Abstract
Canine hemangiosarcoma (HSA) is a progressive malignant neoplasm of dogs for which there is currently no effective treatment. A recent study suggested that receptor tyrosine kinases (RTKs), the PI3K/Akt/m-TOR and MAPK pathways are all activated in canine and human HSA. The aim of the present study was to investigate the overexpression of these proteins by immunohistochemistry in canine splenic HSA to identify potential molecular therapeutic targets. A total of 10 splenic HSAs and two normal splenic samples surgically resected from dogs were sectioned and stained with hematoxylin and eosin for histological diagnosis or analyzed using immunohistochemistry. The expression of RTKs, c-kit, VEGFR-2 and PDGFR-2, as well as PI3K/Akt/m-TOR and MEK was higher in canine splenic HSAs compared to normal spleens. These proteins may therefore be potential therapeutic targets in canine splenic HSA.
Keywords: canine hemangiosarcoma dog, immunohistochemistry, MAPK pathway, PI3K/Akt/m-TOR pathway, receptor tyrosine kinase
Canine hemangiosarcoma (HSA) is a progressive malignant neoplasm that originates from vascular endothelial cells and accounts for 12–21% of canine mesenchymal tumors [5, 10, 25]. Tumors most commonly arise from or metastasize to the spleen, liver, heart or lungs; their highly metastatic behavior results in a 1-year survival rate of <10% and a median survival time of 19–86 days in dogs treated with surgery alone and 179 days in those treated with combined chemotherapy and surgery [6, 13, 29, 30, 33,34,35, 37]. An effective treatment for HSA is yet to be established.
Receptor tyrosine kinases (RTKs) are often activated aberrantly in human cancers [11, 15], and inhibition of RTKs, such as c-kit, vascular endothelial growth factor receptor-2 (VEGFR-2) and platelet derived growth factor receptor-2 (PDGFR-2), reduces canine HSA cell viability [9]. Among canine tumors, anal sac apocrine gland adenocarcinoma was previously found to be positive for PDGFR-2 expression by immunohistochemistry [7], although RTK expression has not been investigated in canine HSA.
The phosphatidylinositol 3 kinase/Akt/mammalian target of rapamycin (PI3K/Akt/m-TOR) and mitogen-activated protein kinase (MAPK) pathways are activated by RTKs and are considered major oncogenic drivers in human hematological malignancies [26]. Inhibitors of the PI3K/Akt/m-TOR pathway are classic examples of targeted therapeutic agents [31]. This pathway has been a particular focus in studies of avian sarcoma tumorigenesis [8]. Furthermore, it was recently shown that the phosphorylation levels of Akt, m-TOR and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) were higher in canine HSA cell lines than in normal canine endothelial cells by western blotting [27], and the overexpression of phosphorylated Akt (p-Akt), phosphorylated m-TOR (p-m-TOR), 4E-BP1 and eukaryotic initiation factor 4E (eIF4E) was observed in canine dermal HSA by immunohistochemistry [28]. Both 4E-BP1 and eIF4E are downstream of the Akt/m-TOR pathway. However, activation of the PI3K/Akt/m-TOR pathway has not been demonstrated in canine splenic HSA.
Canine cardiac HSA tumor grafts were found to be sensitive to the MEK inhibitor PD0325901, suggesting that MEK signaling is necessary for the growth of HSA in mouse models and might therefore be a therapeutic target [2]. However, activation of the MAPK pathway has not been demonstrated in canine splenic HSA.
The aim of the present study was to investigate the overexpression of RTKs, the Akt/m-TOR pathway and the MAPK pathway in canine splenic HSA by using immunohistochemistry in order to identify possible molecular therapeutic targets.
MATERIALS AND METHODS
Samples: A total of 10 surgically resected splenic HSAs from dogs were used for this study. These samples were submitted to the Laboratory of Veterinary Clinical Pathology at Hokkaido University for histological diagnosis between April 2012 and September 2014. In addition, two normal splenic samples taken from dogs that had died for reasons unrelated to this study were used for comparative analysis. Formalin-fixed tissues were processed routinely and embedded in paraffin wax. Samples were stained with hematoxylin and eosin for histological diagnosis or used in immunohistochemical analysis. The present study was approved by the Institutional Review Board of Hokkaido University Veterinary Teaching Hospital. All pet owners understood that the organ samples obtained from their dogs would be used in this study and gave their consent on this basis.
