Abstract
Cyclooxygenase (COX) inhibitors have been shown to exert anti-angiogenic and anti-tumor activities on many types of malignant tumors. These anticancer properties make it worthwhile to examine the possible benefit of combining COX inhibitors with other anti-cancer agents. In the present study, we evaluated the potential of deracoxib (DER) in potentiating antitumor activity of doxorubicin (DOX) in canine mammary carcinoma cells (CMT-U27). DER (50–250 µM) enhanced the antiproliferative activity of DOX by reducing the IC50 (approximately 3- to 3.5 fold). Interaction analysis of the data showed that combinations of DOX at 0.9 µM with DER (100–250 µM) produced synergism in the CMT-U27 cell line, with a ratio index ranging from 1.98 to 2.33. In additional studies identifying the mechanism of observed synergistic effect, we found that DER strongly potentiated DOX-caused G0/G1 arrest in cell cycle progression. Also, DER (100–250 µM) augmented apoptosis induction with approximately 1.35- and 1.37- fold increases in apoptotic response caused by DOX in the cells. DER enhanced the antiproliferative effect of DOX in conjunction with induction of apoptosis by modulation of Bcl-2 expression and changes in the cell cycle of the CMT-U27 cell line. Although the exact molecular mechanism of the alterations in the cell cycle and apoptosis observed with DER and DOX combinations require further investigations, the results suggest that the synergistic effect of DOX and DER combinations in CMT therapy may be achieved at relatively lower doses of DOX with lesser side effects. Therefore, combining DER with DOX may prove beneficial in the clinical treatment of canine mammary cancer.
Keywords: apoptosis, canine mammary tumor, cell cycle, deracoxib, doxorubicin
Canine mammary tumors (CMTs) are the most common neoplasms occurring in female dogs, with a prevalence of 0.2% [23, 27]. Histologically, approximately 50–71% of these neoplasms are considered malignant, and distant metastases are common causes of death in cancer patients [67]. Despite their importance and high incidence, their molecular mechanisms have not been completely defined, and effective therapeutic options are scarce [26, 43]. The preferred therapy for CMT is surgical intervention with the intention of tumor extirpation, but it alone yields unsatisfactory results in dogs with malignant mammary tumor, because scattered tumor cells and metastases cannot be eliminated [19, 59, 64]. At present, the use of anticancer drugs to combat the micrometastatic disease is a reasonable consideration, but there are no agents currently approved for the treatment of malignant mammary tumors in dogs. Many of the chemotherapy protocols used in veterinary medicine have been co-opted from protocols used to treat human patients [41, 60]. Among the several types of chemotherapeutic drugs, doxorubicin (DOX), either alone or in combination with other drugs, is a widely used anti-cancer agent for the therapy of these tumors in veterinary practice [33, 66]. It is known that failure of chemotherapy with this agent as a result of development of resistance and dose-limiting toxicity is a major problem in the clinical management of the neoplasms [39, 62]. Therefore, alternative strategies that increase the therapeutic efficacy and minimize the systemic toxicity of the chemotherapeutic agent against mammary tumors in dogs are needed.
Numerous experimental, epidemiological and clinical studies indicate that nonsteroidal anti-inflammatory drugs (NSAIDs), particularly the selective cyclooxygenase (COX)-2 inhibitors, have chemotherapeutic and chemoprophylactic potentials in several different types of cancer, including breast carcinoma. Antitumorigenic and chemotherapeutic effects of these inhibitors have been attributed to both COX-dependent and COX-independent mechanisms relating to induction of cell apoptosis and inhibition of angiogenesis and cell invasion/migration [4, 6, 20, 42, 49, 57]. Also, numerous investigators have explored the therapeutic benefit of combining selective COX-2 inhibitors with chemotherapeutics and have shown that the COX-2 inhibitors are able to enhance the effects of certain cytostatic agents, such as doxorubicin, irinotecan and 5-fluorouracil in various human carcinoma cell lines and in a xenograft animal model [6, 22, 61, 69]. Currently, a number of phase I-III clinical trials are still investigating the efficacy of selective COX-2 inhibitors as single agents or in combination with chemotherapy in cancer therapy [16, 50]. In veterinary medicine, data from experimental and clinic studies exist on the efficacy of selective COX-2 inhibitors (firocoxib, deracoxib) against CMT [2, 46], but to our knowledge there is no published report demonstrating synergistic cytotoxic effects on canine mammary carcinoma cells elicited by anticancer agents in combination with these drugs. If synergistic effects could be achieved by combining selective COX-2 inhibitors with anticancer agents for CMTs, as well as in some types of human cancer [6, 17, 25, 47], they could reduce the doses of anticancer drugs needed and thus occurrence of complications. Therefore, we decided to investigate whether the selective COX-2 inhibitor deracoxib (DER), a drug licensed for veterinary use, could potentiate the inhibitory action of DOX, the standard chemotherapeutic drug, in a canine mammary tumor cell line (CMT-U27).
MATERIALS AND METHODS
Cell line and chemicals: To investigate the potential effects of the selective COX-2 inhibitor DER and cytotoxic anthracycline DOX against canine mammary carcinoma cells, the CMT-U27 cell line was chosen, as it has high proliferative and anti-apoptotic potential [29]. The canine mammary carcinoma cell line (CMT-U27) was kindly supplied by Prof. Eva Héllmen, Uppsala University, Sweden. This cell line was derived from a primary tumor (infiltrating ductal carcinoma), and when inoculated in the fat mammary pad of female nude mice, it metastasized to the lymph nodes, lungs, liver and heart [18]. DER was a generous gift from Novartis Pharmaceuticals Inc. (Basel, Switzerland). Polyclonal anti-Bcl-2 (sc-492), antibody, a secondary antibody kit and 3,3′-diaminobenzidine (DAB) (sc-2018) were purchased from Santa Cruz Biotechnology (Dallas, TX, U.S.A.). Unless otherwise indicated, all reagents were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.).
Cell culture: Cells were cultured in Dulbecco’s modified eagle’s medium (DMEM-F12) supplemented with 10% fetal bovine serum, 100 IUml−1 penicillin G, 100 µgml−1 streptomycin and 2.5 µgml−1 amphotericin B in an atmosphere of 37°C in 5% CO2, and the cells were harvested at approximately 80–90% confluence using 0.25% trypsin-EDTA solution. DOX and DER were dissolved in DMEM-F12 and sterile dimethyl sulfoxide (DMSO), respectively, and further serial dilutions for both drugs were made with DMEM-F12. The final DMSO concentration did not exceed 0.1% (and had no effect on cell growth) in any experimental group, and this condition was used as a control in each experiment (all groups comprised 0.1% DMSO). All of the stock solutions were kept at −20°C.
Cell viability assay: To determine DOX and DER concentrations that would be used in combination studies, an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assay was performed on CMT-U27 cells. For this purpose, cells were seeded at 1 × 104/well in a final volume of 100 µl medium in 96-well flat-bottomed tissue culture plates, in triplicate, and incubated in a humidified atmosphere at 37°C under 5% CO2 and 95% air to allow cell adhesion. After 24 hr incubation, the medium was removed, and cells were treated with various concentrations of DOX (0.1, 1, 10, 50 and 100 µM) and DER (50, 100 and 250 µM) for 72 hr. The concentrations for DOX were chosen on the basis of previous reports about the effects of this drug on the in vitro viability of canine mammary tumor cells [39]. DER concentrations were selected according to our previous study [2]. Cell viability, based on mitochondrial dehydrogenase activity, was determined by colorimetric assay using a MTT Cell Proliferation Kit (Roche Applied Science, Mannheim, Germany) in accordance with the instruction manual. The optical density of each well at 550 nm against a reference wavelength of 650 nm was measured using a microplate reader (ELx800, Biotek Instruments, Winooski, VT, U.S.A.). Cell viability was calculated as follows: Viability (%)=(Absorbance of the treated wells)/(Absorbance of the control wells) ×100. Each concentration was tested in three different experiments and run in triplicate. The dose-response curves were plotted for each drug, and the concentration of drug required for 50% inhibition of cell viability (IC50) was determined graphically. Subsequently, we tested 0.9 µM (IC50) and 0.09 µM (1/10 IC50) of DOX with 50, 100 and 250 µM of DER in combination to learn whether DER could enhance the antiproliferative effects of DOX in CMT-U27 cells. For this purpose, the cells were treated with DOX and DER for 72 hr, and the antiproliferative effects of the combined agents were evaluated according to the MTT assay.
