Abstract
Because the establishment of pregnancy begins at the uterine horn ipsilateral to the corpus luteum (ipsi-horn) in cattle, levels of progesterone (P4) and receptor expression in the endometrial tissue, which regulate the intrauterine environment for embryo development, may differ between the ipsi-horn and the uterine horn contralateral to corpus luteum (contra-horn). The aim of the present study was to determine the endometrial tissue P4 concentrations and nuclear progesterone receptor (PGR), progesterone receptor membrane component 1 (PGRMC1) and PGRMC2 mRNA expressions in the cranial and middle parts of the uterine horns during the luteal phase. The results showed higher endometrial tissue P4 concentrations in the cranial part of the ipsi-horn than in that of the contra-horn (P<0.01); however, no change in the endometrial tissue P4 concentrations was evident during the luteal phase. The PGR mRNA expression was higher during the early luteal phase (P<0.05), but no differences between the horns were evident. However, PGRMC1 mRNA expression during the early luteal phase was higher in the cranial part of the ipsi-horn than in that of the contra-horn (P<0.05). In the middle part, there were no changes in the endometrial tissue P4 concentrations and P4 receptor expressions during the luteal phase. In conclusion, the differences in dynamics of endometrial tissue P4 concentrations and P4 receptor expressions between the uterine horns ipsilateral and contralateral to the ovary containing a corpus luteum may cause differences in the intrauterine environment for both the ipsi- and contra-horns.
Keywords: bovine, progesterone, receptor, uterus
In cattle, embryonic development in the oviduct and uterus during early gestation is an important step for the implantation and establishment of pregnancy. The morula-stage embryo enters the uterus and differentiates to the blastocyst stage at approximately 6 days after fertilization. After hatching, the blastocyst slowly grows into an ovoid or tubular form. The conceptus occupies the entire length of the uterine horn ipsilateral to the corpus luteum (CL) at 15 days after estrus.
Progesterone (P4) released from CL regulates endometrial functions, which are necessary to stimulate and maintain growth of the conceptus, as well as to stimulate implantation [19]. In cattle, secretions from the uterine gland are necessary to maintain conceptus growth [4], and P4 plays important roles in modulating components of the uterine gland secretions [24]. Serum P4 concentrations during the early luteal phase (ELP) markedly affect embryonic survival during early pregnancy [20, 21]. For example, lower P4 concentrations during ELP retard embryonic development in cattle [12, 21]. P4 plays an important role in maintaining pregnancy via the P4 receptor in cattle. Serum P4 concentrations gradually increase with the formation of a CL during the ELP and reach their maximum with the maturation of the CL during the mid-luteal phase (MLP) [8, 15]. The domestic cattle (Bos taurus) is a uniparous species, and in most cases, females produce only one offspring per pregnancy [16]. In addition, intrauterine migration has not been observed in single-ovulation cows [28], whereas it has been observed in ewes [28], pigs [6, 7] and horses [13]. Based on the above reports, pregnancies in cattle are established following the development of one embryo on the side of the uterine horn ipsilateral to the ovary containing a CL. Therefore, the intrauterine environment between the ipsilateral and contralateral horns is different. In fact, a study by Cerbito et al. [5] showed that bovine endometrial tissue P4 concentrations in the horn ipsilateral to the ovary containing a CL are higher than that in the horn contralateral to the ovary containing the CL. Therefore, we hypothesized that the difference in the intrauterine environment between the ipsilateral and contralateral horns may be regulated by P4 and its receptors in the endometrial tissue of the ipsilateral and contralateral horns. In the bovine uterus, various types of progesterone receptors are present, including the nuclear progesterone receptor (PGR) and membrane progesterone receptors (PGRMC1, PGRMC2, mPR-α, mPR-β and mPR-γ) [2, 17, 27]. Progesterone receptors play a role in facilitating successful pregnancy; however, this role is as yet not fully understood in the bovine uterus. The PGR mRNA expression physiologically decreases and reaches a baseline during the MLP of the estrous cycle [27]. In addition, the PGR mRNA expression in the endometrium of pregnant cattle at 13 [25] and 15.5 [2] days after estrus is lower than in that of nonpregnant cattle. The membrane progesterone receptors comprising PGRMC1, PGRMC2, and mPR (mPR-α, mPR-β and mPR-γ) have been confirmed in the bovine endometrium [2, 17]. Few studies have been conducted on comparison of P4 receptor mRNA expression between the ipsilateral and contralateral horns during the estrous cycle in the bovine endometrium. Therefore, we examined whether the expressions of PGR, PGRMC1 and PGRMC2 were different between the ipsilateral and contralateral horns.