Immunohistochemistry: The standard 3-3′-diaminobenzidine-4HCl (DAB) technique was used for immunohistochemistry (Kyodo Byori Inc., Kobe, Japan). Paraffin-embedded samples were sectioned, dehydrated in xylene, rehydrated through a graded series of alcohols and rinsed in phosphate-buffered saline (PBS). For antigen retrieval, the sections were immersed in 10 mM citric acid buffer solution and heated for 10 min at 121°C in an autoclave. Sections were subsequently incubated in 3% H2O2 in distilled water for 15 min at room temperature (RT) and washed in PBS. To prevent nonspecific antibody binding, the sections were treated with Blocking One (Nacalai Tesque, Kyoto, Japan) for 10 min at RT. The sections were then incubated with the primary antibodies: anti-c-kit, anti-VEGFR-2, anti-PDGFR-2, anti-PI3K, anti-p-Akt, anti-p-m-TOR, anti-MEK1, anti-MEK2 or anti-p-extracellular signal-regulated kinase (ERK) (Table 1) overnight at 4°C, and then washed with 0.05 M PBS and incubated with the secondary antibody: a peroxidase-labeled anti-mouse polyclonal antibody or anti-rabbit polyclonal antibody (Nichirei Biosciences Inc., Tokyo, Japan) for 30 min at RT. After an additional wash with PBS, the sections were incubated with DAB for 5 min at RT, washed, counterstained with Mayer’s hematoxylin, dehydrated using sequential 70%, 85% and 100% ethanol solutions, and then incubated in xylene.
Table 1. Primary antibodies used in immunohistochemical analysis.
| Primary antibody | Host (clone) | Dilution | Company |
|---|---|---|---|
| c-kit | Rabbit | 1:50 | Santa Cruz |
| VEGFR-2 | Mouse | 1:250 | Santa Cruz |
| PDGFR-2 | Rabbit | 1:1,000 | Cell Signaling |
| PI3K | Rabbit | 1:500 | Upstate |
| phosphor-Akt | Rabbit (D9E) | 1:50 | Cell Signaling |
| phosphor-m-TOR | Rabbit (49F9) | 1:500 | Cell Signaling |
| MEK1 | Mouse (25/MEK1) | 1:1,000 | BD Transduction Laboratories |
| MEK2 | Mouse (96/MEK2) | 1:1,000 | BD Transduction Laboratories |
| phospho-p44/42 MAPK (ERK1/2) | Rabbit | 1:100 | Cell Signaling |
Immunohistochemical evaluation: Immunohistochemically stained sections were evaluated by microscopy (Biorevo BZ-9000, Keyence, Osaka, Japan). The HSA and normal spleen (control) sections were graded for staining intensity using a BZ-II Analyzer (Keyence). The percentage reflected the total area of positive cells out of the total area of all cells within the three randomly selected fields of view. The HSAs and controls were scored as follows: 1+, 10% to <30% of positive cells; 2+, 30% to <50% of positive cells; and 3+, ≥50% of positive cells. Samples with <10% of positively labeled cells were considered negative.
RESULTS
Clinical information and histological interpretation: The clinical data from dogs with HSA or normal spleens are shown in Table 2. The mean age of the 10 dogs with HSA was 11.4 years (range, 9–15 years), and the mean age of the two normal dogs was 11 years (9 and 13 years). The male to female ratio in the HSA group was 2.3:1. Both of the normal dogs were male.
Table 2. Summary of clinical data.
| No. | Breed | Age (years) | Sex | |
|---|---|---|---|---|
| Hemangiosarcoma | 1 | Miniature dachshund | 9 | FN a) |
| 2 | Miniature schnauzer | 11 | M b) | |
| 3 | Miniature schnauzer | 9 | M | |
| 4 | Labrador retriever | 13 | MN c) | |
| 5 | Labrador retriever | 15 | M | |
| 6 | Labrador retriever | 13 | FN | |
| 7 | Golden retriever | 11 | M | |
| 8 | Maltese | 10 | MN | |
| 9 | Border collie | 13 | M | |
| 10 | Labrador retriever | 10 | FN | |
| Normal spleen | 11 | Beagle | 9 | M |
| 12 | Mixed breed | 13 | MN |
a) FN, neutered female; b) M, male; c) MN, neutered male.
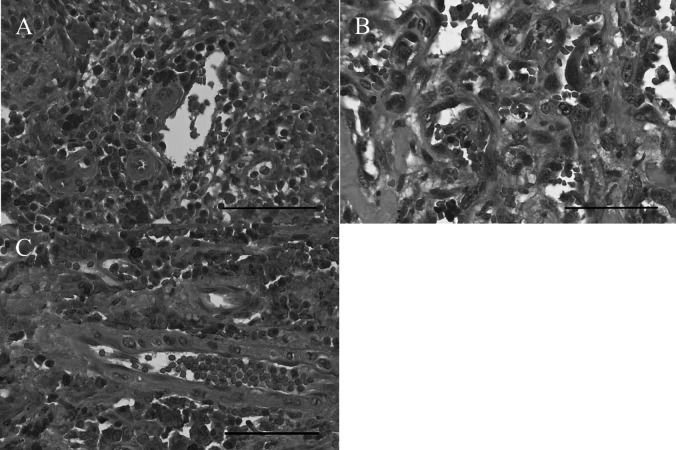
Microscopically, the HSA samples displayed various morphological features including vascular clefts or channels, and contained spindle and polygonal cells (Fig. 1A–1C).
Fig. 1.
Hematoxylin and eosin staining (× 400). (A) Hemangiosarcoma (HSA) showing polygonal tumor cells with some areas of vascular clefts or channels in Case 10. (B) HSA in Case 9. (C) HSA showing some polygonal tumor cells. Red blood cells were observed in vascular clefts or channels in Case 5. Bar, 60 µm.