Drug interaction analysis: The nature of the interaction between DOX (0.9 µM and 0.09 µM) and DER (50, 100 and 250 µM) was determined by calculating the ratio index (RI), which was initially described by Kern et al. [24] and later modified by Romanelli et al. [53]. The RI is calculated as the ratio of expected cell survival (Sexp, defined as the product of the viability observed with drug A alone and the survival observed with drug B alone) to the observed cell survival (Sobs) for the combination of A and B (RI=Sexp/Sobs). Type of interaction was defined as follows: RI≥1.5, synergistic; RI<1.5 to >0.5, additive; and RI≤0.5, antagonistic [31]. This method was selected, because treatment with DER had little effect on cell viability, which meant that other methods, such as the median effect principle and isobologram methods, were not suitable.
Apoptosis assay: To determine the mechanism of interaction between DER and DOX, an apoptosis assay was performed. Flow cytometric analyses of phosphatidylserine exposure were quantitatively performed using an Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (BD Biosciences, San Jose, CA, U.S.A.). The method is based on the binding of Annexin V to phosphatidylserine that is translocated from the inner membrane leaflet to the outer layer in cells undergoing apoptosis [68]. The cells were cultured at a density of 1 × 105/ml in 24-well flat bottom microtiter plates (Jet Biofil, Seoul, Korea) and cultivated in a medium as described above. After 24 hr, the medium was replaced with fresh medium containing DOX (0.9 µM) with or without DER (50–250 µM). The cells were trypsinized 72 hr after treatment, washed twice each with ice-cold phosphate-buffered saline (PBS) consisting of 0.01 M phosphate buffer, 0.0027 M potassium chloride and 0.137 M sodium chloride and then resuspended in 100 µl binding buffer (0.1 M Hepes/NaOH (pH 7.4), 1.4 M NaCl, 25 mM CaCl2), additive supplemented with 5 µl of FITC-Annexin V and 5 µl of propidium iodide (PI). The cell suspension was gently vortexed and incubated for 15 min at room temperature in the dark. Following incubation, 400 µl of binding buffer was added to each tube, and then, the cell suspension was analyzed within 1 hr on a FACScan flow cytometer (BD Biosciences) using the standard optics for detecting FL1 (FITC) and FL3 (PI). Data were analyzed with the CellQuest WinMDI software (BD Biosciences, San Jose, CA, U.S.A.).
Cell cycle analysis: The effects of DOX (0.9 and 0.09 µM) with or without DER (50–250 µM) on the CMT-U27 cell cycle were evaluated by flow cytometry using a Coulter DNA Prep Reagents Kit (Beckman Coulter, High Wycombe, Buckinghamshire, U.K.). The cells were cultured at a density of 1 × 105/ml in 24-well flat bottom microtiter plates and cultivated and treated as described for an apoptosis assay. After the 72 hr treatment, the floating and adherent cells were combined for the analyses. Cells were washed with PBS, and the cell suspensions were resuspended in 100 µl of PBS. The resuspended cells were stained according to the manufacturer’s instructions. The DNA content of the stained cells was immediately analyzed using a FACScan flow cytometer (BD Biosciences). At least 10,000 cells were counted. The percentages of cells in the G0/G1 phase, S phase and G2/M phase were calculated using the CellQuest WinMDI software (BD Biosciences).
Prostaglandin E2 (PGE2 assay): In order to elucidate whether the antiproliferative effect was mediated via the COX pathway, we studied the effect of exogenous addition of PGE2 (1–5 µg/ml) on the in vitro antiproliferative activity of DER alone and in combination with DOX against CMT-U27 cells. The cells were seeded at 1 × 104/well in 100 µl of medium in 96-well plates and incubated overnight. Subsequently, DOX (0.9 and 0.09 µM) was added to the plate in the presence or absence of PGE2 (1–5 µg/ml). Similarly, DER (50–250 µM) in combination with DOX (0.9 and 0.09 µM) was added in the presence or absence of PGE2 (1–5 µg/ml) and incubated for 72 hr, and the antiproliferative activity was assessed by MTT assay.
Immunocytochemistry: In order to examine the protein expression of the apoptosis marker Bcl-2 in CMT-U27 cells, sterilized coverslips were placed on the bottom of a 24-well plate, and the cells were seeded at a density of 1 × 105 cells/ml and incubated overnight. After incubation, cells were treated with 50–250 µM DER alone or in combination with 0.9 µM and 0.09 µM DOX for 72 hr. At the end of treatment, the cells were rinsed with PBS and fixed with cold methanol for 10 min. After fixation, the cells were washed with PBS (pH 7.4, 0.1 M) for 5 min, and the endogenous peroxidase activity was inactivated by incubation with 0.3% H2O2 in methanol for 10 min. Then, the cells were washed three times with PBS and incubated for 10 min with a protein blocking agent to block nonspecific immunolabeling. Afterwards, cells were incubated with polyclonal anti-Bcl-2 (sc-492) antibody at a dilution of 1:200 at room temperature for 90 min. After extensive washing in PBS, the cover slips were incubated with a secondary antibody kit (sc-2018) containing a biotinylated secondary antibody and avidin-peroxidase for 20 min, at room temperature. Finally, cells were rinsed with PBS and incubated with DAB complexes according to the manufacturer’s protocol and then counterstained with Mayer’s hematoxylin and mounted onto glass slides. The cells were observed with a light microscope (Olympus BX50, Olympus, Tokyo, Japan). Intensity of immunolabeling was assessed by examination of 10 representative high-power fields (400 ×). Positive cells were identified by distinct brown nuclear staining. The number of immunoreactive cells was assessed semiquantitatively. For staining density, specimens were classified as negative, + (<10% positive cells), ++ (10–50% positive cells) and +++ (>50% positive cells). Results were considered inconclusive when there were insufficient neoplastic cells available for analysis [73].
Statistical analysis: Samples were assayed at least three times for each determination, and results were expressed as the mean ± SE. The statistical differences between the treatments and the control were tested by one-way analysis of variance (ANOVA) followed by the Student’s t-test using the GraphPad InStat software (GraphPad Software, San Diego, CA, U.S.A.). A difference in means with a P-value of 0.05 or less was considered to be statistically significant.
RESULTS
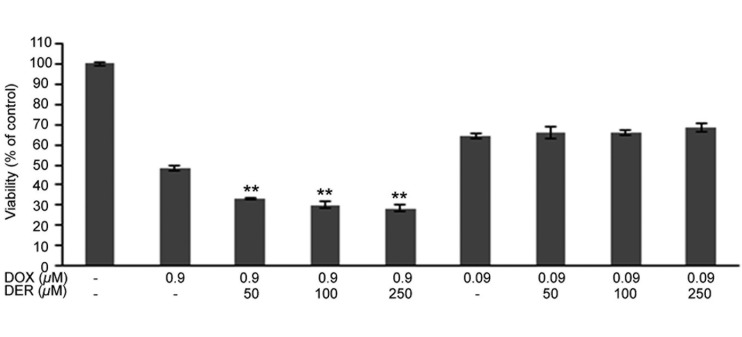
Antiproliferative effects of DER and DOX combinations on CMT-U27 cells: The antiproliferative effects of DER and DOX combinations on CMT-U27 cells were evaluated by MTT assay. The antiproliferative effects of DER and DOX as single agents against CMT-U27 cells were shown in our previous studies [2, 3]. Those studies demonstrated that DER even at concentration as high as 250 µM had weak growth inhibitory activity in the cells (with growth inhibition of 16.49% compared with the control). By contrast, CMT-U27 cells responded to DOX sensitively, with an obvious dose-dependent antiproliferative effect and IC50 of approximately 0.9 µM. Subsequently, to determine whether DER enhances the anti-proliferative effect of DOX on CMT-U27 cells, three doses of DER (50, 100 and 250 µM) were used in combination with DOX (0.9 and 0.09 µM). As shown in Fig. 1, DER potentiated the inhibitory effect of DOX (0.9 µM) on CMT-U27 cells. The influence of DER on the effect of DOX (0.9 µM) appeared to be moderately dose dependent, and the strongest inhibition, approaching 71%, was observed with the combination of DOX at 0.9 µM and DER at 250 µM. Also, the COX inhibitor at the concentrations used enhanced the antiproliferative activity of DOX in CMT-U27 cells, which decreased (2.89- to 3.71-fold) the IC50 value from 0.876 µM (DOX at 0.9 µM) to 0.303 µM, 0.245 µM and 0.236 µM (0.9 µM in combination with 50, 100 and 250 µM DER), respectively.
Fig. 1.
Antiproliferative effects of doxorubicin and deracoxib combinations in the CMT-U27 cell line. Cells were treated with the indicated doses of doxorubicin and deracoxib, and cell viability was assayed 72 hr after treatment. Data are expressed as mean percentage of cell viabilities ± standard error (SE). **P<0.01 compared with the doxorubicin-treated group. DOX, doxorubicin; DER, deracoxib.
