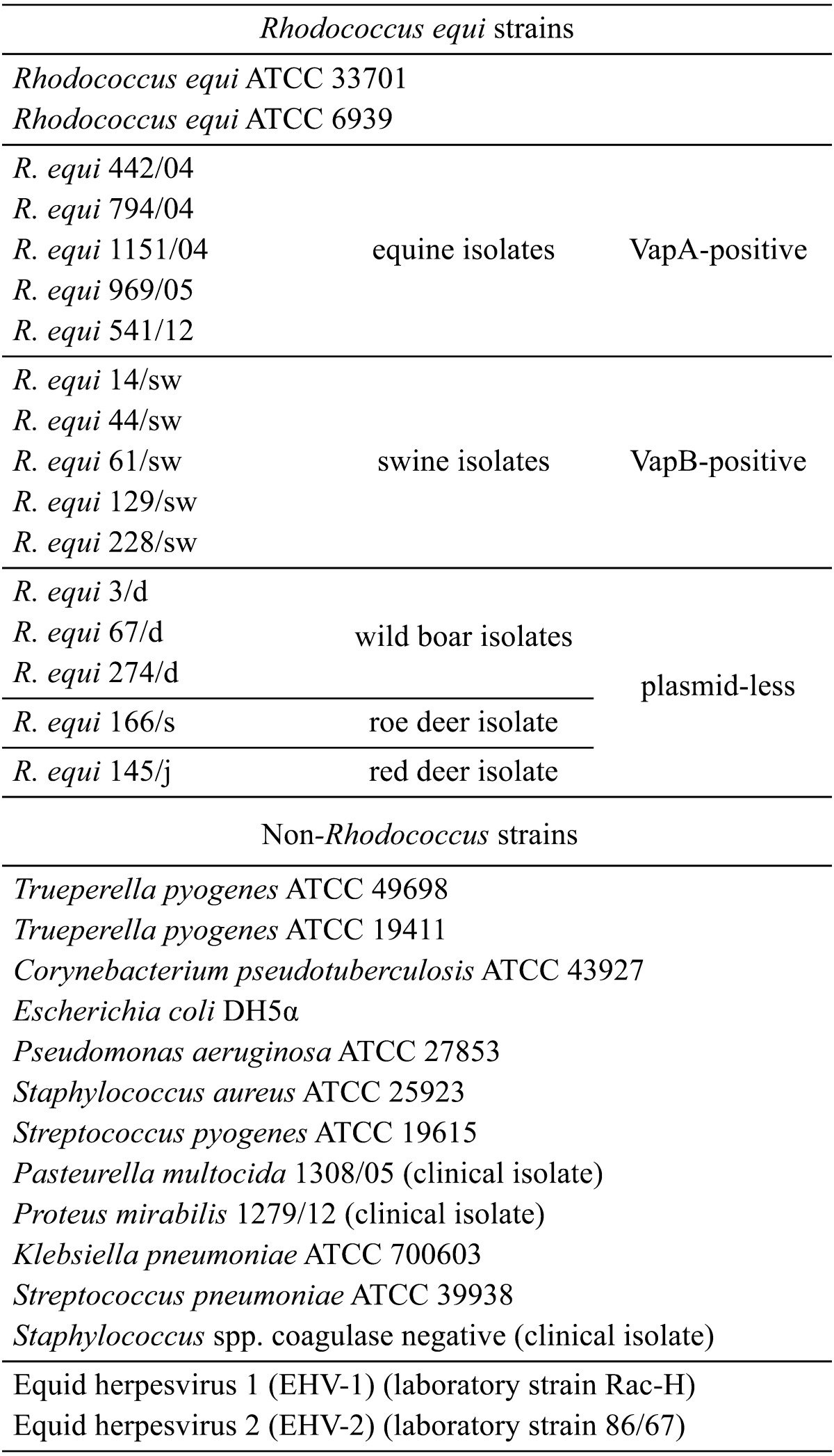
Specificity: The reference strain of R. equi ATCC 33701 served as a positive control in all performed reactions. The inclusivity of the method was evaluated by analyzing two reference R. equi strains (ATCC 33701 and ATCC 6939) and fifteen clinical R. equi isolates of different origin from the collection of the Department of Preclinical Sciences, Faculty of Veterinary Medicine, Warsaw University of Life Sciences (Table 2). The DNA isolated from 12 bacterial non-Rhodococcus strains and two equine viruses (EHV-1 and EHV-2) was used to assess the analytical specificity of the developed real-time PCR assay (Table 2).