Immunohistochemistry: The immunohistochemical staining intensities of the HSAs and normal spleens (controls) are shown in Table 3, and the immunohistochemical scores obtained for the HSAs and controls are shown in Table 4.
Table 3. Immunohistochemical staining intensity.
| No. | c-kit | VEGFR-2 | PDGFR-2 | PI3K | Akt | m-TOR | MEK1 | MEK2 | ERK | |
|---|---|---|---|---|---|---|---|---|---|---|
| HSA (%) a) | 1 | 4.0 | 52.0 | 6.0 | 25.1 | 4.8 | 11.0 | 9.7 | 42.2 | 1.9 |
| 2 | 3.8 | 35.1 | 6.1 | 22.4 | 4.9 | 13.3 | 22.4 | 36.8 | 4.0 | |
| 3 | 1.1 | 51.2 | 3.6 | 27.1 | 3.7 | 15.0 | 19.4 | 49.2 | 5.0 | |
| 4 | 20.9 | 34.7 | 35.9 | 28.5 | 4.7 | 11.6 | 11.4 | 33.7 | 28.3 | |
| 5 | 2.3 | 12.4 | 2.2 | 20.4 | 2.6 | 4.6 | 6.4 | 19.4 | 1.9 | |
| 6 | 2.2 | 31.2 | 36.5 | 32.9 | 1.9 | 6.8 | 27.5 | 48.4 | 2.4 | |
| 7 | 1.6 | 35.5 | 2.7 | 22.5 | 2.4 | 15.9 | 20.1 | 32.1 | 2.0 | |
| 8 | 17.5 | 47.3 | 25.8 | 40.5 | 4.8 | 7.0 | 8.2 | 59.3 | 20.2 | |
| 9 | 2.2 | 36.5 | 24.5 | 26.3 | 1.5 | 17.5 | 12.3 | 35.0 | 1.7 | |
| 10 | 10.8 | 37.4 | 4.1 | 37.2 | 2.7 | 11.7 | 16.8 | 32.7 | 2.1 | |
| Average b) | 6.6 | 37.3 | 14.8 | 28.3 | 3.4 | 11.4 | 15.4 | 38.9 | 7.0 | |
| Control | 11 | 6.0 | 7.3 | 5.0 | 7.7 | 6.6 | 5.9 | 6.5 | 7.2 | 4.6 |
| (%) | 12 | 4.9 | 8.5 | 6.1 | 6.4 | 4.7 | 3.3 | 3.9 | 7.1 | 5.7 |
| Average | 5.4 | 7.9 | 5.5 | 7.1 | 5.7 | 4.6 | 5.2 | 7.2 | 5.1 |
a) %, percentage of the totals of positive area per the totals of all area among three fields of vision chosen at random; b) Average, average of each percentage.
Table 4. Immunohistochemical scores.
| No. | c-kit | VEGFR-2 | PDGFR-2 | PI3K | Akt | m-TOR | MEK1 | MEK2 | ERK | |
|---|---|---|---|---|---|---|---|---|---|---|
| HSA | 1 | - c) | 3+ | - | 1+ | - | 1+ | - | 2+ | - |
| 2 | - | 2+ | - | 1+ | - | 1+ | 1+ | 2+ | - | |
| 3 | - | 3+ | - | 1+ | - | 1+ | 1+ | 2+ | - | |
| 4 | 1+ | 2+ | 2+ | 1+ | - | 1+ | 1+ | 2+ | 1+ | |
| 5 | - | 1+ | - | 1+ | - | - | - | 1+ | - | |
| 6 | - | 2+ | 2+ | 2+ | - | - | 1+ | 2+ | - | |
| 7 | - | 2+ | - | 1+ | - | 1+ | 1+ | 2+ | - | |
| 8 | 1+ | 2+ | 1+ | 2+ | - | - | - | 3+ | 1+ | |
| 9 | - | 2+ | 1+ | 1+ | - | 1+ | 1+ | 2+ | - | |
| 10 | 1+ | 2+ | - | 2+ | - | 1+ | 1+ | 2+ | - | |
| % a) | 1+ d) | 30 | 10 | 20 | 70 | 0 | 70 | 70 | 10 | 20 |
| 2+ e) | 0 | 70 | 20 | 30 | 0 | 0 | 0 | 80 | 0 | |
| 3+ f) | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | |
| Total b) | 30 | 100 | 40 | 100 | 0 | 70 | 70 | 100 | 20 | |
| Control | 11 | - | - | - | - | - | - | - | - | - |
| 12 | - | - | - | - | - | - | - | - | - |
a) %, percentage of specimens with each staining score; b) Total, proportion of specimens with any positive score; c) -, <10% of positive cells; d) 1+, 10% to <30% of positive cells; e) 2+, 30% to <50% of positive cells; f) 3+, >50% of positive cells.