MATERIALS AND METHODS
Animals and collection of endometrial tissue: Reproductive tracts of Holstein cows used in the present study were obtained from a local abattoir. Evidently healthy uteri without a conceptus were transported to the laboratory within 30 min. Days of the estrous cycle were assessed by morphological observations of follicles and CLs in ovaries according to a previous report [23]. For measurement of the P4 concentration and mRNA expression, endometrial tissue was collected from cows at three different stages of the luteal phase. Based on ovarian morphology, the stages of the estrous cycle were estimated as follows: ELP (days 5 and 6, day 0=estrus), MLP (days 8–12) and late luteal phase (LLP, days 15–17).
Endometrial tissue was collected from the cranial and middle parts of uterine horns both ipsilateral and contralateral to the ovary containing a CL (the ipsi-horn and contra-horn, respectively; ELP, n=6; MLP, n=6; and LLP, n=5). Cranial endometrial tissue samples were collected at approximately 2 cm from the uterotubal junction. Middle endometrial tissue samples were collected from the uterus just above the external bifurcation. Endometrial tissue samples were cut into small pieces, weighed and rinsed in 1 ml of physiological saline and then placed on absorbent paper to dry. Approximately 50 mg of each uterine endometrial tissue was frozen in liquid nitrogen and stored at −80°C until processed for P4 measurement and RNA isolation.
The experimental procedures complied with the Guide for Care and Use of Agricultural Animals of Obihiro University.
Endometrial P4 concentration: The concentrations of P4 in the endometrial tissue were determined in duplicate using a second-antibody enzyme immunoassay (EIA). All EIA procedures were established previously [22]. Endometrial tissue samples were placed into a glass tube with the assay buffer for the steroid EIA (0.1 g tissue per 1 ml buffer solution) in cold water and homogenized using a MultiPro (DREMEL Germany) at 15,000 rpm for 2 min. The homogenate was centrifuged at 1,450 ×g for 15 min, and the supernatant was collected. Steroid hormones in the supernatant were extracted with diethyl ether. The recovery rate of P4 was 80%. The standard curve ranged from 0.05 ng/ml to 50 ng/ml for P4, the median effective dose (ED50) of the assay was 2.4 ng/ml for P4, and the intra- and interassay CVs were 8.2% and 10.5% at 1.5 ng/ml, respectively.
RNA isolation and cDNA synthesis: Frozen uterine endometrial tissue samples were individually ground using a mortar and pestle and then transferred immediately to total RNA extraction solution (ISOGEN, Nippon Gene, Tokyo, Japan). Total RNA was prepared according to the instructions of the manufacturer. The yield of total RNA was quantified by measuring the absorbance at 260 nm using a NanoDrop Spectrophotometer (NanoDrop 2000c, Thermo Fisher Scientific, Waltham, MA, U.S.A.). Total RNA (1 µg) was treated with DNase using a commercial kit (RQ1 DNase, Promega, Madison, WI, U.S.A.). Single-stranded cDNA was reverse transcribed from total RNA using a first-strand cDNA synthesis kit (SuperScript VILO Reaction Mix, Invitrogen, Carlsbad, CA, U.S.A.). The reverse transcription conditions consisted of 10 min of annealing at 25°C, 60 min of cDNA synthesis at 42°C and 5 min of inactivation at 85°C.
Real-time polymerase chain reaction: Gene expression was determined by real-time polymerase chain reaction (PCR) using a Rotor-Gene Q (QIAGEN, Tokyo, Japan) and SYBR® Premix Ex Taq™ II (TAKARA, Tokyo, Japan). The primers for real-time PCR were designed using Primer3 based on the bovine sequences and are shown in Table 1. The amplification program consisted of 30 sec activation at 95°C, followed by 40 cycles of PCR steps (15 sec denaturation at 94°C, 30 sec annealing and 20 sec extension at 72°C). Annealing temperatures for each primer set are shown in Table 1. Positive and negative controls were used as quality controls of the process. The values were normalized using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the housekeeping gene. The relative quantification of mRNA expression of all the factors was performed according to the method of Pfaffl [26].