The nature of the interaction between DOX and DER was analyzed using the RI, which quantitatively measures the interaction of two drugs. The combinations of DOX at 0.9 µM and DER at 50–250 µM exhibited synergistic activity against CMT-U27 cells (Table 1). The synergistic effect was most prominent when 0.9 µM DOX was combined with 250 µM DER (RI=2.33). On the other hand, the combinations of a low dose (0.09 µM) of DOX with DER (50–250 µM) resulted in additive interaction (RI=1.08–1.15)
Table 1. Type of interaction between doxorubicin and deracoxib in CMT-U27 cells.
| Combination of DOX with DER | RI value |
|---|---|
| DOX (0.9 µM) | |
| DOX+ DER (50 µM) | 2.24 (synergistic) |
| DOX+ DER (100 µM) | 1.98 (synergistic) |
| DOX+ DER (250 µM) | 2.33 (synergistic) |
| DOX (0.09 µM) | |
| DOX+ DER (50 µM) | 1.15 (additive) |
| DOX+ DER (100 µM) | 1.13 (additive) |
| DOX+ DER (250 µM) | 1.08 (additive) |
DOX, doxorubicin; DER, deracoxib; RI, ratio index. Type of interaction: RI≥1.5, synergistic; RI<1.5 to >0.5, additive; RI≤0.5, antagonistic interaction.
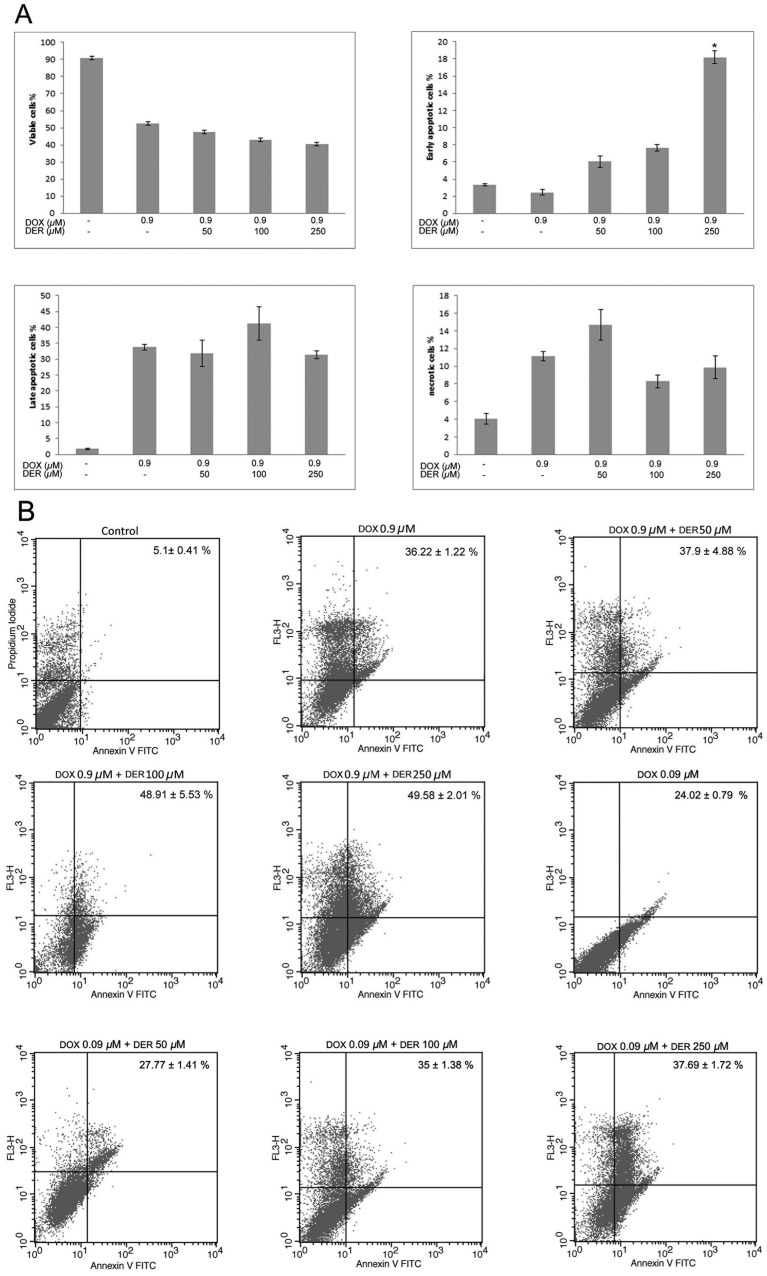
Effects of DER and DOX combinations on apoptosis in CMT-U27 cells: The apoptotic effects of DER as a single agent against CMT-U27 cells were shown in our previous study [2]. To explore the mechanisms of synergistic effects of DOX and DER combinations, an apoptosis assay was performed using specific concentrations of DOX (0.9 µM) and DER (50–250 µM) that cannot lead to toxicity. Treatment with DOX (0.9 µM) alone induced 36.22% apoptosis in CMT-U27 cells. The combined treatment of DOX and DER (100 µM and 250 µM) significantly augmented apoptosis induction in CMT-U27 cells (sum of early and late apoptotic cells; 48.91% and 49.58%, respectively), and approximately 1.35- and 1.37-fold increases, respectively, in apoptotic response were observed as compared with that caused by DOX. Treatment with DOX (0.09 µM) alone induced 24.02% apoptosis in CMT-U27 cells. The combined treatment of DOX (at 0.09 µM) and DER (at 50 µM, 100 µM and 250 µM) induced 27.77%, 35% and 37.69% apoptosis, respectively, in CMT-U27 cells in terms of the sum of early and late apoptotic cells (Fig. 2A and 2B).
Fig. 2.
Effects of doxorubicin and deracoxib combination treatment on apoptosis of CMT-U27 cells. (A) The number of viable, early apoptotic, late apoptotic and necrotic cells due to treatment with doxorubicin and deracoxib combinations for 72 hr in the CMT-U27 cell line. The experiment was conducted in three replicates. Data are expressed as the mean ± standard error (SE). *P<0.05 compared with the 0.9 µM doxorubicin-treated group. DOX, doxorubicin; DER, deracoxib. (B) Representative cytograms of the CMT-U27 cell line double stained with Annexin V-FITC and propidium iodide (PI). The numbers written on histograms represent the sum of early and late apoptotic cells (%).
Effects of DOX and DER combinations on the cell cycle distribution of CMT-U27 cells: To confirm the mechanism of such synergistic effects of DOX and DER combinations, we also examined the effects of their combinations on cell cycle parameters under the same experimental conditions. As shown in Table 2, the percentage of cells in the G0/G1 phase and S phase of the cell cycle increased with DOX (0.9 µM) treatment as a single agent, while the proportion of cells in the G2/M phase decreased compared with the control. The combined treatments of DOX and DER altered the cell cycle profile in CMT-U27 cells. The most remarkable change in cell cycle progression was seen with the combination of 0.9 µM DOX and 250 µM DER. This combination led to a further increase in the G0/G1 phase (from 56.6 to 91.34%) with a concomitant decrease in the S phase (from 34.83 to 7.16) compared with DOX alone (0.9 µM).
Table 2. Effects of doxorubicin and deracoxib combination treatment for 72 hr on cell cycle kinetics of CMT-U27 cells.
| Drugs | Concentration | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|---|
| Control | - | 54.51 ± 2.68 | 29.05 ± 1.68 | 16.44 ± 1.24 |
| DOX | 0.9 µM | 56.6 ± 4.24 | 34.83 ± 2.53 | 8.51 ± 1.14 |
| DOX + DER | 0.9 µM + 50 µM | 84.49 ± 3.60 | 15.30 ± 2.08 | 0.21 ± 0.04a) |
| DOX + DER | 0.9 µM + 100 µM | 84.96 ± 1.45 | 14.83 ± 2.65 | 0.21 ± 0.03a) |
| DOX + DER | 0.9 µM + 250 µM | 91.34 ± 3.64 | 7.16 ± 1.32a) | 1.49 ± 0.18a) |
| DOX | 0.09 µM | 86.93 ± 4.99 | 12.92 ± 1.72 | 0.14 ± 0.04 |
| DOX + DER | 0.09 µM + 50 µM | 89.58 ± 5.46 | 10.30 ± 1.06 | 0.12 ± 0.03 |
| DOX + DER | 0.09 µM + 100 µM | 89.13 ± 4.49 | 10.84 ± 1.50 | 0.20 ± 0.04 |
| DOX + DER | 0.09 µM + 250 µM | 89.79 ± 2.85 | 10.1 ± 1.46 | 0.10 ± 0.02 |
Each value represents the mean ± SE of three experiments. a) P<0.05 compared with the 0.9 µM doxorubicin-treated group. DOX, doxorubicin; DER, deracoxib.