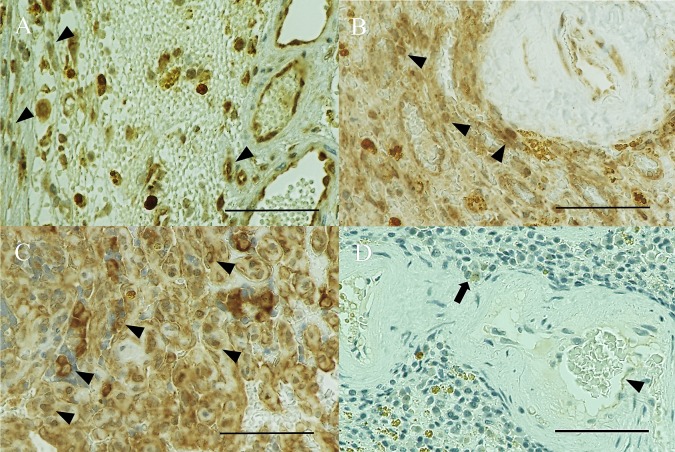
All 10 of the HSA samples (100%) expressed VEGFR-2 (1+, Fig. 2A), and 90% of these samples showed strong expression (2+, Fig. 2B; and 3+, Fig. 2C). Conversely, both normal spleens were negative for VEGFR-2 (control, Fig. 2D). VEGFR-2 expression was higher in HSAs than in normal spleens. Only HSAs expressed c-kit (30%) and PDGFR-2 (40%).
Fig. 2.
VEGFR-2 immunohistochemical staining (× 400). (A) Hemangiosarcoma (HSA) showing weak (1+) cytoplasmic and nuclear expression (arrowhead) in Case 5. (B) HSA showing strong (2+) cytoplasmic and nuclear expression (arrowheads) in Case 4. (C) HSA showing strong (3+) cytoplasmic and nuclear expression (arrowheads) in Case 1. (D) A normal spleen showing no expression (control), which may reflect the presence of VEGFR-2-expressing endothelial cells (arrowhead) and macrophages (arrow). The nuclei of lymphocytes show dense blue stain in Case 12. Bar, 60 µm.
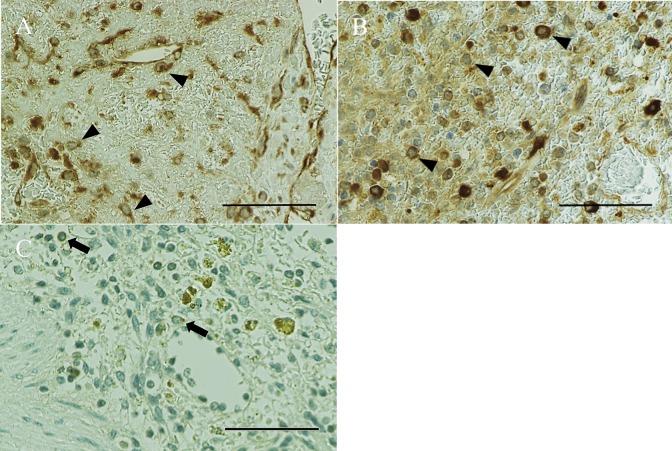
All of the HSAs expressed PI3K (1+, Fig. 3A), with strong expression observed in 30% of these cases (2+, Fig. 3B). In contrast, PI3K was not expressed in the normal spleens (control, Fig. 3C). A total of 70% of the HSAs were positive for p-m-TOR, while normal spleens were negative. PI3K and m-TOR expression was higher in HSAs than in normal spleens. None of the specimens stained positively for p-Akt compared to the positive control (not shown).
Fig. 3.
PI3K immunohistochemical staining (× 400). (A) Hemangiosarcoma (HSA) showing weak (1+) cytoplasmic and nuclear expression (arrowheads) in Case 1. (B) HSA showing strong (2+) cytoplasmic and nuclear expression (arrowheads) in Case 6. (C) A normal spleen showing no expression (control), which may reflect the presence of PI3K-expressing leukocytes (arrows), in Case 11. Normal endothelial cells show no expression. Bar, 60 µm.
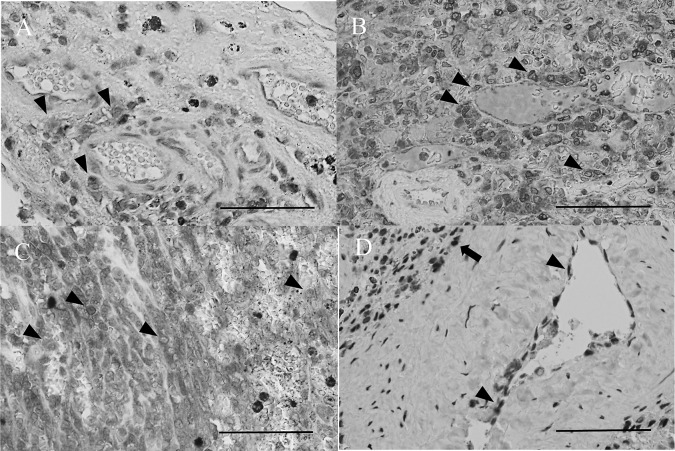
All of the HSAs expressed MEK2 (1+, Fig. 4A). Strong expression was observed in 80% of the specimens (2+, Fig. 4B; and 3+, Fig. 4C), while normal spleens were negative for MEK2 (control, Fig. 4D). A total of 70% of the HSAs expressed MEK1 and 20% expressed p-ERK. In contrast, both MEK1 and p-ERK were not expressed in normal spleens.
