Abstract
Endometritis is one of the major diseases causing infertility in the cow. Intrauterine infusion of povidone-iodine (PVP-I) is a common treatment. However, the optimal concentration of PVP-I for treating endometritis effectively remains unknown. We tested concentrations of 2.0% or 0.5% PVP-I for treating clinical endometritis in dairy cattle. In Experiment 1, bacteria isolated from the uterus were incubated with either 2.0% or 0.5% PVP-I, and the numbers of bacterial colonies were counted. In Experiment 2, 18 cows with clinical endometritis were treated with either 2.0% or 0.5% PVP-I (n=9 in each group). Cytology samples and bacteria were collected using a cytobrush on weeks 0 (W0), 1 (W1) and 2 (W2) after treatment. Subsequent reproductive performance was compared between the two groups. In Experiment 1, both concentrations had a similar antiseptic outcome. In Experiment 2, the percentage of polymorphonuclear neutrophils (PMN%) in the endometrial epithelium at W2 in the 2.0% group was significantly lower (P<0.05) than in the 0.5% group, although the PMN% decreased significantly from W0 to W2 (P<0.01) in both groups. Decreases in bacterial infection rates from W0 to W2 were similar in both groups. The first service conception rate was higher, numbers of services per conception were fewer, and time to conception was shorter in the 2.0% group than in the 0.5% group. Thus, an intrauterine infusion of 2.0% PVP-I was better than 0.5% in treating clinical endometritis in these dairy cattle.
Keywords: clinical endometritis, cow, endometrial cytology, polymorphonuclear neutrophil, povidone-iodine
Bovine clinical endometritis is defined as the presence of a purulent uterine discharge in the vagina and an enlarged cervix beyond 3 weeks postpartum [24, 25]. Generally, uterine inflammation, such as endometritis or metritis, is caused by bacterial infections in the uterine lumen during the early postpartum period. These persist if they are left untreated and sometimes develop into pyometra [8, 21, 25]. Most of the cows suffering from these uterine diseases will exhibit anestrous with an extended luteal phase and can develop subfertility or infertility [4, 24]. Therefore, robust treatment interventions are required. Many therapeutic methods are employed, including the administration of hormones, such as prostaglandin F2α (PGF2α) or estradiol, intrauterine infusions of antibiotics [14] or povidone-iodine (polyvinylpyrrolidone-iodine or PVP-I) [17,18,19, 26]. For problems with residual hormones or antibiotics [10, 20], antibiotic-resistant bacteria [9] and withdrawal periods, safe and proper treatments to treat these uterine diseases are needed. Conversely, PVP-I has advantages that it does not require a withdrawal period and does not pass into the milk except in the case of excess administration [1, 2]. A concentration of 2.0% PVP-I is commonly used for treating endometritis and pyometra [20]. However, while intrauterine 0.5% PVP-I treatment has positive effects on bovine reproductive performance [17], detrimental effects of 2.0% PVP-I treatment on fertility have also been reported [19].
To exert an antiseptic effect, a high concentration of free iodine released from PVP-I is essential. The concentration of this antibacterial element peaks at 0.1% PVP-I [5, 28]. Based on this, lower concentrations of PVP-I are recommended for treating infections at surgical sites in human medicine [29]. However, iodine is deactivated easily by environmental organic matter [5]. Therefore, even while it shows the strongest antiseptic effect, 0.1% PVP-I might be too dilute because of possible presence of organic materials, such as pus or estrous mucus, in the uterus and not suitable in the field as the optimal treatment for endometritis or pyometra. Therefore, the adequate concentration of PVP-I and an optimal treatment protocol should be developed. Moreover, a more accurate method for monitoring uterine conditions is required to evaluate the therapeutic effects of PVP-I. In human and veterinary medicine, polymorphonuclear neutrophils (PMNs) collected using a cytobrush are commonly studied as biomarkers to monitor the severity of inflammation, and the relationship between a high PMN% in the female genital tract and reproductive disturbances [7, 22]. PMNs exhibit rapid chemotaxis towards pathogenic bacteria along with macrophages, and uterine inflammation can be detected rapidly as PMNs migrate into the endometrium [13].
The aim of this study was to determine whether 2.0% or 0.5% PVP-I was better for treating clinical endometritis diagnosed by endometrial cytology in dairy cattle.