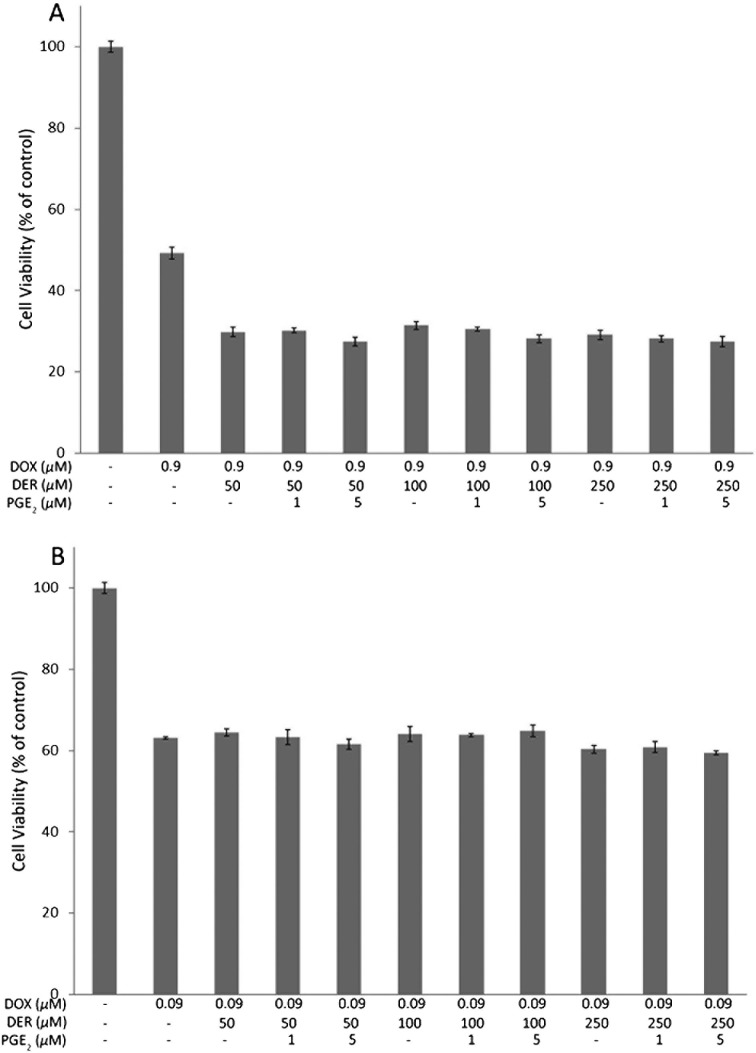
Effects of supplementation of PGE2 on growth inhibitory activity of DOX enhanced by DER in CMT-U27 cells: The effects of supplementation of PGE2 on the growth inhibitory activity of DOX enhanced by DER in CMT-U27 cells may be due to COX-2 inhibition. To examine this, we determined whether the COX-2 end product PGE2 could reverse the observed effects. Therefore, exogenous PGE2 (1–5 µM) was added to the medium in order to take into account the fact that some PGE2 may degrade or be internalized into cells. As shown in Fig. 3A and 3B, cell viability was not changed significantly with the addition of PGE2. PGE2 at all tested concentrations failed to reverse the enhancing effect of DER on the growth inhibitory activity of DOX.
Fig. 3.
Effects of supplementation of PGE2 on enhancement of growth inhibitory activity of doxorubicin caused by deracoxib in CMT-U27 cells. Cells were treated with or without deracoxib (50-250 µM) in the absence or presence of PGE2 (1 or 5 µM) and 0.9 µM doxorubicin (A) or 0.09 µM doxorubicin (B) alone or in combination for 72 hr before determination of cell viability by MTT assay. DOX, doxorubicin; DER, deracoxib.
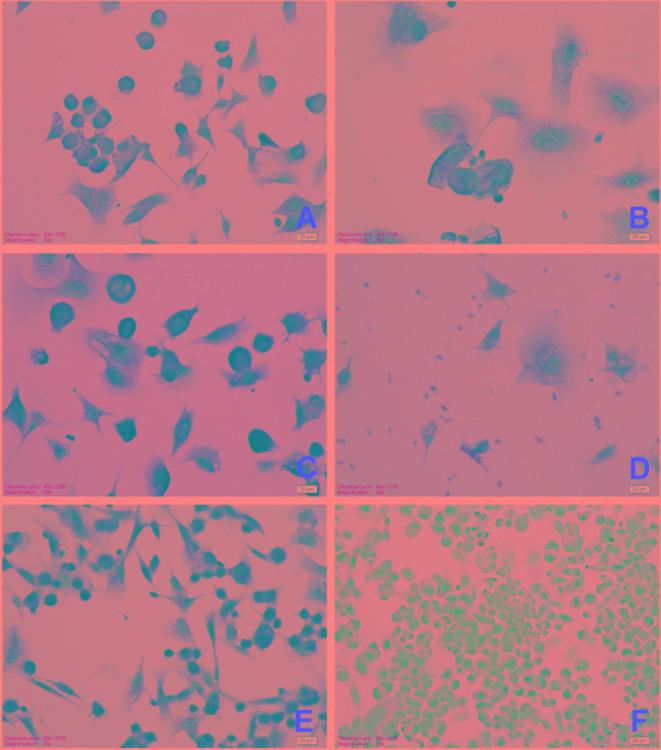
Effects of DOX and DER alone or in combination on Bcl-2 expression in CMT U27 cells: To determine the apoptotic pathway activated by DER, we examined the apoptosis-related target Bcl-2 in CMT-U27 cells. Immunocytochemical staining revealed that increasing the concentrations of DER inhibited expression of the anti-apoptotic protein Bcl-2 (Fig. 4). These effects were more pronounced in the combinations of DOX (at 0.9 µM) with DER (at 100 µM and 250 µM) (Table 3). However, the combinations of DOX at 0.09 µM with DER (at 100 µM and 250 µM) decreased the expression of Bcl-2 slightly.
Fig. 4.
Immunocytochemical expression of Bcl-2 in CMT U27 cells. A) Deracoxib 50 µM, moderate immunopositivity; B) Deracoxib 250 µM, slight immunopositivity; C) Doxorubicin 0.09 µM + deracoxib 50 µM, strong immunopositivity; D) Doxorubicin 0.9 µM + deracoxib 250 µM slight immunopositivity; E) Control cells, very strong immunopositivity; F) Negative control cells, no reaction. Bar=20 µm.
Table 3. Effects of deracoxib or doxorubicin alone or in combination on Bcl-2 expression in CMT-U27 cells.
| Drugs | Concentration | Bcl-2 |
|---|---|---|
| Control | - | +++ |
| DOX | 0.9 µM | ++ |
| DER | 50 µM | ++ |
| DER | 100 µM | + |
| DER | 250 µM | + |
| DOX + DER | 0.9 µM + 50 µM | ++ |
| DOX + DER | 0.9 µM + 100 µM | + |
| DOX + DER | 0.9 µM + 250 µM | + |
| DOX | 0.09 µM | +++ |
| DOX + DER | 0.09 µM + 50 µM | +++ |
| DOX + DER | 0.09 µM + 100 µM | ++ |
| DOX + DER | 0.09 µM + 250 µM | ++ |
DOX, doxorubicin; DER, deracoxib. Results are categorized as negative, + (<10% positive cells), + + (10–50% positive cells) and +++ (>50% positive cells).
DISCUSSION
Despite many advances in the field of cancer therapeutics, canine malignant mammary tumors continue to be a leading cause of death in dogs, which is in part due to the failure of chemotherapy. Clearly, new therapeutic modalities are needed to both improve the outcome of dogs suffering from the tumors and to reduce the long-term toxicities associated with the current standard of treatment. Drug development strategies include the evaluation of new drug combinations that may have improved efficacy compared with single agents. By treating a tumor with a combination of agents that employ different mechanisms of action and have different spectra of normal tissue toxicity, the overall response can be enhanced without an increase in toxicity [35, 37]. Besides enhanced cytotoxicity, combinations of chemotherapeutic agents may minimize or delay the induction of drug resistance [10, 21]. Furthermore, drug combinations that use lower doses of individual agents may improve selectivity and reduce the severity of undesired side effects of chemotherapy [45].