Fig. 4.
MEK2 immunohistochemical staining (× 400). (A) Hemangiosarcoma (HSA) showing weak (1+) cytoplasmic and nuclear expression (arrowheads) in Case 5. (B) HSA showing strong (2+) cytoplasmic and nuclear expression (arrowheads) in Case 2. (C) HSA showing strong (3+) cytoplasmic and nuclear expression (arrowheads) in Case 8. (D) A normal spleen showing no expression (control), which may reflect the presence of weak MEK2-expressing endothelial cells (arrowheads) and macrophages (arrow), in Case 12. Bar, 60 µm.
DISCUSSION
It was previously shown that all human HSAs and hemangiomas (HAs) expressed VEGFR-2, although the staining intensity was stronger in HSAs [24]. In the present study, all HSA specimens expressed VEGFR-2, and 90% of these cases showed strong expression; however, VEGFR-2 was not expressed in normal spleens. Our findings are therefore consistent with those of the previous study, as VEGFR-2 expression was strong in canine HSAs.
Strong and focally positive c-kit staining has been demonstrated in most HSAs by immunohistochemistry [24]. Additionally, the RTK, c-kit and PDGFR-2 inhibitors, imatinib and dasatinib were shown to reduce the viability of canine HSA cell lines [9]. In the present study, 30% of HSAs displayed weak expression of c-kit and 40% of HSAs expressed PDGFR-2, with strong expression in 20% of these cases. However, normal spleens were negative for c-kit and PDGFR-2. Our findings suggest that c-kit and PDGFR-2 are specific antigens for canine splenic HSA. The RTKs VEGFR-2, c-kit, and PDGFR-2 are therefore potential molecular targets for HSA treatment. In addition, VEGFR-2 inhibition decreased murine renal cell and colon carcinoma burden by preventing vascularization and growth of micrometastases in vivo rather than by preventing the establishment of micrometastases. This previous study showed that the VEGFR-2 pathway contributed to the vascularization and growth of micrometastases [22]. There is evidence that cyclophosphamide- and etoposide-based metronomic chemotherapy (daily oral low-dose chemotherapy) can prolong survival in canines with splenic HSA [21]. However, a previous study showed that combinations of doxorubicin-based conventional protocols and cyclophosphamide-based metronomic protocols appeared to be more effective than either type of chemotherapy alone, although the increased survival times resulting from the current protocols were modest [36]. Treatment with the lower dose anticancer agent, metronomic chemotherapy, prevented vascularization of the tumor similar to VEGFR-2 inhibition. It has been suggested that the combination of such therapies that prevent vascularization, VEGFR-2-targeted therapy and metronomic chemotherapy may be effective for the treatment of canine HSA.
Previous immunohistochemical studies have also suggested that the Akt/m-TOR pathway is activated in human HSA [20], and activation of this pathway has been reported in cell lines derived from cases of canine melanoma [19] and osteosarcoma [12]. Furthermore, a recent immunohistochemical study found that the Akt/m-TOR pathway was activated in canine dermal HSA [28], and the PI3K signaling pathway was shown to be crucial for the proliferation of canine MCT cell lines [1]. In the present study, all HSA specimens showed expression of PI3K, 70% showed expression of m-TOR, and 30% displayed strong expression of PI3K. Therefore, the PI3K/Akt/m-TOR pathway might be an ideal candidate for molecularly targeted therapy in canine splenic HSA.
We found no p-Akt expression in HSAs or normal spleens. A previous study demonstrated that the detection of phosphorylated proteins in formalin-fixed tissues was difficult, especially in surgically obtained clinical tissue samples [4]. This is because the majority of phosphorylated proteins are lost within 60 min of collection [18]. A more recent study found that canine dermal HSA samples were small enough to be fixed quickly in order to retain phosphorylated proteins, and more than 75% of these samples were shown to express p-Akt by immunohistochemistry [28]. Unfortunately, it is unknown whether the samples used in our study were fixed within several minutes of resection. The present study was limited in terms of the use of samples submitted to the comparative pathologic laboratory that were not quickly resected during surgery. Formalin-fixed sections or fresh cryosections obtained within 60 min of surgery are required for immunohistochemical evaluation.