Table 1. Primers used in real-time PCR.
| Gene | Sequence of nucleotide (5′–3′) | Size (bp) | Annealing temperature (°C) |
Accession no. | |
|---|---|---|---|---|---|
| PGR | Fa) | TAATCTGTGGGGATGAAGCA | 181 | 58 | NM 001205356 |
| Rb) | CAGCACTTTCTAAGGCGACA | ||||
| PGRMC1 | Fa) | AGGAGTGAGGTCGGAAAGGT | 165 | 59 | NM 001075133 |
| Rb) | ATCAATGGCAAGGTGTTCG | ||||
| PGRMC2 | Fa) | CCAGAGGACTGGCAACATTT | 167 | 56 | NM 001099060 |
| Rb) | ACGGTTCTTCCCCTGGTTT | ||||
| GAPDH | Fa) | CCACTTGATGTTGGCAGGAT | 66 | 59 | XM 001252511 |
| Rb) | GAAGCTCGTCATCAATGGAAA | ||||
a) Forward, b) Reverse.
Statistical analysis: Statistical analyses were performed using StatView Version 5.0 (SAS Institute, Cary, NC, U.S.A.). For endometrial tissue P4 concentrations and PGR, PGRMC1 and PGRMC2 mRNA expression levels, a two-way factorial ANOVA was used to determine the main effects of location (ipsi-horn vs contra-horn) and estrous stage (ELP vs MLP vs LLP) and their interaction, respectively. When a significant interaction was detected, Scheffe’s F-test was used as a multiple comparison test to detect significant differences between locations, within stages and among stages within locations. Correlations between endometrial tissue P4 and PGR, PGRMC1 and PGRMC2 mRNA expressions were assessed using the nonparametric Spearman rank correlation test.
RESULTS
Endometrial tissue P4 concentration: The endometrial tissue P4 concentrations in the cranial and middle parts of the uterine horn are presented in Tables 2 and 3, respectively. In the cranial part, the endometrial tissue P4 concentrations were higher in the ipsi-horn than in the contra-horn during all stages of the luteal phase (P<0.01). In both the ipsi- and contra-horns, the endometrial tissue P4 concentrations tended to be high at the ELP and then gradually decreased at the MLP and LLP. However, there were no statistical differences in endometrial tissue P4 concentrations among the three stages of the luteal phase. In addition, the interaction of location by stages was not significant. In the middle part, the endometrial tissue P4 concentrations in both the ipsi- and contra- horns tended to be high at the ELP and then decreased from the ELP to MLP. The location and stages had no effect on the endometrial tissue P4 concentrations.
Table 2. Concentration of endometrial tissue P4 in the cranial part of the ipsilateral and contralateral horns.
| Stages (n) | Endometrial tissue P4 (ng/g) | ||
|---|---|---|---|
| Ipsi-horn | Contra-horn | Overall means | |
| ELP (6) | 32.6 ± 3.7 | 15.9 ± 2.0 | 24.3 ± 3.2 |
| MLP (6) | 25.8 ± 1.4 | 14.4 ± 2.7 | 20.1 ± 2.2 |
| LLP (5) | 18.8 ± 3.7 | 12.5 ± 2.7 | 13.7 ± 2.4 |
| Overall means | 26.1 ± 2.1a) | 14.4 ± 1.4b) | |
a, b) Significant differences at P<0.01 between the horn ipsilateral to a CL (ipsi-horn) and horn contralateral to the CL (contra-horn). The luteal phase was divided into three stages (ELP, early luteal phase, n=6; MLP, mid-luteal phase, n=6; and LLP, late luteal phase, n=5). Results are presented as the mean ± SEM.
Table 3. Concentration of endometrial tissue P4 in the middle part of the ipsilateral and contralateral horns.
| Stages (n) | Endometrial tissue P4 (ng/g) | ||
|---|---|---|---|
| Ipsi-horn | Contra-horn | Overall means | |
| ELP (6) | 16.4 ± 4.2 | 14.4 ± 2.7 | 15.4 ± 2.4 |
| MLP (6) | 11.9 ± 2.6 | 9.2 ± 2.3 | 10.6 ± 1.7 |
| LLP (5) | 15.6 ± 4.7 | 8.9 ± 3.0 | 12.3 ± 2.9 |
| Overall means | 14.6 ± 2.1 | 10.9 ± 1.6 | |
The luteal phase was divided into three stages (ELP, early luteal phase, n=6; MLP, mid-luteal phase, n=6; and LLP, late luteal phase, n=5). Results are presented as the mean ± SEM.