Selective COX-2 inhibitors exert antitumor effects on tumor cells directly via inhibition of cell proliferation, induction of apoptosis and reduction of cell motility and adhesion and also have anti-angiogenic activity by suppressing tumor angiogenesis [36, 56, 69, 72]. These anticancer properties make it worthwhile to examine the possible benefit of combining selective COX-2 inhibitors with conventional anticancer therapies, such as chemotherapy. Related studies have shown that selective COX-2 inhibitors in particular strengthen the effectiveness of chemotherapy treatment in various types of human tumors, including breast tumors [5, 9, 56, 69]. Similarly, during the last decade, various preclinical and clinical trials in the field of veterinary oncology have been conducted to study the use of COX-2 selective inhibitors in combination with other agents in early and advanced cancers and have been shown to improve treatment outcome in dogs with transitional cell carcinoma and osteosarcoma [34, 70]. However, to our knowledge, there is no published report demonstrating the therapeutic efficiency of selective COX-2 inhibitors with conventional anti-cancer agents in canine mammary tumor. Based on the encouraging results from COX-2 inhibitors as chemotherapeutic and chemopreventive agents in the treatment of several types of human and canine malignancy including mammary tumor [2, 14], we evaluated the potential of a selective COX-2 inhibitor (DER) in potentiating the antitumor activity of a conventional cytotoxic agent (DOX) in vitro in canine mammary carcinoma cells (CMT-U27). For this purpose, we preferred DER, a highly selective canine COX-2 inhibitor accepted as safe and well-tolerated in dogs [52], and DOX, a cytotoxic anthracycline antibiotic commonly used in veterinary clinical treatments for various cancers [62].
DER is widely used in veterinary medicine for the control of pain and inflammation associated with osteoarthritis and orthopedic surgery in dogs [8]. Recently, it has been reported that this drug might be a useful alternative for the prevention and/or treatment of some cancer types in dogs [34, 54]. Similarly, in our previous investigation, we proved that DER had a clear antiproliferative and apoptotic effect on canine mammary carcinoma cells in vitro [2]. These effects have only been observed at high concentrations (250–1,000 µM) of DER as well as seen in other NSAIDs (e.g., indomethacin, meloxicam), which are 10- to 1,000-fold those required to inhibit PG synthesis or COX enzymes [38, 51, 63]. The clinical importance of the direct cytotoxicity observed in vitro is presently unknown, as it is not known what plasma concentrations of DER would be necessary to achieve effective concentrations (≤250 µM) intratumorally or what duration of drug exposure is necessary to induce the observed cytotoxicity. However, it is known that plasma concentrations of DER can reach as much as 20 µM when DER is administrated at a dose of 4 mg/kg daily (i.e. the dosage used for postoperative orthopedic pain) [54]. It is also accepted that use of the drug at higher than approved dosages, especially long-term use, can lead to an increased risk of toxicity [48]. On the other hand, the concentration in the tumor may differ from that in plasma. It has been reported that the concentrations of NSAIDs, extensively bound to proteins, in the acidic environment of inflammation (such as in a tumor) relating to high protein content might be higher than in plasma [28, 70]. Similarly, tumor cells are able to produce angiogenic proteins like the vascular endothelial growth factor (VEGF), which could lead to a preferential accumulation of NSAIDs, so it is possible that the concentrations in the tumor cells are higher than in plasma [70]. Immunohistochemical studies have demonstrated that VEGF is highly expressed in CMT cells [27]. It has been reported that VEGF and a beta-galactoside-binding protein (galectin-3, an important mediator of VEGF) are highly expressed in CMT-U27 cells [7]. Based on this information, we used the lowest possible concentrations (50 µM, 100 µM and 250 µM) of DER, which are likely the most practical for clinical use, in our combination studies.
DOX is a cytotoxic anthracycline antibiotic that is commonly used in both veterinary and human cancer chemotherapy protocols [40, 62]. This medication has antitumor activity against a wide variety of carcinomas including mammary tumors, however, its utility is limited due to acute and chronic toxicities, such as myelosuppression, immunosuppression and dose-cumulative cardiotoxicity [11, 66]. Therefore, it is important to use this drug in in vitro therapeutic tests with the purpose of searching for a new methodology for combination protocols with nontoxic drugs, as it could enable the dose of DOX to be lowered due to its good antitumor activity, which is mainly observed in the metastatic mammary tumor. Our previous study indicated that a broad concentration range (0.1–100 µM) of DOX inhibited canine mammary carcinoma cell proliferation in vitro [3]. The IC50 of 876 nM determined for DOX in CMT-U27 cells was within the range of clinically relevant concentrations, as dogs that were treated with 1 mg/kg DOX achieved plasma concentrations of 0.7 µg/ml (1.2 µM) 5 min after intravenous administration [70]. The concentrations of DOX for a desirable pharmacological effect in canine mammary tumor cell lines in vitro were reported to be 280 nM and 840 nM [58, 70]. The IC50 value for CMT-U27 cells is higher than reported for the same compound in different CMT cells. We suggest that DOX is less potent in CMT-U27 cells than other mammary tumor cell types. CMT-U27 cells have a high growth rate and anti-apoptotic potential associated with enhanced expression of genes involved in the Ca2+ signaling pathway and growth hormone cellular pathway [68]. The high anti-apoptotic potential of these cells has been shown to be related to elevated expression ABR, which interacts with the tumor protein P53 and TMD1 genes, which are involved in drug resistance in tumor cells [30]. Prior studies have also demonstrated that CMT-U27 cells express high levels of anti-apoptotic Bcl-2 protein, which is linked to resistance to several therapeutic modalities [30, 68]. These data confirm our finding that highly metastatic CMT-U27 cells have low sensitivity to DOX. Therefore, we concluded that CMT-U27 cells could be an in vitro model for canine mammary cancer refractory to DOX chemotherapy.
In this study, we tested whether a COX-2 inhibitor drug could restore the response of a chemotherapeutic agent in canine mammary cancer. Our results demonstrated that DER enhanced the cytotoxic action of DOX at 0.9 µM in CMT-U27 cells in a dose dependent manner, which decreased the IC50 value from 0.876 µM (DOX at 0.9 µM) to 0.303 µM, 0.245 µM and 0.236 µM (0.9 µM in combination with 50, 100 and 250 µM DER), respectively. These results suggested that DER sensitized CMT-U27 cells to the action of DOX. The addition of DER to 0.9 µM DOX, even at concentrations that did not affect cell viability, achieved at least the same level of cytotoxicity as an approximately 3- to 3.5-fold higher dose of DOX alone in the cell line. In contrast, DER exerted no effect on the action of DOX at 0.09 µM in the cell line. These results suggest that the enhancement of cytotoxicity in combination treatment of DOX with DER depends on the DOX chemotherapy dose and that the addition of DER (50–250 µM) to DOX (0.9 µM) therapy may be beneficial to reduce the chemotherapy dose necessary to achieve a cytotoxic response in canine mammary cancer. To determine the nature of the interaction between DOX and DER, we used the method of Kern et al. as modified by Romanelli et al. [24, 53]. This method is accepted as the only correct method to evaluate the type of interaction between two drugs when one or both has a low cytotoxic effect or no dose-response curve [31]. Interaction analysis of the data showed that the combinations of DOX at 0.9 µM with DER produced synergism in the CMT-U27 cell line, with a ratio index ranging from 1.98 to 2.33. DOX at a low concentration (0.09 µM) with DER exhibited interaction in an additive manner. Our results are consistent with the work of Van Wijngaarden et al. [69], who demonstrated that celecoxib enhanced DOX-induced cytotoxicity in MDA-MB231 breast cancer cells. These effects are likely to be mediated via a COX-independent mechanism. The induction of COX-2 and its associated production of PGE2 from arachidonic acid are thought to play a role in the initiation and maintenance of cancer cell survival and growth [17]. The downstream product of the COX-2 catalyzed metabolism of arachidonic acid, PGE2, has been shown to promote the growth of breast carcinoma cells and reverse the growth inhibitory effect of the selective COX-2 inhibitor celecoxib in breast cancer MCF-7 cells [12]. In our study, we found that exogenous addition of PGE2 (1–5 µg/ml) did not reverse the antiproliferative effect of DER in combination with DOX. These results suggest that growth inhibition in CMT-U27 cells by DOX and its combination with DER are not directly associated with their ability to suppress PGE2 production. Our results are supported by the data of Duffy et al. (1998), who showed that exogenous PGE2 did not reverse the synergistic cytotoxicity of DOX with indomethacin against a DLKP cell line [15]. Similarly, PGE2 and the prostaglandin precursor arachidonic acid were shown to not reverse the growth inhibitory effects of the COX-2-selective inhibitor SC236 with DOX on HKESC-1 and HKESC-2 cells [71]. These observations suggest that COX-independent mechanisms are responsible for the in vitro growth inhibitory effects of these compounds. The synergistic and additive effects on CMT-U27 cells elicited by treatment with DOX (0.9 and 0.09 µM, respectively) and DER (especially at the concentrations of 100 and 250 µM) are associated with a marked increase in apoptosis. Our results demonstrated that DER (100 and 250 µM) enhanced the apoptotic activity of DOX at 0.9 and 0.09 µM in the cells, which increased the apoptotic index from 36.22% to 48.91% and 49.58% and from 24.02% to 35% and 37.69%, respectively. Also, our results show that DOX alone caused late apoptosis rather than early apoptosis. However, the early apoptotic cell number was significantly increased by the addition of DER (250 µM). We suggest that the increase in the percentage of early apoptotic cells could be related to DER. DER may cause cell membrane loss and therefore allow DOX to more easily penetrate into cells, and it may sensitize mammary cancer cells to DOX by activating the apoptotic program. Also, the increases in apoptotic activity according to DOX concentration support this suggestion. In support of these possibilities, some reports have [44, 65] suggested that NSAIDs, as disordering agents on membranes, might interfere with the lateral heterogeneity of membranes, disrupting the organization and function of microdomains, which are involved in the regulation of protein location and signaling pathways. Alteration of membrane properties, such as perturbations of membrane fluidity by anti-inflammatory agents, has been recently described and has been indicated as an additional mechanism by which anti-inflammatory agents have pharmacological effects relating to anticancer activity in some publications [32, 65]. In this regard, previous studies have demonstrated that COX-2 inhibitors (e.g., celecoxib, indomethacin, nimesulide) augmented chemotherapeutic drug-induced apoptosis by downregulation of anti-apoptotic mediators, such as Bcl-2, in breast, prostate and osteosarcoma cells [13, 38, 69]. To define the mechanistic role of DER in apoptosis induction, we examined Bcl-2 expression, which is known to promote cell survival and has been correlated with the development of DOX resistance [1], in CMT-U27 cells. We showed that DER (100 and 250 µM) induced downregulation of Bcl-2 at 0.9 µM DOX more strongly than at 0.09 µM DOX in CMT-U27 cells. We concluded that DER may be altered the sensitivity of CMT-U27 cells to DOX-induced apoptosis by downregulating Bcl-2 expression. Other possible functions of DER relating to anti-apoptotic genes need to be further investigated.