Table 2. Rhodococcus equi and non-Rhodococcus strains tested for specificity of the developed assay.
Analytical sensitivity: Analytical sensitivity of the developed assay was determined by analysis of serial dilutions of the R. equi ATCC 33701 genomic DNA (final concentrations of a template ranged from 44 to 0.004 ng/reaction) in six independent repetitions.
Clinical specimens: The diagnostic applicability of the developed assay for a direct detection of R. equi in clinical samples was evaluated by testing 20 specimens collected from swine submaxillary lymph nodes and 20 specimens recovered from diseased horses, including tracheal wash (n=4), abscess (n=3), nasal swab (n=1) and 12 tissue samples (lung, n=9; liver, n=1; spleen, n=1; and lymph node, n=1). The results of the assay were compared to the results of standard laboratory methods, such as a culture of specimens and a conventional PCR technique that targets the choE gene of R. equi. For the R. equi culture, the specimens were inoculated into Columbia agar with sheep blood and a selective CAZ-NB medium (Mueller-Hinton agar base supplemented with ceftazidime−20 µg/ml and novobiocin−25 µg/ml) modified by an addition of cycloheximide−26 µg/ml and 0.005% potassium tellurite. Each R. equi isolate was tested for the vap genes presence by PCR as was described previously [22].
The conventional PCR with primers targeting the choE gene was carried out as described by Ladrón et al. [10]. Amplification reactions were carried out in a 25 µl reaction mixture containing 12.5 µl of DreamTaq Green PCR Master Mix (Fermentas, ThermoFisher Scientific Inc.), 10 pmol of each primer (Genomed S. A., Warsaw, Poland) and 5 µl of total genomic DNA. The PCR thermal profile was: an initial denaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 2 min. After the last cycle, a final extension was performed at 72°C for 10 min. Products of the amplification were separated by electrophoresis through 1.5% agarose gel in TAE buffer, stained with ethidium bromide, visualized and analyzed using a VersaDoc Model 1000 Imaging System and Quantity One software (version 4.4.0; BioRad Laboratories, Hercules, CA, U.S.A.). GeneRuler 100bp DNA Ladder Plus (Fermentas, ThermoFisher Scientific Inc.) was used for estimating the molecular size of the obtained PCR product.
RESULTS
The designed primers and the probe recognized only the target sequence of R. equi and did not show any cross homologies in in silico analysis using the nucleotide blast search. The capacity of the assay to detect only a specific template was also confirmed experimentally with genomic DNA samples extracted from R. equi, including two reference strains and fifteen clinical isolates from different hosts, and 14 non-target templates listed in Table 2. The assay was specific, because all tests with studied R. equi strains gave positive results, and no unspecific amplification signals or cross-reactions with other non-target microorganisms, including common bacterial and viral species, were observed.
The analytical sensitivity of the developed assay was evaluated with serial dilutions of genomic DNA of reference R. equi strain ATCC 33701. A positive signal of amplification was obtained in all DNA dilutions which means that the sensitivity of the assay was at least 0.004 ng of template DNA.
In order to evaluate the usefulness of the developed assay in direct detection of R. equi in specimens, we performed tests for different clinical materials. A total of 20 specimens collected from horses with clinical symptoms of rhodococcal infection and 20 specimens collected from swine were tested using the developed assay, a culture method as the ‘gold standard’ for the laboratory diagnostics and a conventional PCR for amplification of choE gene. The results are shown in Table 3. Among 40 clinical specimens tested by the culture method, 24 were positive for R. equi (60.0%), whereas R. equi DNA was detected by the developed assay in 36 samples (90.0%). The conventional PCR targeting the choE gene has the lowest detection rate, and only 8 studied specimens (20.0%) were positive. The results of both PCR assays were consistent only in 12 samples (30%), while the agreement between developed assay and culture method was 65.0%. Only one sample negative for real-time PCR was culture positive. This sample was also negative in conventional PCR and gave the negative result in the internal control reaction. The range of Cp value for the positive samples was from 32.87 to 35.58. All equine isolates harbored the vapA gene, and swine isolates were vapB-positive.