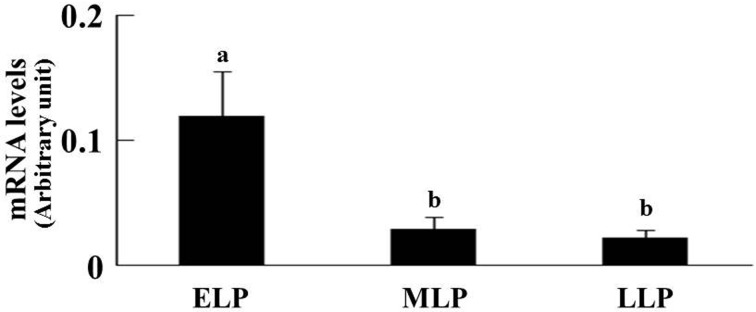
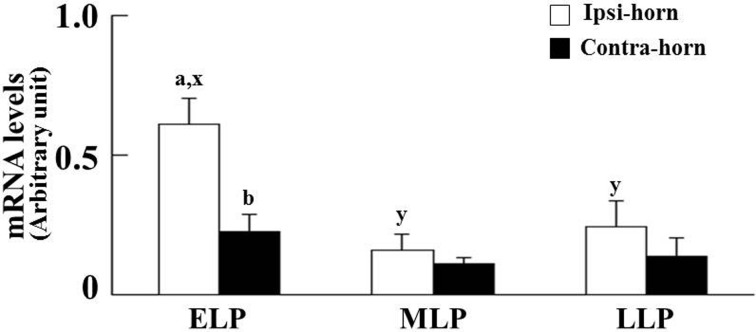
Expression of P4 receptor mRNA in endometrial tissue: The PGR and PGRMC1 mRNA expressions are shown in Figs. 1 and 2, respectively. P4 receptor mRNA expression levels were normalized using the housekeeping gene GAPDH. In the cranial part, the effects of stages on the PGR mRNA expression were significant; however, location effects were not observed. The PGR mRNA expression was higher at the ELP than at the MLP and LLP (Fig. 1). Both main effects (location and stages) and the location by stages interaction on the PGRMC1 mRNA expression were significant in the cranial part. At the ELP, the PGRMC1 mRNA expression in the ipsi-horn was higher compared with the contra-horn (P<0.05, Fig. 2). However, the PGRMC2 mRNA expression was not affected by location and stages in the cranial part of both horns. In the middle part, nevertheless, the location and stages had no effect on the PGR, PGRMC1 and PGRMC2 mRNA expressions.
Fig. 1.
Comparative changes of the relative amounts of PGR mRNA expression during the luteal phase (ELP, n=12; MLP, n=12; LLP, n=10) in the cranial part of the uterus. Since the effect of location was not observed, the data for the ipsilateral (ELP, n=6; MLP, n=6; LLP, n=5) and contralateral horns (ELP, n=6; MLP, n=6; LLP, n=5) were combined. The relative level of PGR mRNA expression was normalized to GAPDH. Significant differences at P<0.05 within the respective stages are indicated by letters (a, b). Results are presented as the mean ± SEM. The luteal phase was divided into three stages (ELP, early luteal phase; MLP, mid-luteal phase; and LLP, late luteal phase).
Fig. 2.
Comparative changes of the relative amounts of the PGRMC1 mRNA expression during the luteal phase (ELP, n=6; MLP, n=6; and LLP, n=5) in the cranial part of the uterus. In the cranial part, both main effects (location and stages) and the location by stages interaction were significant. The relative level of PGRMC1 mRNA expression was normalized to GAPDH. Significant differences at P<0.05 within respective stages between the horns ipsilateral (ipsi-horn) and contralateral (contra-horn) to a CL are indicated by the letters a and b, whereas differences within the ipsi-horn among the three stages are indicated by the letters x and y. Results are presented as the mean ± SEM. The luteal phase was divided into three stages (ELP, early luteal phase; MLP, mid-luteal phase; and LLP, late luteal phase).
The relationship between P4 concentrations and P4 receptors expression in endometrial tissue: In the cranial part of the ipsi-horn at the ELP, PGR mRNA expression had a negative correlation (r=−0.871, P<0.05) with endometrial tissue P4. In addition, there was a significant positive correlation (r=0.913, P<0.05) between PGRMC1 mRNA expression and the endometrial tissue P4 concentrations. However, there was no correlation of PGRMC2 mRNA expression with endometrial tissue P4. In the cranial part of the ipsi-horn at the MLP and LLP, the P4 concentration showed no correlation with PGR, PGRMC1 and PGRMC2 mRNA expression in the endometrial tissue. In the cranial part of the contra-horn and the middle part of both the ipsi- and contra-horns, P4 receptors mRNA expression showed no correlations with endometrial tissue P4 concentrations regardless of the stage of the luteal phase.