MATERIALS AND METHODS
Experiment 1: effect of PVP-I in vitro. Bacterial species were collected using a cytobrush from the endometrium of four Holstein-Friesian cows (Age: 4.3 ± 1.5 and parity: 3.3 ± 1.5; mean ± standard deviation, SD) at 2 to 7 weeks postpartum that had shown normal calving, in a single herd. We identified Trueperella pyogenes, Enterococcus faecalis, Paenibacillus tundrae, Achromobacter denitrificans, Corynebacterium jeikeium, Micrococcus lylae, Streptococcus thoraltensis, Peptoniphilus indolicus and Bifidobacterium pseudolongum. After isolation, bacterial emulsions were prepared from a colony of each species and adjusted to 0.5 McFarland turbidity units (approximately 1 × 106 to 5 × 106 colony forming units/ml) with sterile physiological saline [27]. Aliquots of 50 µl of emulsion derived from each bacterial species were added into 450 µl of one of three different solutions, 2.0% or 0.5% PVP-I, or sterile physiological saline (control). The mixture (500 µl) was then vortexed for 5 sec and then allowed to stand for 30 sec. One hundred µl aliquots of mixture were inoculated onto agar media: Trueperella pyogenes and Streptococcus thoraltensis were placed on Mueller Hinton agar (Eiken Chemical Co., Ltd., Tokyo, Japan) with 5% defibrinated horse blood (Nippon Bio-Test Laboratories, Inc., Tokyo, Japan); Peptoniphilus indolicus and Bifidobacterium pseudolongum were placed on Gifu anaerobic medium agar (NISSUI Pharmaceutical Co., Ltd., Tokyo, Japan); and the others were put on Mueller Hinton agar. These were then incubated at 37°C for 24 hr. After incubation, bacterial colonies in control solution were counted from 1: 1,000 or 1: 10,000 dilution, and those in the two different PVP-I solutions were counted without dilution. The antiseptic effect of the two different PVP-I solutions was defined as the growth inhibition rate (in %) defined as the decreased rate of colony formation for each bacterial species compared with the number of colonies in the control solution, and the number of colony after a contact time of 30 sec with the two PVP-I solutions was counted.
Experiment 2: effects of intrauterine PVP-I in cows with clinical endometritis. Experiment 2 was conducted in the same herd as Experiment 1, where 166 milking Holstein-Friesian cows were kept in a free-stall barn and pasture; milked twice a day. The mean 305-day milk yield of this herd was 8,990 kg. A total of 18 cows of this herd were used. The age and parity (mean ± SD) were 3.7 ± 1.7 years and 2.3 ± 0.7, respectively. All of the 18 cows studied had calved normally, no periparturient disorders and no history of disease until used for this study. After at least 5 weeks postpartum, 34–268 days (83.4 ± 77.0), all of the animals were diagnosed as having pyometra by transrectal ultrasonography (characterized by an accumulation of purulent or mucopurulent exudates in the uterus). They were then treated with intramuscular injections either of PGF2α or 2.0 ml of Resipron® (0.25 mg of cloprostenol/ml; ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) in 16 animals with a functional corpus luteum (CL) or by uterine lavage with 2.0 l of sterile physiological saline in the remaining two animals with no CL. One week after the treatment, all of the cows were confirmed to have no pyometra (no purulent fluid or fluid accumulation in the uterine lumen), no functional CL and no estrous signs and were diagnosed from a cytobrush sample and vaginal mucous score as having clinical endometritis: all cows showed a PMN% >10% [25]. Then, the 18 cows were randomly divided into two groups and treated with either 2.0% (n=9) or 0.5% PVP-I (n=9). The mean days postpartum in each group at the time of PVP-I treatment were 78.1 ± 25.6 and 102.7 ± 26.6 days, respectively, and the difference between the two groups was not significant (P=0.52). At the time of PVP-I treatment (week zero, W0), cytology samples of the endometrial epithelium were collected aseptically using a cytobrush. Cytology slides were prepared by rolling the brush onto two clean microscope slides for each sample, fixed with cytofixative (Cytokeep II, Alfresa Pharma Corp., Osaka, Japan) immediately at the farm, stained with Diff-Quik stain (Sysmex Corp., Kobe, Japan) at laboratory, washed in distilled water and air-dried. Cytological assessment was used to determine the percentage of PMNs by counting a minimum of 200 nucleated cells [15]. Vaginal mucus was collected using a Metricheck® device (Simcro Tech Ltd., Hamilton, New Zealand), and the vaginal mucus score (VMS) was recorded on a 0–4 scale [25]. For bacterial cultures, sterilized swabs were rolled against the cytobrush, placed in transport medium (Seed Swab No. 3, Eiken Chemical Co., Ltd.) on the farm and inoculated onto blood agar plates in the laboratory. These culture medium plates were incubated at 37°C for 48 hr under aerobic and anaerobic conditions. The presence of bacterial infection was recorded as the growth of five or more colonies, and the bacterial infection rate was defined as the proportion of the number of animals with bacterial infection over the total number of animals in each group. After collecting endometrial and vaginal samples, 50 ml of either 2.0% or 0.5% of PVP-I were administered into the uterus at W0. Additional endometrial and vaginal samples were collected and processed at W1 and W2 after treatment in the same manner as at W0. No further treatment was conducted.