Table 3. The results of the R. equi detection in clinical specimens from horses and swine using different techniques.
| Sample IDa) | Specimen type | R. equi detection | Comments | |||
|---|---|---|---|---|---|---|
| Cultureb) | conventional PCRb) | real-time PCRb) | Concordancec) | |||
| 1/h | abscess | + | + | + | PC, PP | |
| 2/h | lung | + | – | + | PC | |
| 3/h | lung | – | – | + | N | frozen material of poor quality |
| 4/h | tracheal wash | + | + | + | PC, PP | |
| 5/h | tracheal wash | – | + | + | PP | frozen material |
| 6/h | abscess | + | + | + | PC, PP | |
| 7/h | nasal swab | – | – | – | PC, PP | |
| 8/h | lung | + | – | + | PC | |
| 9/h | lung | + | – | + | PC | |
| 10/h | lung | + | + | + | PC, PP | |
| 11/h | liver | – | – | – | PC, PP | |
| 12/h | spleen | – | – | – | PC, PP | |
| 13/h | lung | + | – | + | PC | |
| 14/h | lymph node | – | –/+ | + | PP | frozen material of poor quality |
| 15/h | lung | – | – | + | N | frozen material of poor quality |
| 16/h | lung | – | – | + | N | frozen material of poor quality |
| 17/h | abscess | + | – | – | N | predominance of pus |
| 18/h | lung | + | – | + | PC | |
| 19/h | tracheal wash | – | –/+ | + | PP | Proteus spp. growth |
| 20/h | tracheal wash | – | – | + | N | Proteus spp. growth |
| 1/s | submaxillary lymph nodes | + | – | + | PC | |
| 2/s | – | – | + | N | ||
| 3/s | + | – | + | PC | ||
| 4/s | + | – | + | PC | ||
| 5/s | – | – | + | N | ||
| 6/s | – | – | + | N | ||
| 7/s | – | – | + | N | ||
| 8/s | + | – | + | PC | ||
| 9/s | + | – | + | PC | ||
| 10/s | + | – | + | PC | ||
| 11/s | + | – | + | PC | ||
| 12/s | – | – | + | N | ||
| 13/s | + | – | + | PC | ||
| 14/s | + | – | + | PC | ||
| 15/s | + | – | + | PC | ||
| 16/s | – | – | + | N | ||
| 17/s | + | – | + | PC | ||
| 18/s | + | – | + | PC | ||
| 19/s | + | –/+ | + | PC, PP | ||
| 20/s | + | – | + | PC | ||
a) /h, horse origin; /s, swine origin; b) +, positive result; –, negative result; –/+, uncertain result; c) PC, concordance with culture-based method present; PP concordance within PCR present, N, lack of concordance.
The internal control for verification of DNA extraction and purification process was included in the study of all clinical specimens. The amplification of the equine and swine β-2-microglobulin genes in all tested samples, except one, showed that the used method of DNA isolation was effective and that no inhibition of the real-time PCR occurred due to the presence of nonspecific polymerase inhibitors in the reactions.
DISCUSSION
Previously developed molecular tests for species-specific detection of R. equi were applied to amplification of different target sequences, including a region of 85–90 kb VapA plasmid found in virulent strains [5, 6, 15, 20, 21, 24, 26, 29], a R. equi specific fragment of the 16S rRNA gene [24, 36], the choE gene [5, 10, 21] or an unidentified genomic region of R. equi [1]. These methods were used to identify the pure culture of strains or to detect the pathogen in certain type of clinical specimens, such as nasopharyngeal swabs [20, 24], tracheobronchial aspirate [6, 20, 24, 29, 36], bronchoalveolar lavage (BAL) [21], blood, serum [24] or feces [20, 36]. To our knowledge, this is the first paper describing the real-time PCR assay applicable for direct R. equi detection in various specimens, including animal tissues. Moreover, the assay allows to amplify the conserved choE gene of R. equi and thus, to detect all strains, not only virulent ones.