In additional studies identifying the mechanism of observed synergistic and additive effects in treatment with DOX and DER, we found that DER potentiated DOX-caused G0/G1 arrest in cell cycle progression, while DOX-induced cell cycle arrest in the G0/G1 phase, as well as induction of cell death, has been demonstrated in breast cancer MCF-7 cells [55]. We showed that a low concentration of DOX (0.09 µM) induced higher G0/G1 arrest compared with a high concentration of DOX (0.9 µM). Similar effects were observed in the combination study. The current results indicated that cell cycle regulation by DOX occurs differentially according to the concentrations applied. This effect could result from the increased penetration of DOX into CMT-U27 cells caused by DER. On the other hand, the percentages of CMT-U27 cells arrested at the G0/G1 phase are inversely proportional to the DOX concentrations, and this could arise from the density of the viable cells observed with both DOX treatments, because high concentrations of DOX lead to necrosis. This is the first study to demonstrate that DOX and DOX with DER induced cell cycle arrest in CMT-U27 cells, and the molecular mechanism of accumulation of cells in the G0/G1 phase induced by DER in the presence of DOX is unknown. We suggest that the increase in the percentage of CMT-U27 cells in the G0/G1 phase may be related to the accompanying changes in the level of proteins regulating the cell cycle and the decline of cell proliferation activity.
In conclusion, we elucidated that DER enhanced the antiproliferative effect of DOX in conjunction with induction of apoptosis by modulation of Bcl-2 expression and changes in the cell cycle of the CMT-U27 cell line. Although the exact molecular mechanism of the alterations in the cell cycle and apoptosis observed for DER and DOX combinations require further investigations, the results suggest that the synergistic effect of DOX and DER combinations in CMT therapy may be achieved at relatively lower doses of DOX with lesser side effects.
Acknowledgments
This work was supported by grant no. 108O296 from the Scientific and Technological Research Council of Turkey (TUBITAK) and by a grant from the Scientific Research Projects Coordination Unit of Istanbul University (Project no. UDP-6853). The authors would like to thank Professor Eva Hellmén (Division of Anatomy and Physiology, Swedish University of Agricultural Sciences, Sweden) for her kind donation of the CMT-U27 cell line.
REFERENCES
- 1.AbuHammad S., Zihlif M.2013. Gene expression alterations in doxorubicin resistant MCF7 breast cancer cell line. Genomics 101: 213–220. doi: 10.1016/j.ygeno.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 2.Ustün Alkan F., Ustüner O., Bakırel T., Cınar S., Erten G., Deniz G.2012. The effects of piroxicam and deracoxib on canine mammary tumour cell line. Scientific World Journal 2012: 976740. doi: 10.1100/2012/976740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ustün Alkan F., Bakirel T., Ustüner O., Yardibi H.2014. In vitro effects of doxorubicin and deracoxib on oxidative-stress-related parameters in canine mammary carcinoma cells. Acta Vet. Hung. 62: 372–385. doi: 10.1556/AVet.2014.012 [DOI] [PubMed] [Google Scholar]
- 4.Alshafie G. A., Abou-Issa H. M., Seibert K., Harris R. E.2000. Chemotherapeutic evaluation of Celecoxib, a cyclooxygenase-2 inhibitor, in a rat mammary tumor model. Oncol. Rep. 7: 1377–1381. [DOI] [PubMed] [Google Scholar]
- 5.Arun B., Goss P.2004. The role of COX-2 inhibition in breast cancer treatment and prevention. Semin. Oncol. 31Suppl 7: 22–29. doi: 10.1053/j.seminoncol.2004.03.042 [DOI] [PubMed] [Google Scholar]
- 6.Awara W. M., El-Sisi A. E., El-Sayad M. E., Goda A. E.2004. The potential role of cyclooxygenase-2 inhibitors in the treatment of experimentally-induced mammary tumour: does celecoxib enhance the anti-tumour activity of doxorubicin? Pharmacol. Res. 50: 487–498. doi: 10.1016/j.phrs.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 7.Benazzi C., Al-Dissi A., Chau C. H., Figg W. D., Sarli G., de Oliveira J. T., Gärtner F.2014. Angiogenesis in spontaneous tumors and implications for comparative tumor biology. Scientific World Journal 2014: 919570. doi: 10.1155/2014/919570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bienhoff S. E., Smith E. S., Roycroft L. M., Roberts E. S., Baker L. D.2011. Efficacy and safety of deracoxib for the control of postoperative pain and inflammation associated with dental surgery in dogs. ISRN Vet. Sci. 2011: 593015. doi: 10.5402/2011/593015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C., Shen H. L., Yang J., Chen Q. Y., Xu W. L.2011. Preventing chemoresistance of human breast cancer cell line, MCF-7 with celecoxib. J. Cancer Res. Clin. Oncol. 137: 9–17. doi: 10.1007/s00432-010-0854-3 [DOI] [PubMed] [Google Scholar]
- 10.Chou T. C.2010. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 70: 440–446. doi: 10.1158/0008-5472.CAN-09-1947 [DOI] [PubMed] [Google Scholar]
- 11.Coppoc G. L.2009. Chemotherapy of neoplastic diseases. pp. 1205–1231. In: Veterinary Pharmacology and Therapeutics, 9th ed. (Riviere, J. E. and Papich, M. G. eds.), Wiley-Blackwell Ames. [Google Scholar]
- 12.Dai Z. J., Ma X. B., Kang H. F., Gao J., Min W. L., Guan H. T., Diao Y., Lu W. F., Wang X. J.2012. Antitumor activity of the selective cyclooxygenase-2 inhibitor, celecoxib, on breast cancer in vitro and in vivo. Cancer Cell Int. 12: 53–60. doi: 10.1186/1475-2867-12-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dandekar D. S., Lopez M., Carey R. I., Lokeshwar B. L.2005. Cyclooxygenase-2 inhibitor celecoxib augments chemotherapeutic drug-induced apoptosis by enhancing activation of caspase-3 and -9 in prostate cancer cells. Int. J. Cancer 115: 484–492. doi: 10.1002/ijc.20878 [DOI] [PubMed] [Google Scholar]
- 14.Dang C. T., Shapiro C. L., Hudis C. A.2002. Potential role of selective COX-2 inhibitors in cancer management. Oncology (Huntingt.) 16Suppl 4: 30–36. [PubMed] [Google Scholar]
- 15.Duffy C. P., Elliott C. J., O’Connor R. A., Heenan M. M., Coyle S., Cleary I. M., Kavanagh K., Verhaegen S., O’Loughlin C. M., NicAmhlaoibh R., Clynes M.1998. Enhancement of chemotherapeutic drug toxicity to human tumour cells in vitro by a subset of non-steroidal anti-inflammatory drugs (NSAIDs). Eur. J. Cancer 34: 1250–1259. doi: 10.1016/S0959-8049(98)00045-8 [DOI] [PubMed] [Google Scholar]
- 16.de Groot D. J., de Vries E. G., Groen H. J. M., de Jong S.2007. Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: from lab to clinic. Crit. Rev. Oncol. Hematol. 61: 52–69. doi: 10.1016/j.critrevonc.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 17.Haynes A., Shaik M. S., Chatterjee A., Singh M.2003. Evaluation of an aerosolized selective COX-2 inhibitor as a potentiator of doxorubicin in a non-small-cell lung cancer cell line. Pharm. Res. 20: 1485–1495. doi: 10.1023/A:1025774630993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellmén E.1993. Canine mammary tumour cell lines established in vitro. J. Reprod. Fertil. Suppl. 47: 489–499. [PubMed] [Google Scholar]