To evaluate the effect of treatments on subsequent reproductive performance, the intervals from the treatment to first artificial insemination (AI) service (days to first service), the first service conception rate, the number of AI services per conception and days to conception were recorded. All of the animals used in Experiment 2 were inseminated and diagnosed for pregnancy by ultrasonography at 30 to 36 days after AI, and all of them became pregnant during the experimental period.
Statistical analysis: Statistical analysis was performed using the statistical software EZR version 1.27 (http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html) [12]. Differences between two groups or within groups were evaluated by Welch’s unequal variances t test (Experiment 1), the Mann-Whitney nonparametric U test (PMN%, VMS and subsequent reproductive performance in Experiment 2), Two-way analysis of variance without replication was applied to the PMN% and VMS values in Experiment 2, and Fisher’s exact probability test was used for the bacterial infection rate in Experiment 2. Results are expressed as the mean ± standard error of the mean (SEM) or as a percentage.
RESULTS
Experiment 1: In both groups, bacterial growth inhibition rates were 99.8–100%, with no significant differences (Table 1). There were also no significant differences in the numbers of colonies between the two groups (Table 2).
Table 1. Growth inhibition rates (%) by 2.0% PVP-I and 0.5% PVP-I on bacteria isolated from bovine uterus.
| Number of Colony | Growth inhibition rate (%) | ||||
|---|---|---|---|---|---|
| Control a) | 2.0% PVP-I | 0.5% PVP-I | 2.0% PVP-I | 0.5% PVP-I | |
| Trueperella pyogenes | 5.3 × 106 | Null b) | Null | 100 | 100 |
| Enterococcus spp. | 6.6 × 104 | 70 | 81 | 99.9 | 99.9 |
| Paenibacillus spp. | 2.3 × 104 | 55 | 56 | 99.8 | 99.8 |
| Achromobacter spp. | 5.0 × 104 | 67 | 63 | 99.9 | 99.9 |
| Corynebacterium spp. | 5.8 × 104 | 77 | 59 | 99.9 | 99.9 |
| Micrococcus spp. | 3.3 × 105 | 42 | 63 | 100 | 100 |
| Streptococcus spp. | 8.3 × 104 | Null | Null | 100 | 100 |
| Peptoniphilus spp. | 1.8 × 106 | Null | Null | 100 | 100 |
| Bifidobacterium spp. | 1.1 × 106 | Null | Null | 100 | 100 |
a) Bacterial colonies were counted from 1: 1,000 or 1: 10,000 dilution. b) No bacterial colony.
Table 2. Number of colony after a contact time of 30 sec with 2.0% PVP-I and 0.5% PVP-I in each emulsion of the bacteria isolated from bovine uteri.