The results of the present study show that the use of the developed real-time PCR assay is a rapid, accurate and useful tool for direct detection of that R. equi in different clinical specimens. The specificity of the assay was confirmed with DNA extracted from other clinically relevant bacterial and viral pathogens, especially those associated with pulmonary infections in horses, as well as DNA samples extracted directly from various clinical specimens from horses and swine. We showed the advantages of the developed approach over the culture method and conventional PCR assay. The DNA of R. equi was detected in 23 of 24 culture-positive samples and in 13 of 16 culture-negative samples. Although the real-time PCR assay targets the same region of the R. equi genome as conventional PCR, the results of both tests were consistent for only 11 of 40 samples. That confirms the high sensitivity of the developed assay and its advantages over the conventional PCR based on the same target. In contrast to number of PCR for detecting equine isolates harboring a VapA plasmid, the present real-time PCR assay is designed to the conserved choE gene that is present in all R. equi strains, including those isolated from other hosts.
The sensitivity of the culture-based method can be imperfect, because results are strictly dependent on bacterial growth and thus on quality of specimens. Optimal sample preparation procedures, especially appropriate storage and transport conditions of specimens, are required to reach good sensitivity of a culture, since dead or inhibited bacteria cannot be detected by this method. It should be noted that the samples shipped to the laboratory do not always meet quality standards or may be analyzed immediately. In case of 5 PCR-positive samples where specimens were frozen and of poor quality, no growth of R. equi was observed. Inhibition of bacterial growth may be noted also in clinical specimens obtained during administration of antibiotics [1]. Molecular methods allow detecting the presence of nucleic acids from viable as well as non-viable cells, even in a case of low bacterial number, and thus may exhibit the highest sensitivity. Besides the physiological status of cultured cells, false negative results can also be ascribed to the presence of multiple bacteria species contaminating clinical specimens and overgrowth of fast growing microorganisms that quickly dominate and impair the ability to isolate of R. equi. This situation was observed in two other PCR-positive samples in which R. equi detection by culture was impossible as a result of Proteus spp. growth.
PCR methods are more specific and sensitive to detect a small number of bacteria, even before development of clinical signs of infection and below an amount that could be detected in microbial cultures. On the other hand, the sensitivity of PCR methods may be influenced by the presence of PCR inhibitors, such as DNA-degrading enzymes in a template or in reaction reagents, and by the insufficient quality of a DNA template. The presence of inhibitors interfering with nucleic acid extraction and/or amplification has been observed in different clinical samples, especially in tissue specimens [3, 8]. The presence of large amounts of eukaryotic DNA in a template has also been shown to have inhibitory properties [8]. Difficulties in isolation of DNA from Gram-positive bacteria, such as R. equi and an excess of co-extracted equine DNA from tissue specimens may pose a problem leading to false-negative results in PCR. The optimal sample preparation, monitoring and accurate assessment of the presence of PCR inhibitors are required in order to eliminate false negative results. The inclusion of the internal control to the PCR confirms correct extraction and purification steps and thus acceptable quality of a template. Therefore, in the present real-time PCR assay, the internal control with oligonucleotides specific for equine and swine β-2-microglobulins was performed for all clinical samples to check the presence of inhibitors and to eliminate false negative results. The internal control assay gave positive result for 39 samples, which indicated the lack of inhibitor components in the reaction mixture. The PCR negative samples were collected from abscess with predominance of pus, which is known inhibitor of PCR reaction [8]. The developed assay can also be used to detect R. equi in specimens collected from hosts other than horses and pigs, however, the appropriate internal control (e.g. the primers/probe set targeting the host species-specific reference gene), should be included.
The developed real-time PCR assay is a useful and rapid tool applicable to direct detection of R. equi in various specimens, including tissue samples, and to identification of isolates of different origin, also those carrying virulence-associated plasmids different than VapA or plasmid-less. The detection of environmental avirulent isolates, which may be present in upper respiratory tract of horse as normal biota, could be some limitation of the newly developed assay as a diagnostic tool for analysis of some specimens in foals, such as tracheal washes or nasal swabs. On the other hand, R. equi isolation from tissue samples of other hosts can be problematic, as was shown by testing submaxillary lymph nodes specimens of swine origin. Moreover, in the pathogenesis of R. equi infections in humans may be included mechanisms other than those associated with Vaps. For example, the mycolic acids in the cell wall play a crucial role in the intracellular survival of these bacteria and the formation of granulomatous lesions in patients [25]. Takai et al. (1994) showed that 31 of 39 clinical isolates of R. equi from patients with and without AIDS did not carry the virulence plasmid and were avirulent in mice [25]. Thus, detection of both, virulent as well as avirulent strains causes that the test is a good tool for the detection of R. equi in case of other host, including humans. The assay allowed to detect R. equi in more clinical samples when compared to the culture method and conventional PCR assay and thus, it could complement the standard bacteriological methods and improve the diagnostics of R. equi infections.