- 19.Hermo G. A., Torres P., Ripoll G. V., Scursoni A. M. K., Gomez D. E., Alonso D. F., Gobello C.2008. Perioperative desmopressin prolongs survival in surgically treated bitches with mammary gland tumours: a pilot study. Vet. J. 178: 103–108. doi: 10.1016/j.tvjl.2007.06.015 [DOI] [PubMed] [Google Scholar]
- 20.Howe L. R., Dannenberg A. J.2003. COX-2 inhibitors for the prevention of breast cancer. J. Mammary Gland Biol. Neoplasia 8: 31–43. doi: 10.1023/A:1025731204719 [DOI] [PubMed] [Google Scholar]
- 21.Huang G. S., Lopez-Barcons L., Freeze B. S., Smith A. B., 3rd, Goldberg G. L., Horwitz S. B., McDaid H. M.2006. Potentiation of taxol efficacy and by discodermolide in ovarian carcinoma xenograft-bearing mice. Clin. Cancer Res. 12: 298–304. doi: 10.1158/1078-0432.CCR-05-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irie T., Tsujii M., Tsuji S., Yoshio T., Ishii S., Shinzaki S., Egawa S., Kakiuchi Y., Nishida T., Yasumaru M., Iijima H., Murata H., Takehara T., Kawano S., Hayashi N.2007. Synergistic antitumor effects of celecoxib with 5-fluorouracil depend on IFN-γ. Int. J. Cancer 121: 878–883. doi: 10.1002/ijc.22720 [DOI] [PubMed] [Google Scholar]
- 23.Itoh T., Uchida K., Ishikawa K., Kushima K., Kushima E., Tamada H., Moritake T., Nakao H., Shii H.2005. Clinicopathological survey of 101 canine mammary gland tumors: differences between small-breed dogs and others. J. Vet. Med. Sci. 67: 345–347. doi: 10.1292/jvms.67.345 [DOI] [PubMed] [Google Scholar]
- 24.Kern D. H., Morgan C. R., Hildebrand-Zanki S. U.1988. In vitro pharmacodynamics of 1-β-D-arabinofuranosylcytosine: synergy of antitumor activity with cis-diamminedichloroplatinum(II). Cancer Res. 48: 117–121. [PubMed] [Google Scholar]
- 25.Kim H. J., Yim G. W., Nam E. J., Kim Y. T.2014. Synergistic effect of COX-2 inhibitor on paclitaxel-induced apoptosis in the human ovarian cancer cell line OVCAR-3. Cancer Res. Treat. 46: 81–92. doi: 10.4143/crt.2014.46.1.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klopfleisch R., Gruber A. D.2009. Differential expression of cell cycle regulators p21, p27 and p53 in metastasizing canine mammary adenocarcinomas versus normal mammary glands. Res. Vet. Sci. 87: 91–96. doi: 10.1016/j.rvsc.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 27.Klopfleisch R., von Euler H., Sarli G., Pinho S. S., Gärtner F., Gruber A. D.2011. Molecular carcinogenesis of canine mammary tumors: news from an old disease. Vet. Pathol. 48: 98–116. doi: 10.1177/0300985810390826 [DOI] [PubMed] [Google Scholar]
- 28.Knottenbelt C., Chambers G., Gault E., Argyle D. J.2006. The in vitro effects of piroxicam and meloxicam on canine cell lines. J. Small Anim. Pract. 47: 14–20. doi: 10.1111/j.1748-5827.2006.00006.x [DOI] [PubMed] [Google Scholar]
- 29.Król M., Pawlowski K. M., Skierski J., Rao N. A. S., Hellmen E., Mol J. A., Motyl T.2009. Transcriptomic profile of two canine mammary cancer cell lines with different proliferative and anti-apoptotic potential. J. Physiol. Pharmacol. 60Suppl 1: 95–106. [PubMed] [Google Scholar]
- 30.Król M., Polańska J., Pawłowski K. M., Turowski P., Skierski J., Majewska A., Ugorski M., Morty R. E., Motyl T.2010. Transcriptomic signature of cell lines isolated from canine mammary adenocarcinoma metastases to lungs. J. Appl. Genet. 51: 37–50. doi: 10.1007/BF03195709 [DOI] [PubMed] [Google Scholar]
- 31.Leonetti C., Scarsella M., Zupi G., Zoli W., Amadori D., Medri L., Fabbri F., Rosetti M., Ulivi P., Cecconetto L., Bolla M., Tesei A.2006. Efficacy of a nitric oxide-releasing nonsteroidal anti-inflammatory drug and cytotoxic drugs in human colon cancer cell lines in vitro and xenografts. Mol. Cancer Ther. 5: 919–926. doi: 10.1158/1535-7163.MCT-05-0536 [DOI] [PubMed] [Google Scholar]
- 32.Lichtenberger L. M., Zhou Y., Jayaraman V., Doyen J. R., Doyen J. R., O’Neil R. G., Dial E. J., Volk D. E., Gorenstein D. G., Boggara M. B., Krishnamoorti R.1821. 2012. Insight into NSAID-induced membrane alterations, pathogenesis and therapeutics: Characterization of interaction of NSAIDs with phosphatidylcholine. BBA-. Mol. Cell. Biol. L: 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKnight J. A.2003. Principles of chemotherapy. Clin. Tech. Small Anim. Pract. 18: 67–72. doi: 10.1053/svms.2003.36617 [DOI] [PubMed] [Google Scholar]
- 34.McMillan S. K., Boria P., Moore G. E., Widmer W. R., Bonney P. L., Knapp D. W.2011. Antitumor effects of deracoxib treatment in 26 dogs with transitional cell carcinoma of the urinary bladder. J. Am. Vet. Med. Assoc. 239: 1084–1089. doi: 10.2460/javma.239.8.1084 [DOI] [PubMed] [Google Scholar]
- 35.Morris J., Dobson J.2001. Small Animal Oncology, 1st ed., Blackwell Science, Oxford. [Google Scholar]
- 36.Mross K., Steinbild S.2012. Metronomic anti-cancer therapy −An ongoing treatment option for advanced cancer patients. J. Cancer Ther. Res. 1: 1–32. doi: 10.7243/2049-7962-1-32 [DOI] [Google Scholar]
- 37.Narang A. J., Desai D. S.2009. Pharmaceutical perspectives of cancer therapeutics. pp. 49–92. In: Anticancer Drug Development Unique Aspects of Pharmaceutical Development, (Lu, Y. and Mahato R. I. eds.), Springer, New York. [Google Scholar]
- 38.Naruse T., Nishida Y., Ishiguro N.2007. Synergistic effects of meloxicam and conventional cytotoxic drugs in human MG-63 osteosarcoma cells. Biomed. Pharmacother. 61: 338–346. doi: 10.1016/j.biopha.2007.02.011 [DOI] [PubMed] [Google Scholar]
- 39.Pagnini U., Florio S., Lombardi P., d’Angelo D., Avallone L., Galdiero M., Iovane G., Tortora G., Pagnini G.2000. Modulation of anthracycline activity in canine mammary tumour cells in vitro by medroxyprogesterone acetate. Res. Vet. Sci. 69: 255–262. doi: 10.1053/rvsc.2000.0421 [DOI] [PubMed] [Google Scholar]
- 40.Pang L. Y., Cervantes-Arias A., Else R. W., Argyle D. J.2011. Canine Mammary Cancer Stem Cells are Radio- and Chemo- Resistant and Exhibit an Epithelial-Mesenchymal Transition Phenotype. Cancers (Basel) 3: 1744–1762. doi: 10.3390/cancers3021744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paoloni M., Khanna C.2008. Translation of new cancer treatments from pet dogs to humans. Nat. Rev. Cancer 8: 147–156. doi: 10.1038/nrc2273 [DOI] [PubMed] [Google Scholar]
- 42.Patel M. I., Subbaramaiah K., Du B., Chang M., Yang P., Newman R. A., Cordon-Cardo C., Thaler H. T., Dannenberg A. J.2005. Celecoxib inhibits prostate cancer growth: evidence of a cyclooxygenase-2-independent mechanism. Clin. Cancer Res. 11: 1999–2007. doi: 10.1158/1078-0432.CCR-04-1877 [DOI] [PubMed] [Google Scholar]