DISCUSSION
The results of the present study show the differences in P4 concentrations and PGR and PGRMC1 mRNA expressions in the bovine endometrial tissue between the ipsilateral and contralateral horns through each stage of the luteal phase. The endometrial tissue P4 concentrations were higher in the cranial part of the ipsilateral horn than in the contralateral horn, consistent with the results of previous reports [31]. The present study showed that the endometrial tissue P4 concentrations tended to be higher at the ELP in the cranial part of ipsilateral horn compared with the MLP and LLP. However, during the luteal phase, the endometrial tissue P4 concentration did not change significantly. On the other hand, a previous study reported that endometrial tissue collected during the early stage of the luteal phase (from day 1 to day 5) showed the highest concentration of P4 as compared with the mid and late stages in the ipsilateral horn [17]. In the present study, the ELP samples were collected from day 5 to day 6. The differences in the timing of tissue collection may have resulted in the differences in profile of P4 concentrations of endometrial tissue between the two studies.
Differences in endometrial tissue P4 between the ipsilateral horn and the contralateral horn during the luteal phase in the present study indicate the locational effects of the CL on the P4 concentrations in the endometrium of uterine horns in cattle. The P4 concentration in the uterine artery in the ipsilateral horn was previous reported to be higher than that in the contralateral horn [31]. However, the reasons for high levels of P4 in the uterine artery, which provides P4 to the endometrial tissue in the ipsilateral horn in cattle, are as yet unclear. There is a possibility of a counter current transfer system among the ovarian vein, ovarian artery and uterine artery. In ewes, P4 has been shown to transfer from the utero-ovarian vein into the uterine artery [30]. In cattle, estradiol-17β transfers from the ovarian vein into the ovarian artery [18]. The ovarian artery and a branch of the uterine artery are interconnected by a prominent anastomotic vessel in cattle [14]. These aforementioned studies show that steroid hormones in the ovarian vein may be transported into the ovarian artery at the arterial anastomosis, which connects to the ovarian artery and the neighboring branch of the uterine artery supplying the oviduct and uterine horn. Therefore, P4 released from the CL may migrate into the ovarian vein, ovarian artery and uterine artery and then reach the uterine endometrium in cattle. Finally, the P4 supply to the uterus by the counter current transfer system may contribute to high concentrations of P4 in the ipsilateral horn in cattle.
Interestingly, there were no differences in the endometrial tissue P4 concentrations among the ELP, MLP and LLP. Generally, bovine serum P4 concentrations gradually increase during the ELP and rise to the maximum level during the MLP and LLP [8, 15]. The results of the present study indicate that the dynamics of the endometrial tissue P4 concentrations may be different from those of the serum P4 concentrations during the luteal phase, both in the ipsilateral and contralateral horn. It is unclear why the dynamics of the endometrial tissue P4 concentrations were different from those of the serum P4 concentrations. In the bovine, the blood flow toward the uterus during the periestrous period is increased [11]. This may efficiently enhance the supply of P4 from the developing CL to the uterine horn. It is additionally speculated that higher P4 receptor mRNA expression at the ELP in the endometrial tissue allows the sequestration of a large amount of circulating P4. Consequently, the blood flow toward the uterus and/or expression of the P4 receptors in the bovine endometrium may create differences in concentration between tissue P4 and serum P4. However, further investigation of P4 concentrations in the uterine artery during the estrous cycle and P4 binding ability in the endometrial tissue is required.
P4 action provides an intrauterine environment that is suitable for pregnancy succession in cattle [29]. In cattle, P4 receptor expression was recognized as an important regulatory factor for pregnancy establishment [3]. The decrease of the PGR mRNA expression in the endometrium at the MLP and LLP is consistent with previous reports that demonstrate a decrease in PGR mRNA expression at 8–10 days after estrus [27]. Bridges et al. [2] reported that disappearance of the PGR mRNA expression allows for increased secretion of uterine gland products, which is required to support development of the conceptus in the ipsilateral horn.
A previous report demonstrated that PGRMC1 and PGRMC2 gene expressions in the bovine endometrium did not differ among the stages of the estrous cycle [17].In both the ipsi- and contra-horns, there was no change in the PGRMC2 mRNA expression during the luteal phase in the present study. These results in the ipsi-horn were consistent with previous reports [17]. On the other hand, PGRMC1 mRNA expression in the cranial part of the ipsi-horn was higher at the ELP. The reason why the profiles of PGRMC1 mRNA expression during the luteal phase are different between the previous study and the present study is not clear. In the present study, the tissues from both the cranial and middle parts of the uterus were collected and analyzed separately. However, the location of the endometrial tissue collected in the previous study was not mentioned clearly. Therefore, the differences in results between the previous study and the present study might be caused by differences in the sampling location within the uterine horn.