| Concentration of PVP-I (%) | Number of Colony | |
|---|---|---|
| Trueperella pyogenes | 2.0 | 0 |
| 0.5 | 41 | |
| Enterococcus spp. | 2.0 | 37 |
| 0.5 | 43 | |
| Paenibacillus spp. | 2.0 | 43 |
| 0.5 | 66 | |
| Achromobacter spp. | 2.0 | 56 |
| 0.5 | 52 | |
| Corynebacterium spp. | 2.0 | 0 |
| 0.5 | 0 | |
| Micrococcus spp. | 2.0 | 0 |
| 0.5 | 0 | |
| Streptococcus spp. | 2.0 | 0 |
| 0.5 | 0 |
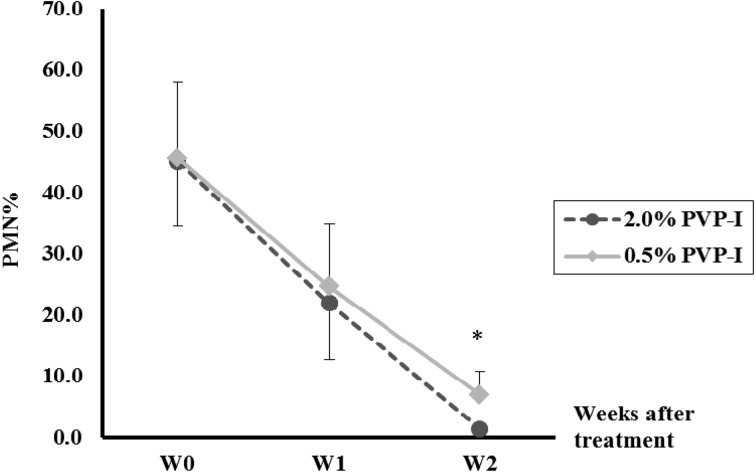
Experiment 2: PMN%. Changes in PMN% before and after PVP-I treatment are shown in Fig. 1. By W2, the PMN% had decreased significantly (P<0.01) compared with that at W0 in both groups (44.9 ± 9.3% to 1.4 ± 0.5% and 45.6 ± 12.5% to 7.0 ± 3.7%, in the 2.0% group and 0.5% groups, respectively). In addition, the PMN% at W2 in the 2.0% group (1.4 ± 0.5%) was significantly (P<0.05) lower than in the 0.5% group (7.0 ± 3.7%).
Fig. 1.
Changes in the percentages of polymorphonuclear neutrophils (PMN%) before and after intrauterine infusion of 2.0% povidone-iodine (PVP-I) or 0.5% PVP-I in Holstein cows with clinical endometritis. W0, week of infusion; W1, 1 week after infusion; W2, 2 weeks after infusion. * Significant difference (P<0.05) between the two groups.
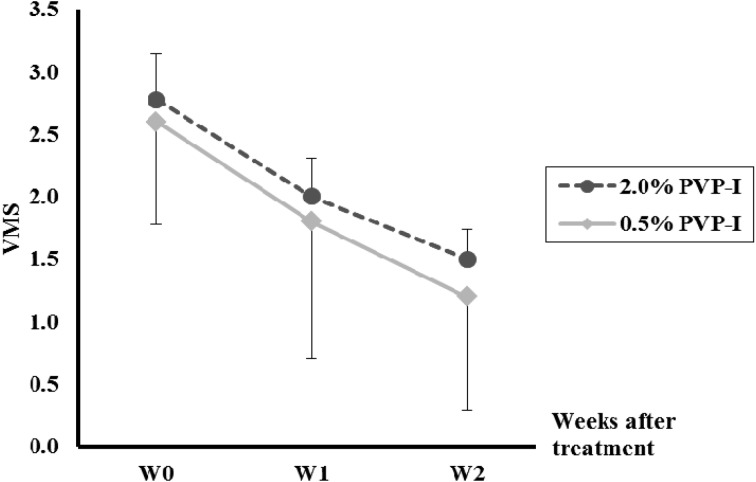
Experiment 2: VMS. In both groups, the VMS decreased steadily after treatment until W2, and there was no significant difference between the two groups (Fig. 2).
Fig. 2.
Changes in vaginal mucus score (VMS) before and after intrauterine infusion of 2.0% povidone-iodine (PVP-I) or 0.5% PVP-I in Holstein cows with clinical endometritis.