All the HSA samples in this study expressed MEK2, and 90% of these demonstrated strong expression. Of the samples, 70% showed weak expression of MEK1. It was previously shown that canine cardiac HSA tumor grafts were sensitive to the MEK inhibitor PD0325901 and that MEK signaling was necessary for the growth of HSA in vivo [2]. In addition, eIF4E, a downstream target of the PI3K/Akt/m-TOR and MAPK pathways, showed stronger expression in canine dermal HSAs compared to HAs by immunohistochemistry [28]. Our findings indicate that the MEK pathway could be a suitable target in the treatment of canine splenic HSA. Interestingly, canine cardiac HSA cellular isolates were previously shown to have higher levels of p-ERK2 than p-ERK1 by immunoblotting [2]. This is consistent with published data indicating that ERK2 may play a more prominent role in canine cardiac HSA. ERK is downstream of MEK; thus, MEK2 may play a more prominent role than MEK1 in canine splenic HSA. Overexpression of downstream components of the RTK pathways, such as the PI3K/Akt/m-TOR and MAPK pathways, indicates that a combination of inhibitors of these pathways may be effective for the treatment of canine HSA. In addition, a previous study demonstrated that mutation of exon-11 in c-kit was detected in high-grade canine fine needle aspiration (FNA)-mast cell tumors (MCTs) but not in low grade MCTs by polymerase chain reaction (PCR), and detection of this mutation by PCR might enable noninvasive grade evaluation of canine MCT [32]. It was recently shown that the phosphorylation levels of Akt and m-TOR were higher in canine HSA cell lines than in normal canine endothelial cells by western blotting [27]. In canine HSA, it has been suggested that obtaining samples by FNA of the tumor (e.g., splenic or hepatic tumors) and measuring the expression of the PI3K/Akt/m-TOR pathway (e.g., p-Akt and p-m-TOR) or the MAPK pathway (e.g., MEK2), or both using PCR may enable noninvasive and quick differential diagnosis of HSA from other benign tumors.
We found that only 20% of HSAs exhibited weak expression of p-ERK. In an earlier study, activation of the ras/MAPK pathway was observed in murine HSA using microarray and reverse transcriptase-PCR [16, 17], although p-MAPK expression was far lower in human HSA [3]. The same mechanism may regulate p-MAPK in canine splenic HSA. Moreover, three major MAPK subfamilies have been identified (p38, ERK and c-Jun N-terminal kinase in mouse glial and neuronal cultures by western blotting [14, 23], and p-ERK2 was shown to be expressed in canine cardiac HSA by immunoblotting [2]. Unfortunately, in the present study, it was unknown whether samples were fixed within several minutes of resection. This is a limitation of the present study and was true for p-Akt. If the sections were formalin-fixed or if fresh cryosections were small enough to be rapidly fixed in order to retain phosphorylated proteins, we may have found that canine splenic HSA was positive for this antigen.
In conclusion, our findings show that signaling of the RTKs, c-kit, VEGFR-2, PDGFR-2 and the PI3K/Akt/m-TOR pathway, and MEK pathway is increased in canine splenic HSA compared to normal spleens. These findings could provide the basis for molecularly targeted therapy in canine HSA.
Acknowledgments
This work was supported by T. Kimura, K. Aoshima, S. Nakamura and M. Furukawa from the Laboratory of Comparative Pathology, Hokkaido University, and S. Konnai from the Laboratory of Infectious Disease, Hokkaido University.
REFERENCES
- 1.Amagai Y., Tanaka A., Matsuda A., Oida K., Jung K., Matsuda H.2013. The phosphoinositide 3-kinase pathway is crucial for the growth of canine mast cell tumors. J. Vet. Med. Sci. 75: 791–794. doi: 10.1292/jvms.12-0540 [DOI] [PubMed] [Google Scholar]
- 2.Andersen N. J., Nickoloff B. J., Dykema K. J., Boguslawski E. A., Krivochenitser R. I., Froman R. E., Dawes M. J., Baker L. H., Thomas D. G., Kamstock D. A., Kitchell B. E., Furge K. A., Duesbery N. S.2013. Pharmacologic inhibition of MEK signaling prevents growth of canine hemangiosarcoma. Mol. Cancer Ther. 12: 1701–1714. doi: 10.1158/1535-7163.MCT-12-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbiser J. L., Weiss S. W., Arbiser Z. K., Bravo F., Govindajaran B., Caceres-Rios H., Cotsonis G., Recavarren S., Swerlick R. A., Cohen C.2001. Differential expression of active mitogen-activated protein kinase in cutaneous endothelial neoplasms: implications for biologic behavior and response to therapy. J. Am. Acad. Dermatol. 44: 193–197. doi: 10.1067/mjd.2000.111632 [DOI] [PubMed] [Google Scholar]