Acknowledgments
This work was supported by a grant from the National Science Centre, Poland, a research project No. N N308 131638. The authors thank Barbara Chojnacka and Alicja Grzechnik for excellent technical assistance.
REFERENCES
- 1.Arriaga J. M., Cohen N. D., Derr J. N., Chaffin M. K., Martens R. J.2002. Detection of Rhodococcus equi by polymerase chain reaction using species-specific nonproprietary primers. J. Vet. Diagn. Invest. 14: 347–353. doi: 10.1177/104063870201400416 [DOI] [PubMed] [Google Scholar]
- 2.von Bargen K., Haas A.2009. Molecular and infection biology of the horse pathogen Rhodococcus equi. FEMS Microbiol. Rev. 33: 870–891. doi: 10.1111/j.1574-6976.2009.00181.x [DOI] [PubMed] [Google Scholar]
- 3.Barouni A. S., Saridakis H. O., Vidotto M. C.2004. Detection of Mycobacterium in clinical samples by multiprimer polymerase chain reaction. Braz. J. Microbiol. 35: 29–32. doi: 10.1590/S1517-83822004000100004 [DOI] [Google Scholar]
- 4.Giguère S., Cohen N. D., Chaffin M. K., Hines S. A., Hondalus M. K., Prescott J. F., Slovis N. M.2011. Rhodococcus equi: clinical manifestations, virulence, and immunity. J. Vet. Intern. Med. 25: 1221–1230. doi: 10.1111/j.1939-1676.2011.00804.x [DOI] [PubMed] [Google Scholar]
- 5.Halbert N. D., Reitzel R. A., Martens R. J., Cohen N. D.2005. Evaluation of a multiplex polymerase chain reaction assay for simultaneous detection of Rhodococcus equi and the vapA gene. Am. J. Vet. Res. 66: 1380–1385. doi: 10.2460/ajvr.2005.66.1380 [DOI] [PubMed] [Google Scholar]
- 6.Harrington J. R., Golding M. C., Martens R. J., Halbert N. D., Cohen N. D.2005. Evaluation of a real-time quantitative polymerase chain reaction assay for detection and quantitation of virulent Rhodococcus equi. Am. J. Vet. Res. 66: 755–761. doi: 10.2460/ajvr.2005.66.755 [DOI] [PubMed] [Google Scholar]
- 7.Hondalus M. K.1997. Pathogenesis and virulence of Rhodococcus equi. Vet. Microbiol. 56: 257–268. doi: 10.1016/S0378-1135(97)00094-1 [DOI] [PubMed] [Google Scholar]
- 8.Honoré-Bouakline S., Vincensini J. P., Giacuzzo V., Lagrange P. H., Herrmann J. L.2003. Rapid diagnosis of extrapulmonary tuberculosis by PCR: impact of sample preparation and DNA extraction. J. Clin. Microbiol. 41: 2323–2329. doi: 10.1128/JCM.41.6.2323-2329.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes M. S., Ball N. W., McCarroll J., Erskine M., Taylor M. J., Pollock J. M., Skuce R. A., Neill S. D.2005. Molecular analyses of mycobacteria other than the M. tuberculosis complex isolated from Northern Ireland cattle. Vet. Microbiol. 108: 101–112. doi: 10.1016/j.vetmic.2005.03.001 [DOI] [PubMed] [Google Scholar]
- 10.Ladrón N., Fernández M., Agüero J., González Zörn B., Vázquez-Boland J. A., Navas J.2003. Rapid identification of Rhodococcus equi by a PCR assay targeting the choE gene. J. Clin. Microbiol. 41: 3241–3245. doi: 10.1128/JCM.41.7.3241-3245.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letek M., Ocampo-Sosa A. A., Sanders M., Fogarty U., Buckley T., Leadon D. P., González P., Scortti M., Meijer W. G., Parkhill J., Bentley S., Vázquez-Boland J. A.2008. Evolution of the Rhodococcus equi vap pathogenicity island seen through comparison of host-associated vapA and vapB virulence plasmids. J. Bacteriol. 190: 5797–5805. doi: 10.1128/JB.00468-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMinn E. J., Alderson G., Dodson H. I., Goodfellow M., Ward A. C.2000. Genomic and phenomic differentiation of Rhodococcus equi and related strains. Antonie van Leeuwenhoek 78: 331–340. doi: 10.1023/A:1010282616110 [DOI] [PubMed] [Google Scholar]