The PGR mRNA expression was not different between the ipsilateral horn and the contralateral horn. It is well known that PGR expression is downregulated by treatment with P4 [25] in the bovine endometrium. In the present study, there were no differences in PGR mRNA expression between the ipsilateral and contralateral horns, although the endometrial tissue P4 concentration was higher in the ipsilateral horn than in the contralateral horn. In fact, a negative correlation between P4 concentration and PGR mRNA expression was observed in endometrial tissue at the ELP only in the ipsilateral horn. From these results, downregulation of the PGR mRNA expression may be independent from tissue P4 concentrations. The PGR mRNA expression in pregnant cattle was lower than that in nonpregnant cattle [2, 25]. Moreover, Forde et al. [12] reported that low P4 concentrations delay the downregulation of PGR mRNA expression. In view of this fact, downregulation of PGR mRNA expression was attributed to high levels of endometrial tissue P4 in the ipsilateral horn, which may create the intrauterine environment for pregnancy succession in the ipsilateral horn.
In the present study, there was a positive correlation between the P4 concentration and PGRMC1 mRNA expression in the cranial part of the uterine horn at the ELP. However, the factor regulating PGRMC1 expression has not yet been investigated in cattle. In ovariectomized mice, PGRMC1 mRNA expression was found to be upregulated in the uterus by P4 treatment [32]. The present study indicates the possibility that PGRMC1 mRNA expression in the cranial part of the ipsilateral horn may be upregulated locally by a higher P4 concentration in the endometrial tissue.
In the cranial part, the PGR and PGRMC1 mRNA expression and P4 concentrations in the ipsilateral horn at the ELP were different from those of the contralateral horn and other stages. The role of changes in PGR and membrane progesterone receptor expressions and endometrial tissue P4 concentration in pregnancy succession is not well known. Okumu et al. [25] reported high P4 causes early downregulation of the PGR expression in the bovine endometrium, which is a critical event for the expression of genes required for embryo development. Because PGR and PGRMC1 mRNA expressions may be regulated differently between the ipsi- and contra-horns, the action of P4 on the PGR and PGRMC1 may create differences in the uterine environment for embryo development between the ipsi- and contra-horns.
On the other hand, there was a possibility that some other route exists via mPR-α, mPR-β and mPR-γ to provide P4 functions [10]. In humans, mPR-α, mPR-β and mPR-γ mRNA expressions were confirmed in the endometrium. In particular, the mPR-α expression was higher at the early-mid secretory phase than the proliferative phase [9]. In the ovine endometrium, the mPR-α mRNA expression increased 4-fold at day 4 of the estrous cycle compared with 3 hr post onset of estrus [1]. There were also no differences in mRNA expressions of mPR-α, mPR-β and mPR-γ in endometrial tissue at 15.5 days between pregnant and nonpregnant cattle [2]. However, the mPR-α, mPR-β and -γ mRNA expressions were not analyzed in the present study. To elucidate the mechanism of the intrauterine environment for pregnancy succession, analysis for P4 receptor expression in the bovine endometrium, not only PGR and PGRMCs but also mPR-α, mPR-β and mPR-γ, during the early luteal phase is required.
In conclusion, the present study demonstrates differences in dynamics of endometrial tissue P4 concentrations and expression of the P4 receptors between the two horns ipsilateral and contralateral to the ovary containing a CL. The PGR or PGRMC1 mRNA expression showed a negative or positive relationship, respectively, with endometrial tissue P4 concentrations of the cranial part of the ipsilateral horn. Differences in dynamics of endometrial tissue P4 concentrations and expression of P4 receptors between the horns ipsilateral and contralateral to the ovary containing a CL may cause differences in intrauterine environment for both the ipsi- and contra-horns.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (26660234) from the Japan Society for the Promotion of Science (JSPS).