- 4.Baker A. F., Dragovich T., Ihle N. T., Williams R., Fenoglio-Preiser C., Powis G.2005. Stability of phosphoprotein as a biological marker of tumor signaling. Clin. Cancer Res. 11: 4338–4340. doi: 10.1158/1078-0432.CCR-05-0422 [DOI] [PubMed] [Google Scholar]
- 5.Bastianello S. S.1983. A survey on neoplasia in domestic species over a 40-year period from 1935 to 1974 in the Republic of South Africa. VI. Tumours occurring in dogs. Onderstepoort J. Vet. Res. 50: 199–220. [PubMed] [Google Scholar]
- 6.Brown N. O., Patnaik A. K., MacEwen E. G.1985. Canine hemangiosarcoma: retrospective analysis of 104 cases. J. Am. Vet. Med. Assoc. 186: 56–58. [PubMed] [Google Scholar]
- 7.Brown R. J., Newman S. J., Durtschi D. C., Leblanc A. K.2012. Expression of PDGFR-β and Kit in canine anal sac apocrine gland adenocarcinoma using tissue immunohistochemistry. Vet. Comp. Oncol. 10: 74–79. doi: 10.1111/j.1476-5829.2011.00286.x [DOI] [PubMed] [Google Scholar]
- 8.Chang H. W., Aoki M., Fruman D., Auger K. R., Bellacosa A., Tsichlis P. N., Cantley L. C., Roberts T. M., Vogt P. K.1997. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science 276: 1848–1850. doi: 10.1126/science.276.5320.1848 [DOI] [PubMed] [Google Scholar]
- 9.Dickerson E. B., Thomas R., Fosmire S. P., Lamerato-Kozicki A. R., Bianco S. R., Wojcieszyn J. W., Breen M., Helfand S. C., Modiano J. F.2005. Mutations of phosphatase and tensin homolog deleted from chromosome 10 in canine hemangiosarcoma. Vet. Pathol. 42: 618–632. doi: 10.1354/vp.42-5-618 [DOI] [PubMed] [Google Scholar]
- 10.Dorn C. R., Taylor D. O., Schneider R., Hibbard H. H., Klauber M. R.1968. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J. Natl. Cancer Inst. 40: 307–318. [PubMed] [Google Scholar]
- 11.Faivre S., Kroemer G., Raymond E.2006. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 5: 671–688. doi: 10.1038/nrd2062 [DOI] [PubMed] [Google Scholar]
- 12.Gordon I. K., Ye F., Kent M. S.2008. Evaluation of the mammalian target of rapamycin pathway and the effect of rapamycin on target expression and cellular proliferation in osteosarcoma cells from dogs. Am. J. Vet. Res. 69: 1079–1084. doi: 10.2460/ajvr.69.8.1079 [DOI] [PubMed] [Google Scholar]
- 13.Hammer A. S., Couto C. G., Filppi J., Getzy D., Shank K.1991. Efficacy and toxicity of VAC chemotherapy (vincristine, doxorubicin, and cyclophosphamide) in dogs with hemangiosarcoma. J. Vet. Intern. Med. 5: 160–166. doi: 10.1111/j.1939-1676.1991.tb00943.x [DOI] [PubMed] [Google Scholar]
- 14.Huang Y. N., Ho Y. J., Lai C. C., Chiu C. T., Wang J. Y.2015. 1,25-Dihydroxyvitamin D3 attenuates endotoxin-induced production of inflammatory mediators by inhibiting MAPK activation in primary cortical neuron-glia cultures. J. Neuroinflammation 12: 147–158. doi: 10.1186/s12974-015-0370-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang B. H., Liu L. Z.2008. Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist. Updat. 11: 63–76. doi: 10.1016/j.drup.2008.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin M., Takahashi M., Moto M., Muguruma M., Ito K., Watanabe K., Kenmochi Y., Kono T., Hasumi K., Mitsumori K.2007. Carcinogenic susceptibility of rasH2 mice to troglitazone. Arch. Toxicol. 81: 883–894. doi: 10.1007/s00204-007-0218-1 [DOI] [PubMed] [Google Scholar]
- 17.Jin M., Matsumoto S., Dewa Y., Nishimura J., Saekusa Y., Hasumi K., Mitsumori K.2008. Extremely weak tumor-promoting effect of troglitazone on splenic hemangiosarcomas in rasH2 mice induced by urethane. Arch. Toxicol. 82: 771–777. doi: 10.1007/s00204-008-0293-y [DOI] [PubMed] [Google Scholar]
- 18.Jones R. J., Boyce T., Fennell M., Jacobs V., Pinto F., Duffield E., Clack G., Green T., Kelly J., Robertson J.2008. The impact of delay in cryo-fixation on biomarkers of Src tyrosine kinase activity in human breast and bladder cancers. Cancer Chemother. Pharmacol. 61: 23–32. doi: 10.1007/s00280-007-0440-9 [DOI] [PubMed] [Google Scholar]
- 19.Kent M. S., Collins C. J., Ye F.2009. Activation of the AKT and mammalian target of rapamycin pathways and the inhibitory effects of rapamycin on those pathways in canine malignant melanoma cell lines. Am. J. Vet. Res. 70: 263–269. doi: 10.2460/ajvr.70.2.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahat G., Dhuka A. R., Hallevi H., Xiao L., Zou C., Smith K. D., Phung T. L., Pollock R. E., Benjamin R., Hunt K. K., Lazar A. J., Lev D.2010. Angiosarcoma: clinical and molecular insights. Ann. Surg. 251: 1098–1106. doi: 10.1097/SLA.0b013e3181dbb75a [DOI] [PubMed] [Google Scholar]