- 13.Meijer W. G., Prescott J. F.2004. Rhodococcus equi. Vet. Res. 35: 383–396. doi: 10.1051/vetres:2004024 [DOI] [PubMed] [Google Scholar]
- 14.Miranda-Casoluengo R., Coulson G. B., Miranda-Casoluengo A., Vázquez-Boland J. A., Hondalus M. K., Meijer W. G.2012. The hydroxamate siderophore rhequichelin is required for virulence of the pathogenic actinomycete Rhodococcus equi. Infect. Immun. 80: 4106–4114. doi: 10.1128/IAI.00678-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miranda-CasoLuengo A. A., Miranda-CasoLuengo R., Lieggi N. T., Luo H., Simpson J. C., Meijer W. G.2013. A real-time impedance based method to assess Rhodococcus equi virulence. PLoS ONE 8: e60612. doi: 10.1371/journal.pone.0060612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muscatello G.2012. Rhodococcus equi pneumonia in the foal—part 2: diagnostics, treatment and disease management. Vet. J. 192: 27–33. doi: 10.1016/j.tvjl.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 17.Niwa H., Lasker B. A., Hinrikson H. P., Franzen C. G., Steigerwalt A. G., Whitney A. M., Brown J. M.2012. Characterization of human clinical isolates of Dietzia species previously misidentified as Rhodococcus equi. Eur. J. Clin. Microbiol. Infect. Dis. 31: 811–820. doi: 10.1007/s10096-011-1379-7 [DOI] [PubMed] [Google Scholar]
- 18.Pilares L., Agüero J., Vázquez-Boland J. A., Martínez-Martínez L., Navas J.2010. Identification of atypical Rhodococcus-like clinical isolates as Dietzia spp. by 16S rRNA gene sequencing. J. Clin. Microbiol. 48: 1904–1907. doi: 10.1128/JCM.01730-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez M. G. V., Vassilev T., Kemmerly S. A.2002. Rhodococcus equi infection in transplant recipients: a case of mistaken identity and review of the literature. Transpl. Infect. Dis. 4: 52–56. doi: 10.1034/j.1399-3062.2002.01001.x [DOI] [PubMed] [Google Scholar]
- 20.Pusterla N., Wilson W. D., Mapes S., Leutenegger C. M.2007. Diagnostic evaluation of real-time PCR in the detection of Rhodococcus equi in faeces and nasopharyngeal swabs from foals with pneumonia. Vet. Rec. 161: 272–275. doi: 10.1136/vr.161.8.272 [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Lázaro D., Lewis D. A., Ocampo-Sosa A. A., Fogarty U., Makrai L., Navas J., Scortti M., Hernández M., Vázquez-Boland J. A.2006. Internally controlled real-time PCR method for quantitative species-specific detection and vapA genotyping of Rhodococcus equi. Appl. Environ. Microbiol. 72: 4256–4263. doi: 10.1128/AEM.02706-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rzewuska M., Witkowski L., Cisek A. A., Stefańska I., Chrobak D., Stefaniuk E., Kizerwetter-Świda M., Takai S.2014. Characterization of Rhodococcus equi isolates from submaxillary lymph nodes of wild boars (Sus scrofa), red deer (Cervus elaphus) and roe deer (Capreolus capreolus). Vet. Microbiol. 172: 272–278. doi: 10.1016/j.vetmic.2014.04.020 [DOI] [PubMed] [Google Scholar]
- 23.Sakai M., Ohno R., Higuchi C., Sudo M., Suzuki K., Sato H., Maeda K., Sasaki Y., Kakuda T., Takai S.2012. Isolation of Rhodococcus equi from wild boars (Sus scrofa) in Japan. J. Wildl. Dis. 48: 815–817. doi: 10.7589/0090-3558-48.3.815 [DOI] [PubMed] [Google Scholar]