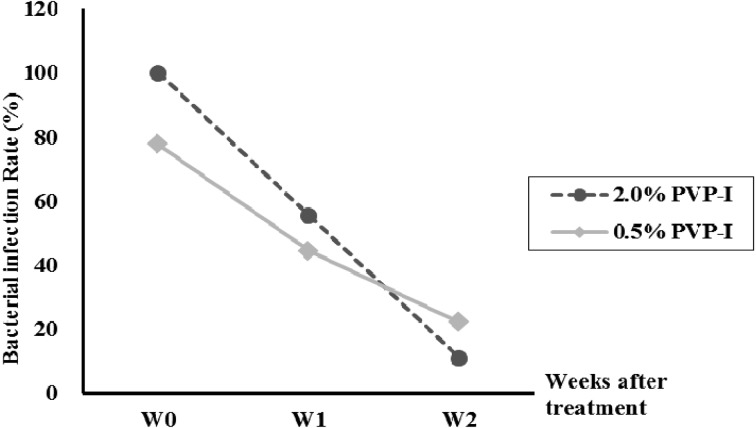
Experiment 2: bacterial infection rates. At W0, bacterial infection rates were 100% in the 2.0% group and 77.8% in the 0.5% group. The rates decreased to 11.1% in the 2.0% group and 22.2% in the 0.5% group by W2. Isolated bacteria were T. pyogenes (81.3%), Staphylococcus spp. (9.4%), Escherichia coli (6.3%) and Achinetobacter spp. (3.1%) (Fig. 3 and Table 3).
Fig. 3.
Changes in bacterial infection rate (percentage of cows with bacteria isolated from the uterus) before and after intrauterine infusions of 2.0% povidone-iodine (PVP-I) or 0.5% PVP-I in Holstein cows with clinical endometritis.
Table 3. Isolated bacteria after treatment by 2.0% or 0.5% PVP-I in cows with clinical endometritis.
| Group | W0 | W1 | W2 | ||||
|---|---|---|---|---|---|---|---|
| 2.0% PVP-I (n=9) | No. of infected cows/ total No. | 9/9 | 5/9 | 1/9 | |||
| Isolated bacteria species | Trueperella pyogenes | 8/9 a) | Trueperella pyogenes | 5/5 | Trueperella pyogenes | 1/1 | |
| Staphylococcus spp. | 1/9 | Staphylococcus spp. | 0/5 | Staphylococcus spp. | 0/1 | ||
| Acinetobacter spp. | 1/5 | ||||||
| 0.5% PVP-I (n=9) | No. of infected cows/ total No. | 7/9 | 4/9 | 2/9 | |||
| Isolated bacteria species | Trueperella pyogenes | 7/7 | Trueperella pyogenes | 4/4 | Trueperella pyogenes | 2/2 | |
| Staphylococcus spp. | 2/7 | Staphylococcus spp. | 0/4 | Staphylococcus spp. | 0/2 | ||
a) No.of the cows infected with the bacterial species described in the left column/ No.of infected cows.
Experiment 2: subsequent reproductive performance. Differences in all four parameters of subsequent reproductive performance (days to first AI service, first AI service conception rate, number of AI services per conception and days to conception) were not significant between the two groups. However, the first AI service conception rate was 11% higher, and the number of AI services per conception was 0.8 times lower in the 2.0% group than in the 0.5% group (Table 4). In addition, the mean time to conception following AI in the 2.0% group (95.1 ± 18.4 days) tended to be shorter (P=0.06) than in the 0.5% group (127.2 ± 35.7 days).
Table 4. Subsequent reproductive performance after intrauterine infusions of 2.0% or 0.5% PVP-I.
| Subsequent reproductive performance | PVP-I treatment group | |
|---|---|---|
| 2.0% PVP-I (n=9) | 0.5% PVP-I (n=9) | |
| Days to first service (Mean ± SE) | 46.8 ± 5.8 | 45.4 ± 5.9 |
| First service conception rate (%) | 44.4 | 33.3 |
| Number of service per conception (Mean ± SE) | 2.0 ± 0.4 | 2.4 ± 0.6 |
| Days to conception (Mean ± SE) | 95.1 ± 18.4a) | 127.2 ± 35.7b) |
a, b) P=0.06.
DISCUSSION
In this study, we validated a 2.0% concentration of PVP-I to treat clinical endometritis in the dairy cow.
In Experiment 1, the 0.5% PVP-I killed bacteria as effectively as 2.0% PVP-I. Both concentrations showed high antiseptic effects by inhibiting more than 99.9% of bacterial growth, against all of the bacteria isolated from endometrial membranes. With regard to contact time, exposure for 30 sec showed a very effective bacterial growth inhibition for all of the bacterial species examined in this study. Bacterial exposure to 2.5% PVP-I for 15 sec showed a sufficient antiseptic effect [11]. Here, we found that the antiseptic effect of PVP-I was immediate and that 0.5% had the same effect as 2.0% in vitro.