REFERENCES
- 1.Ashley R. L., Arreguin-Arevalo J. A., Nett T. M.2009. Binding characteristics of the ovine membrane progesterone receptor alpha and expression of the receptor during the estrous cycle. Reprod. Biol. Endocrinol. 7: 42. doi: 10.1186/1477-7827-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bridges G. A., Mussard M. L., Pate J. L., Ott T. L., Hansen T. R., Day M. L.2012. Impact of preovulatory estradiol concentrations on conceptus development and uterine gene expression. Anim. Reprod. Sci. 133: 16–26. doi: 10.1016/j.anireprosci.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 3.Bridges G. A., Day M. L., Geary T. W., Cruppe L. H.2013. Triennial Reproduction Symposium: deficiencies in the uterine environment and failure to support embryonic development. J. Anim. Sci. 91: 3002–3013. doi: 10.2527/jas.2013-5882 [DOI] [PubMed] [Google Scholar]
- 4.Brooks K., Burns G., Spencer T. E.2014. Conceptus elongation in ruminants: roles of progesterone, prostaglandin, interferon tau and cortisol. J. Anim. Sci. Biotechnol. 5: 53. doi: 10.1186/2049-1891-5-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerbito W. A., Quero F. V., Jr, Balagapo C. R., Jr, Miyazawa K., Sato K.1994. Spatial distribution of progesterone in bovine uterus in relation to corpus luteum location and function. Theriogenology 41: 1663–1671. doi: 10.1016/0093-691X(94)90831-3 [DOI] [Google Scholar]
- 6.Dhindsa D. S., Dziuk P. J., Norton H. W.1967. Time of transuterine migration and distribution of embryos in the pig. Anat. Rec. 159: 325–330. doi: 10.1002/ar.1091590309 [DOI] [PubMed] [Google Scholar]
- 7.Dziuk P. J., Polge C., Rowson L. E.1964. Intra-uterine migration and mixing of embryos in swine following egg transfer. J. Anim. Sci. 23: 37–42. [Google Scholar]
- 8.Endo N., Nagai K., Tanaka T., Kamomae H.2012. Comparison between lactating and non-lactating dairy cows on follicular growth and corpus luteum development, and endocrine patterns of ovarian steroids and luteinizing hormone in the estrous cycles. Anim. Reprod. Sci. 134: 112–118. doi: 10.1016/j.anireprosci.2012.08.018 [DOI] [PubMed] [Google Scholar]
- 9.Fernandes M. S., Pierron V., Michalovich D., Astle S., Thornton S., Peltoketo H., Lam E. W., Gellersen B., Huhtaniemi I., Allen J., Brosens J. J.2005. Regulated expression of putative membrane progestin receptor homologues in human endometrium and gestational tissues. J. Endocrinol. 187: 89–101. doi: 10.1677/joe.1.06242 [DOI] [PubMed] [Google Scholar]
- 10.Fernandes M. S., Brosens J. J., Gellersen B.2008. Honey, we need to talk about the membrane progestin receptors. Steroids 73: 942–952. doi: 10.1016/j.steroids.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 11.Ford S. P., Chenault J. R.1981. Blood flow to the corpus luteum-bearing ovary and ipsilateral uterine horn of cows during the oestrous cycle and early pregnancy. J. Reprod. Fertil. 62: 555–562. doi: 10.1530/jrf.0.0620555 [DOI] [PubMed] [Google Scholar]
- 12.Forde N., Beltman M. E., Duffy G. B., Duffy P., Mehta J. P., O’Gaora P., Roche J. F., Lonergan P., Crowe M. A.2011. Changes in the endometrial transcriptome during the bovine estrous cycle: effect of low circulating progesterone and consequences for conceptus elongation. Biol. Reprod. 84: 266–278. doi: 10.1095/biolreprod.110.085910 [DOI] [PubMed] [Google Scholar]
- 13.Ginther O. J.1983. Mobility of the early equine conceptus. Theriogenology 19: 603–611. doi: 10.1016/0093-691X(83)90180-2 [DOI] [PubMed] [Google Scholar]
- 14.Ginther O. J., Del Campo C. H.1974. Vascular anatomy of the uterus and ovaries and the unilateral luteolytic effect of the uterus: cattle. Am. J. Vet. Res. 35: 193–203. [PubMed] [Google Scholar]
- 15.Kastelic J. P., Bergfelt D. R., Ginther O. J.1990. Relationship between ultrasonic assessment of the corpus luteum and plasma progesterone concentration in heifers. Theriogenology 33: 1269–1278. doi: 10.1016/0093-691X(90)90045-U [DOI] [Google Scholar]
- 16.Komisarek J., Dorynek Z.2002. Genetic aspects of twinning in cattle. J. Appl. Genet. 43: 55–68. [PubMed] [Google Scholar]