- 21.Lana S., U’ren L., Plaza S., Elmslie R., Gustafson D., Morley P., Dow S.2007. Continuous low-dose oral chemotherapy for adjuvant therapy of splenic hemangiosarcoma in dogs. J. Vet. Intern. Med. 21: 764–769. doi: 10.1111/j.1939-1676.2007.tb03019.x [DOI] [PubMed] [Google Scholar]
- 22.Lee Y. J., Karl D. L., Maduekwe U. N., Rothrock C., Ryeom S., D’Amore P. A., Yoon S. S.2010. Differential effects of VEGFR-1 and VEGFR-2 inhibition on tumor metastases based on host organ environment. Cancer Res. 70: 8357–8367. doi: 10.1158/0008-5472.CAN-10-1138 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Leow-Dyke S., Allen C., Denes A., Nilsson O., Maysami S., Bowie A. G., Rothwell N. J., Pinteaux E.2012. Neuronal Toll-like receptor 4 signaling induces brain endothelial activation and neutrophil transmigration in vitro. J. Neuroinflammation 9: 230–240. doi: 10.1186/1742-2094-9-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L., Kakiuchi-Kiyota S., Arnold L. L., Johansson S. L., Wert D., Cohen S. M.2013. Pathogenesis of human hemangiosarcomas and hemangiomas. Hum. Pathol. 44: 2302–2311. doi: 10.1016/j.humpath.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 25.MacVean D. W., Monlux A. W., Anderson P. S., Jr, Silberg S. L., Roszel J. F.1978. Frequency of canine and feline tumors in a defined population. Vet. Pathol. 15: 700–715. doi: 10.1177/030098587801500602 [DOI] [PubMed] [Google Scholar]
- 26.Matsumura I., Mizuki M., Kanakura Y.2008. Roles for deregulated receptor tyrosine kinases and their downstream signaling molecules in hematologic malignancies. Cancer Sci. 99: 479–485. doi: 10.1111/j.1349-7006.2007.00717.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murai A., Asa S. A., Kodama A., Hirata A., Yanai T., Sakai H.2012. Constitutive phosphorylation of the mTORC2/Akt/4E-BP1 pathway in newly derived canine hemangiosarcoma cell lines. BMC Vet. Res. 8: 128–141. doi: 10.1186/1746-6148-8-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murai A., Abou Asa S., Kodama A., Sakai H., Hirata A., Yanai T.2012. Immunohistochemical analysis of the Akt/mTOR/4E-BP1 signalling pathway in canine haemangiomas and haemangiosarcomas. J. Comp. Pathol. 147: 430–440. doi: 10.1016/j.jcpa.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 29.Ogilvie G. K., Powers B. E., Mallinckrodt C. H., Withrow S. J.1996. Surgery and doxorubicin in dogs with hemangiosarcoma. J. Vet. Intern. Med. 10: 379–384. doi: 10.1111/j.1939-1676.1996.tb02085.x [DOI] [PubMed] [Google Scholar]
- 30.Prymak C., McKee L. J., Goldschmidt M. H., Glickman L. T.1988. Epidemiologic, clinical, pathologic, and prognostic characteristics of splenic hemangiosarcoma and splenic hematoma in dogs: 217 cases (1985). J. Am. Vet. Med. Assoc. 193: 706–712. [PubMed] [Google Scholar]
- 31.Ravandi F., Talpaz M., Estrov Z.2003. Modulation of cellular signaling pathways: prospects for targeted therapy in hematological malignancies. Clin. Cancer Res. 9: 535–550. [PubMed] [Google Scholar]
- 32.Sailasuta A., Ketpun D., Piyaviriyakul P., Theerawatanasirikul S., Theewasutrakul P., Rungsipipat A.2014. The Relevance of CD117-Immunocytochemistry Staining Patterns to Mutational Exon-11 in c-kit Detected by PCR from Fine-Needle Aspirated Canine Mast Cell Tumor Cells. Vet. Med. Int. 2014: 787498. doi: 10.1155/2014/787498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorenmo K. U., Baez J. L., Clifford C. A., Mauldin E., Overley B., Skorupski K., Bachman R., Samluk M., Shofer F.2004. Efficacy and toxicity of a dose-intensified doxorubicin protocol in canine hemangiosarcoma. J. Vet. Intern. Med. 18: 209–213. doi: 10.1111/j.1939-1676.2004.tb00162.x [DOI] [PubMed] [Google Scholar]
- 34.Spangler W. L., Culbertson M. R.1992. Prevalence, type, and importance of splenic diseases in dogs: 1,480 cases (1985–1989). J. Am. Vet. Med. Assoc. 200: 829–834. [PubMed] [Google Scholar]
- 35.Spangler W. L., Kass P. H.1997. Pathologic factors affecting postsplenectomy survival in dogs. J. Vet. Intern. Med. 11: 166–171. doi: 10.1111/j.1939-1676.1997.tb00085.x [DOI] [PubMed] [Google Scholar]
- 36.Wendelburg K. M., Price L. L., Burgess K. E., Lyons J. A., Lew F. H., Berg J.2015. Survival time of dogs with splenic hemangiosarcoma treated by splenectomy with or without adjuvant chemotherapy: 208 cases (2001–2012). J. Am. Vet. Med. Assoc. 247: 393–403. doi: 10.2460/javma.247.4.393 [DOI] [PubMed] [Google Scholar]
- 37.Wood C. A., Moore A. S., Gliatto J. M., Ablin L. A., Berg R. J., Rand W. M.1998. Prognosis for dogs with stage I or II splenic hemangiosarcoma treated by splenectomy alone: 32 cases (1991-1993). J. Am. Anim. Hosp. Assoc. 34: 417–421. doi: 10.5326/15473317-34-5-417 [DOI] [PubMed] [Google Scholar]