In Experiment 2, we attempted for the first time to determine the proper concentration of PVP-I as a means of treating bovine endometritis by successive weekly observations of uterine cytology collected using a cytobrush. All of the cows used in this experiment had recuperated from pyometra a week previously. After evacuating the accumulation of pus in the uterus at W0, the cows in both groups had a very high PMN%. This was more than four times the threshold level reported for bovine endometritis [25] and could be regarded as indicating severe endometritis. Therefore, the animals used in this study served as ideal patients to evaluate the efficacy of treatment for this disease. In this experiment, the PMN%, VMS and bacterial infection rate decreased after treatment by intrauterine infusions of PVP-I in both groups, and the treatment was proved to be effective both cytologically and bacteriologically. In a previous report, the PMN% of postpartum cows decreased over time, normally showing less than 6.0% at 5 weeks postpartum and less than 4.0% at 8 weeks [3]. In other words, a PMN% of >4.0% at 8 weeks indicates a pathological level of uterine inflammation, namely subclinical endometritis. All of the cows in this study were at more than 8 weeks postpartum. If they had recovered to normal by the PVP-I treatment, the PMN% should have been lower than 4.0% by W2. Therefore, the diagnostic cut-off point for endometritis was set as 4.0%. In fact, although the PMN% in the 0.5% group decreased significantly after treatment, the PMN% at W2 was significantly higher than in the 2.0% group, as was the bacterial infection rate. Thus, bacterial infection as well as endometrial inflammation persisted at W2 in the 0.5% group.
In theory, concentrations of PVP-I close to 0.1%—the concentration known to release maximum free iodine and show antiseptic effects in vitro—should be more antiseptic and less toxic for endometrial epithelium [28, 29]. However, the results of Experiment 2 contradicted this. Presumably, the efficacy of free iodine might have been reduced by the presence of even such a small amount of organic materials as ultrasonography cannot detect them in the uterus after the removal of accumulated pus. Therefore, the final concentration of iodine in the 2.0% group might have been closer to 0.1% [23], so 2.0% PVP-I was more effective in treating cows with severe endometritis.
In Experiment 2, one of the most commonly isolated bacterial species from cows with endometritis was Trueperella pyogenes (formerly Arcanobacterium pyogenes). This is among the most typical bacteria species causing endometritis in dairy cattle, although other previously reported pathogenic bacteria, such as Fusobacterium necrophorum, Mannheimia haemolytica, Bacteroides spp. and Prevotella spp. [4, 6, 30], were not detected. As the bacterial infection rate in both groups declined gradually after treatment, and all the cows, including the three cows from which T. pyogenes was isolated at W2, became pregnant without further treatment, intrauterine infusions of 2.0% and 0.5% PVP-I effectively inactivated T. pyogenes, consistent with the results of Experiment 1. Effectiveness of intrauterine infusions of PVP-I in curing endometritis caused by other pathogenic bacteria needs to be elucidated in future research.
There was a significant difference (P<0.05) where the PMN% was higher at W2, in the 0.5% group, and there was a tendency (P=0.06) that the time to conception after AI treatment was longer. Together with the results on cytology and bacteriology, we suggest that intrauterine infusion of 2.0% PVP-I appears to be more effective than 0.5% PVP-I as the choice of treatment for bovine endometritis. Even though we did not use infusions of sterile physiological saline as controls in this study, the finding that 2.0% PVP-I performed better than 0.5% PVP-I suggests that this is an effective treatment for severe clinical endometritis, such as conditions immediately after cure from pyometra. An association between the PMN% at 4 hr after AI and reproductive performance has been reported [16]. However, the threshold PMN% at the time of AI that could affect subsequent reproductive performance as well as indicating the appropriate timing for treating endometritis should be clarified in future studies.
In conclusion, intrauterine infusion of 2.0% PVP-I was more effective than 0.5% PVP-I for treating severe clinical endometritis in these dairy cattle.
Acknowledgments
This research was supported in part by a Grant-in-Aid for Scientific Research (No. 24580457 to T. O.) from the Japan Society for the Promotion of Science. We thank the Miyazaki Prefecture Livestock Public Corporation for its cooperation in this study.