- 17.Kowalik M. K., Slonina D., Rekawiecki R., Kotwica J.2013. Expression of progesterone receptor membrane component (PGRMC) 1 and 2, serpine mRNA binding protein 1 (SERBP1) and nuclear progesterone receptor (PGR) in the bovine endometrium during the estrous cycle and the first trimester of pregnancy. Reprod. Biol. 13: 15–23. doi: 10.1016/j.repbio.2013.01.170 [DOI] [PubMed] [Google Scholar]
- 18.Krzymowski T., Stefanczyk S., Kotwica J., Czarnocki J., Glazer T., Janowski T., Chmiel J.1982. 3H −oestradiol −17β counter current transfer from the ovarian vein into the ovarian artery in cows. Anim. Reprod. Sci. 4: 199–206. doi: 10.1016/0378-4320(82)90003-3 [DOI] [Google Scholar]
- 19.Lonergan P.2011. Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology 76: 1594–1601. doi: 10.1016/j.theriogenology.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 20.Lonergan P., Forde N.2014. Maternal-embryo interaction leading up to the initiation of implantation of pregnancy in cattle. Animal 8Suppl 1: 64–69. doi: 10.1017/S1751731114000470 [DOI] [PubMed] [Google Scholar]
- 21.Mann G. E., Lamming G. E.2001. Relationship between maternal endocrine environment, early embryo development and inhibition of the luteolytic mechanism in cows. Reproduction 121: 175–180. doi: 10.1530/rep.0.1210175 [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto A., Okuda K., Schweigert F. J., Schams D.1992. Effects of basic fibroblast growth factor, transforming growth factor-beta and nerve growth factor on the secretory function of the bovine corpus luteum in vitro. J. Endocrinol. 135: 103–114. doi: 10.1677/joe.0.1350103 [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto Y., Skarzynski D. J., Okuda K.2000. Is tumor necrosis factor α a trigger for the initiation of endometrial prostaglandin F2α release at luteolysis in cattle? Biol. Reprod. 62: 1109–1115. doi: 10.1095/biolreprod62.5.1109 [DOI] [PubMed] [Google Scholar]
- 24.Mullen M. P., Bazer F. W., Wu G., Parr M. H., Evans A. C., Crowe M. A., Diskin M. G.2014. Effects of systemic progesterone during the early luteal phase on the availabilities of amino acids and glucose in the bovine uterine lumen. Reprod. Fertil. Dev. 26: 282–292. doi: 10.1071/RD12319 [DOI] [PubMed] [Google Scholar]
- 25.Okumu L. A., Forde N., Fahey A. G., Fitzpatrick E., Roche J. F., Crowe M. A., Lonergan P.2010. The effect of elevated progesterone and pregnancy status on mRNA expression and localisation of progesterone and oestrogen receptors in the bovine uterus. Reproduction 140: 143–153. doi: 10.1530/REP-10-0113 [DOI] [PubMed] [Google Scholar]
- 26.Pfaffl M. W.2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: e45. doi: 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson R. S., Mann G. E., Lamming G. E., Wathes D. C.2001. Expression of oxytocin, oestrogen and progesterone receptors in uterine biopsy samples throughout the oestrous cycle and early pregnancy in cows. Reproduction 122: 965–979. doi: 10.1530/rep.0.1220965 [DOI] [PubMed] [Google Scholar]
- 28.Scanlon P. F.1972. Frequency of transuterine migration of embryos in ewes and cows. J. Anim. Sci. 34: 791–794. [DOI] [PubMed] [Google Scholar]
- 29.Ulbrich S. E., Wolf E., Bauersachs S.2012. Hosting the preimplantation embryo: potentials and limitations of different approaches for analysing embryo-endometrium interactions in cattle. Reprod. Fertil. Dev. 25: 62–70. doi: 10.1071/RD12279 [DOI] [PubMed] [Google Scholar]
- 30.Walsh S. W., Yutrzenka G. J., Davis J. S.1979. Local steroid concentrating mechanism in the reproductive vasculature of the ewe. Biol. Reprod. 20: 1167–1171. doi: 10.1095/biolreprod20.5.1167 [DOI] [PubMed] [Google Scholar]
- 31.Weems C. W., Lee C. N., Weems Y. S., Vincent D. L.1988. Distribution of progesterone to the uterus and associated vasculature of cattle. Endocrinol. Jpn. 35: 625–630. doi: 10.1507/endocrj1954.35.625 [DOI] [PubMed] [Google Scholar]
- 32.Zhang L., Kanda Y., Roberts D. J., Ecker J. L., Losel R., Wehling M., Peluso J. J., Pru J. K.2008. Expression of progesterone receptor membrane component 1 and its partner serpine 1 mRNA binding protein in uterine and placental tissues of the mouse and human. Mol. Cell. Endocrinol. 287: 81–89. doi: 10.1016/j.mce.2008.02.012 [DOI] [PubMed] [Google Scholar]