- 24.Sellon D. C., Besser T. E., Vivrette S. L., McConnico R. S.2001. Comparison of nucleic acid amplification, serology, and microbiologic culture for diagnosis of Rhodococcus equi pneumonia in foals. J. Clin. Microbiol. 39: 1289–1293. doi: 10.1128/JCM.39.4.1289-1293.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takai S., Sasaki Y., Ikeda T., Uchida Y., Tsubaki S., Sekizaki T.1994. Virulence of Rhodococcus equi isolates from patients with and without AIDS. J. Clin. Microbiol. 32: 457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takai S., Ikeda T., Sasaki Y., Watanabe Y., Ozawa T., Tsubaki S., Sekizaki T.1995a. Identification of virulent Rhodococcus equi by amplification of gene coding for 15- to 17-kilodalton antigens. J. Clin. Microbiol. 33: 1624–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takai S., Imai Y., Fukunaga N., Uchida Y., Kamisawa K., Sasaki Y., Tsubaki S., Sekizaki T.1995b. Identification of virulence-associated antigens and plasmids in Rhodococcus equi from patients with AIDS. J. Infect. Dis. 172: 1306–1311. doi: 10.1093/infdis/172.5.1306 [DOI] [PubMed] [Google Scholar]
- 28.Takai S.1997. Epidemiology of Rhodococcus equi infections: a review. Vet. Microbiol. 56: 167–176. doi: 10.1016/S0378-1135(97)00085-0 [DOI] [PubMed] [Google Scholar]
- 29.Takai S., Vigo G., Ikushima H., Higuchi T., Hagiwara S., Hashikura S., Sasaki Y., Tsubaki S., Anzai T., Kamada M.1998. Detection of virulent Rhodococcus equi in tracheal aspirate samples by polymerase chain reaction for rapid diagnosis of R. equi pneumonia in foals. Vet. Microbiol. 61: 59–69. doi: 10.1016/S0378-1135(98)00163-1 [DOI] [PubMed] [Google Scholar]
- 30.Torres-Tortosa M., Arrizabalaga J., Villanueva J. L., Gálvez J., Leyes M., Valencia M. E., Flores J., Peña J. M., Pérez-Cecilia E., Quereda C., Grupo Andaluz para el estudio de las Enfermedades InfecciosasGrupo de estudio de SIDA of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica. 2003. Prognosis and clinical evaluation of infection caused by Rhodococcus equi in HIV-infected patients: a multicenter study of 67 cases. Chest 123: 1970–1976. doi: 10.1378/chest.123.6.1970 [DOI] [PubMed] [Google Scholar]
- 31.Weinstock D. M., Brown A. E.2002. Rhodococcus equi: an emerging pathogen. Clin. Infect. Dis. 34: 1379–1385. doi: 10.1086/340259 [DOI] [PubMed] [Google Scholar]
- 32.Witkowski L., Rzewuska M., Cisek A. A., Chrobak-Chmiel D., Kizerwetter-Świda M., Czopowicz M., Welz M., Kita J.2015. Prevalence and genetic diversity of Rhodococcus equi in wild boars (Sus scrofa), roe deer (Capreolus capreolus) and red deer (Cervus elaphus) in Poland. BMC Microbiol. 15: 110. doi: 10.1186/s12866-015-0445-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamshchikov A. V., Schuetz A., Lyon G. M.2010. Rhodococcus equi infection. Lancet Infect. Dis. 10: 350–359. doi: 10.1016/S1473-3099(10)70068-2 [DOI] [PubMed] [Google Scholar]
- 34.Valero-Rello A., Hapeshi A., Anastasi E., Alvarez S., Scortti M., Meijer W. G., MacArthur I., Vázquez-Boland J. A.2015. An inverton-like linear plasmid mediates intracellular survival and virulence in bovine isolates of Rhodococcus equi. Infect. Immun. 83: 2725–2737. doi: 10.1128/IAI.00376-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vázquez-Boland J. A., Giguère S., Hapeshi A., MacArthur I., Anastasi E., Valero-Rello A.2013. Rhodococcus equi: the many facets of a pathogenic actinomycete. Vet. Microbiol. 167: 9–33. doi: 10.1016/j.vetmic.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 36.Vivrette S. L., Sellon D. C., Gibbons D. S.2000. Clinical application of a polymerase chain reaction assay in the diagnosis of pneumonia caused by Rhodococcus equi in a horse. J. Am. Vet. Med. Assoc. 217: 1348–1350. doi: 10.2460/javma.2000.217.1348 [DOI] [PubMed] [Google Scholar]