REFERENCES
- 1.Carleton C. L., Threlfall W. R., Schwarze R. A.2008. Iodine in milk and Serum following intrauterine infusion of Lugol’s solution. Int. J. Appl. Res. Vet. Med. 6: 121–129. [Google Scholar]
- 2.Chanoine J. P., Boulvain M., Bourdoux P., Pardou A., Van Thi H. V., Ermans A. M., Delange F.1988. Increased recall rate at screening for congenital hypothyroidism in breast fed infants born to iodine overloaded mothers. Arch. Dis. Child. 63: 1207–1210. doi: 10.1136/adc.63.10.1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubuc J., Duffield T. F., Leslie K. E., Walton J. S., LeBlanc S. J.2010. Definitions and diagnosis of postpartum endometritis in dairy cows. J. Dairy Sci. 93: 5225–5233. doi: 10.3168/jds.2010-3428 [DOI] [PubMed] [Google Scholar]
- 4.Farin P. W., Ball L., Olson J. D., Mortimer R. G., Jones R. L., Adney W. S., McChesney A. E.1989. Effect of Actinomyces pyogenes and gram-negative anaerobic bacteria on the development of bovine pyometra. Theriogenology 31: 979–989. doi: 10.1016/0093-691X(89)90481-0 [DOI] [PubMed] [Google Scholar]
- 5.Favero M. S.1983. Chemical disinfection of medical and surgical materials. pp. 469–492. In: Disinfection, Sterilization and Preservation, 3rd ed., Lea & Febiger, Philadelphia. [Google Scholar]
- 6.Földi J., Kulcsár M., Pécsi A., Huyghe B., de Sa C., Lohuis J. A., Cox P., Huszenicza G.2006. Bacterial complications of postpartum uterine involution in cattle. Anim. Reprod. Sci. 96: 265–281. doi: 10.1016/j.anireprosci.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 7.Galvão K. N., Frajblat M., Brittin S. B., Butler W. R., Guard C. L., Gilbert R. O.2009. Effect of prostaglandin F2α on subclinical endometritis and fertility in dairy cows. J. Dairy Sci. 92: 4906–4913. doi: 10.3168/jds.2008-1984 [DOI] [PubMed] [Google Scholar]
- 8.Gilbert R. O., Shin S. T., Guard C. L., Erb H. N., Frajblat M.2005. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 64: 1879–1888. doi: 10.1016/j.theriogenology.2005.04.022 [DOI] [PubMed] [Google Scholar]
- 9.Haimerl P., Heuwieser W.2014. Invited review: Antibiotic treatment of metritis in dairy cows: a systematic approach. J. Dairy Sci. 97: 6649–6661. doi: 10.3168/jds.2014-8462 [DOI] [PubMed] [Google Scholar]
- 10.Jones G. M., Seymour E. H.1988. Cowside antibiotic residue testing. J. Dairy Sci. 71: 1691–1699. doi: 10.3168/jds.S0022-0302(88)79734-9 [DOI] [PubMed] [Google Scholar]
- 11.Zamora J. L.1986. Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am. J. Surg. 151: 400–406. doi: 10.1016/0002-9610(86)90477-0 [DOI] [PubMed] [Google Scholar]
- 12.Kanda Y.2013. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48: 452–458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasimanickam R., Duffield T. F., Foster R. A., Gartley C. J., Leslie K. E., Walton J. S., Johnson W. H.2004. Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology 62: 9–23. doi: 10.1016/j.theriogenology.2003.03.001 [DOI] [PubMed] [Google Scholar]
- 14.Kasimanickam R., Duffield T. F., Foster R. A., Gartley C. J., Leslie K. E., Walton J. S., Johnson W. H.2005. The effect of a single administration of cephapirin or cloprostenol on the reproductive performance of dairy cows with subclinical endometritis. Theriogenology 63: 818–830. doi: 10.1016/j.theriogenology.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 15.Kasimanickam R., Duffield T. F., Foster R. A., Gartley C. J., Leslie K. E., Walton J. S., Johnson W. H.2005. A comparison of the cytobrush and uterine lavage techniques to evaluate endometrial cytology in clinically normal postpartum dairy cows. Can. Vet. J. 46: 255–259. [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann T. B., Drillich M., Tenhagen B. A., Forderung D., Heuwieser W.2009. Prevalence of bovine subclinical endometritis 4h after insemination and its effects on first service conception rate. Theriogenology 71: 385–391. doi: 10.1016/j.theriogenology.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 17.Koujan A., Eissa H. M., Hussein M. A., Ayoub M. M., Afiefy M. M.1996. Therapeutic efficacy of povidone-iodine (Betadine) and dichloroxylenol (Septocid) in Holstein cows affected with endometritis and/or cervicitis. Acta Vet. Hung. 44: 111–119. [PubMed] [Google Scholar]
- 18.McDougall S., de Boer M., Compton C., Leblanc S. J.2013. Clinical trial of treatment programs for purulent vaginal discharge in lactating dairy cattle in New Zealand. Theriogenology 79: 1139–1145. doi: 10.1016/j.theriogenology.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 19.Nakao T., Moriyoshi M., Kawata K.1988. Effect of postpartum intrauterine treatment with 2% polyvinyl-pyrrolidone-iodine solution on reproductive efficiency in cows. Theriogenology 30: 1033–1043. doi: 10.1016/0093-691X(88)90277-4 [DOI] [PubMed] [Google Scholar]
- 20.Ohtani S., Okuda K.1997. Effect of intrauterine Infusion of polyvinyl-pyrrolidone iodine and intramuscular injection of prostaglandin F2α on reproductive performance in cows. Reprod. Domest. Anim. 32: 259–262. doi: 10.1111/j.1439-0531.1997.tb01291.x [DOI] [Google Scholar]
- 21.Opsomer G., Gröhn Y. T., Hertl J., Coryn M., Deluyker H., de Kruif A.2000. Risk factors for post partum ovarian dysfunction in high producing dairy cows in Belgium: a field study. Theriogenology 53: 841–857. doi: 10.1016/S0093-691X(00)00234-X [DOI] [PubMed] [Google Scholar]
- 22.Ramsey P. S., Lyon M. D., Goepfert A. R., Cliver S., Schwebke J., Andrews W. W., Goldenberg R. L., Hauth J. C.2005. Use of vaginal polymorphonuclear to epithelial cell ratios for the prediction of preterm birth. Obstet. Gynecol. 105: 139–144. doi: 10.1097/01.AOG.0000148269.36622.0a [DOI] [PubMed] [Google Scholar]
- 23.Satomura K., Kitamura T., Kawamura T., Shimbo T., Watanabe M., Kamei M., Takano Y., Tamakoshi A., Great Cold Investigators-I. 2005. Prevention of upper respiratory tract infections by gargling: a randomized trial. Am. J. Prev. Med. 29: 302–307. doi: 10.1016/j.amepre.2005.06.013 [DOI] [PubMed] [Google Scholar]
- 24.Sheldon I. M., Cronin J., Goetze L., Donofrio G., Schuberth H. J.2009. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 81: 1025–1032. doi: 10.1095/biolreprod.109.077370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheldon I. M., Lewis G. S., LeBlanc S., Gilbert R. O.2006. Defining postpartum uterine disease in cattle. Theriogenology 65: 1516–1530. doi: 10.1016/j.theriogenology.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 26.Sheldon I. M., Noakes D. E.1998. Comparison of three treatments for bovine endometritis. Vet. Rec. 142: 575–579. doi: 10.1136/vr.142.21.575 [DOI] [PubMed] [Google Scholar]
- 27.Sosis M. B., Braverman B.1993. Growth of Staphylococcus aureus in four intravenous anesthetics. Anesth. Analg. 77: 766–768. doi: 10.1213/00000539-199310000-00019 [DOI] [PubMed] [Google Scholar]
- 28.Takikawa K., Nakao M., Arita T.1978. Change in apparent permeability of iodine in the presence of polyvinylpyrrolidone. Chem. Pharm. Bull. (Tokyo) 26: 874–879. doi: 10.1248/cpb.26.874 [DOI] [Google Scholar]
- 29.von Keudell A., Canseco J. A., Gomoll A. H.2013. Deleterious effects of diluted povidone-iodine on articular cartilage. J. Arthroplasty 28: 918–921. doi: 10.1016/j.arth.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 30.Williams E. J., Fischer D. P., Pfeiffer D. U., England G. C., Noakes D. E., Dobson H., Sheldon I. M.2005. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 63: 102–117. doi: 10.1016/j.theriogenology.2004.03.017 [DOI] [PubMed] [Google Scholar]