- 43.Pawłowski K. M., Mucha J., Majchrzak K., Motyl T., Król M.2013. Expression and role of PGP, BCRP, MRP1 and MRP3 in multidrug resistance of canine mammary cancer cells. BMC Vet. Res. 9: 119–128. doi: 10.1186/1746-6148-9-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira-Leite C., Nunes C., Reis S.2013. Interaction of nonsteroidal anti-inflammatory drugs with membranes: in vitro assessment and relevance for their biological actions. Prog. Lipid Res. 52: 571–584. doi: 10.1016/j.plipres.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 45.Pinto A. C., Moreira J. N., Simões S.2011. Combination chemotherapy in cancer: Principles, evaluation and drug delivery strategies. pp. 693–714. In: Current Cancer Treatment−Novel Beyond Conventional Approaches, (Ozdemir, O. ed.), InTech, Rijeka. [Google Scholar]
- 46.Polton G.2009. Mammary tumours in dog. Ir. Vet. J. 62: 50–56. [Google Scholar]
- 47.Rahman M., Selvarajan K., Hasan M. R., Chan A. P., Jin C., Kim J., Chan S. K., Le N. D., Kim Y. B., Tai I. T.2012. Inhibition of COX-2 in colon cancer modulates tumor growth and MDR-1 expression to enhance tumor regression in therapy-refractory cancers in vivo. Neoplasia 14: 624–633. doi: 10.1593/neo.12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radi Z. A., Khan N. K.2006. Effects of cyclooxygenase inhibition on the gastrointestinal tract. Exp. Toxicol. Pathol. 58: 163–173. doi: 10.1016/j.etp.2006.06.004 [DOI] [PubMed] [Google Scholar]
- 49.Rao P., Knaus E. E.2008. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharm. Sci. 11: 81s–110s. [DOI] [PubMed] [Google Scholar]
- 50.Rayburn E. R., Ezell S. J., Zhang R.2009. Anti-Inflammatory agents for cancer therapy. Mol. Cell Pharmacol. 1: 29–43. doi: 10.4255/mcpharmacol.09.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raz A.2002. Is inhibition of cyclooxygenase required for the anti-tumorigenic effects of nonsteroidal, anti-inflammatory drugs (NSAIDs)? In vitro versus in vivo results and the relevance for the prevention and treatment of cancer. Biochem. Pharmacol. 63: 343–347. doi: 10.1016/S0006-2952(01)00857-7 [DOI] [PubMed] [Google Scholar]
- 52.Roberts E. S., Van Lare K. A., Marable B. R., Salminen W. F.2009. Safety and tolerability of 3-week and 6-month dosing of Deramaxx (deracoxib) chewable tablets in dogs. J. Vet. Pharmacol. Ther. 32: 329–337. doi: 10.1111/j.1365-2885.2008.01043.x [DOI] [PubMed] [Google Scholar]
- 53.Romanelli S., Perego P., Pratesi G., Carenini N., Tortoreto M., Zunino F.1998. In vitro and in vivo interaction between cisplatin and topotecan in ovarian carcinoma systems. Cancer Chemother. Pharmacol. 41: 385–390. doi: 10.1007/s002800050755 [DOI] [PubMed] [Google Scholar]
- 54.Royals S. R., Farese J. P., Milner R. J., Lee-Ambrose L., van Gilder J.2005. Investigation of the effects of deracoxib and piroxicam on the in vitro viability of osteosarcoma cells from dogs. Am. J. Vet. Res. 66: 1961–1967. doi: 10.2460/ajvr.2005.66.1961 [DOI] [PubMed] [Google Scholar]
- 55.Rusetskaya N. V., Lukyanova N. Y., Chekhun V. F.2009. Molecular profile and cell cycle in MCF-7 and MCF-7/Dox cells exposed to conventional and liposomal forms of doxorubicin. Exp. Oncol. 31: 140–143. [PubMed] [Google Scholar]
- 56.Rüegg C.2006. Non steroidal anti-inflammatory drugs and Cox-2 inhibitors as anti-angiogenic drugs. Haemotologica Reports 2: 45–47. [Google Scholar]
- 57.Sahin M., Sahin E., Gümüslü S.2009. Cyclooxygenase-2 in cancer and angiogenesis. Angiology 60: 242–253. [DOI] [PubMed] [Google Scholar]
- 58.Simon D., Knebel J. W., Baumgartner W., Aufderheide M., Meyer-Lindenberg A., Nolte I.2001. In vitro efficacy of chemotherapeutics as determined by 50% inhibitory concentrations in cell cultures of mammary gland tumors obtained from dogs. Am. J. Vet. Res. 62: 1825–1830. doi: 10.2460/ajvr.2001.62.1825 [DOI] [PubMed] [Google Scholar]
- 59.Simon D., Schoenrock D., Baumgärtner W., Nolte I.2006. Postoperative adjuvant treatment of invasive malignant mammary gland tumors in dogs with doxorubicin and docetaxel. J. Vet. Intern. Med. 20: 1184–1190. [DOI] [PubMed] [Google Scholar]
- 60.Sorenmo K.2003. Canine mammary gland tumors. Vet. Clin. North Am. Small Anim. Pract. 33: 573–596. doi: 10.1016/S0195-5616(03)00020-2 [DOI] [PubMed] [Google Scholar]
- 61.Soriano A. F., Helfrich B., Chan D. C., Heasley L. E., Bunn P. A., Jr, Chou T. C.1999. Synergistic effects of new chemopreventive agents and conventional cytotoxic agents against human lung cancer cell lines. Cancer Res. 59: 6178–6184. [PubMed] [Google Scholar]
- 62.Spee B., Jonkers M. D. B., Arends B., Rutteman G. R., Rothuizen J., Penning L. C.2006. Specific down-regulation of XIAP with RNA interference enhances the sensitivity of canine tumor cell-lines to TRAIL and doxorubicin. Mol. Cancer 5: 34–43. doi: 10.1186/1476-4598-5-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki Y., Inoue T., Ra C.2010. NSAIDs, mitochondria and calcium signaling: Special focus on aspirin/salicylates. Pharmaceuticals (Ott.) 3: 1594–1613. doi: 10.3390/ph3051594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tavares W. L. F., Lavalle G. E., Figueiredo M. S., Souza A. G., Bertagnolli A. C., Viana F. A. B., Paes P. R. O., Carneiro R. A., Cavalcanti G. A. O., Melo M. M., Cassali G. D.2010. Evaluation of adverse effects in tamoxifen exposed healthy female dogs. Acta Vet. Scand. 52: 67–72. doi: 10.1186/1751-0147-52-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tavolari S., Munarini A., Storci G., Laufer S., Chieco P., Guarnieri T.2012. The decrease of cell membrane fluidity by the non-steroidal anti-inflammatory drug Licofelone inhibits epidermal growth factor receptor signalling and triggers apoptosis in HCA-7 colon cancer cells. Cancer Lett. 321: 187–194. doi: 10.1016/j.canlet.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 66.Todorova I., Simeonova G., Simeonov R., Dinev D.2005. Doxorubicin and Cyclophosphamide chemotherapy in dogs with spontaneous mammary tumours. Trakia J. Sci. 3: 51–58. [Google Scholar]
- 67.Uyama R., Nakagawa T., Hong S. H., Mochizuki M., Nishimura R., Sasaki N.2006. Establishment of four pairs of canine mammary tumour cell lines derived from primary and metastatic origin and their E-cadherin expression. Vet. Comp. Oncol. 4: 104–113. doi: 10.1111/j.1476-5810.2006.00098.x [DOI] [PubMed] [Google Scholar]
- 68.van Engeland M., Nieland L. J. W., Ramaekers F. C. S., Schutte B., Reutelingsperger C. P. M.1998. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31: 1–9. doi: [DOI] [PubMed] [Google Scholar]
- 69.van Wijngaarden J., van Beek E., van Rossum G., van der Bent C., Hoekman K., van der Pluijm G., van der Pol M. A., Broxterman H. J., van Hinsbergh V. W., Löwik C. W.2007. Celecoxib enhances doxorubicin-induced cytotoxicity in MDA-MB231 cells by NF-kappaB-mediated increase of intracellular doxorubicin accumulation. Eur. J. Cancer 43: 433–442. doi: 10.1016/j.ejca.2006.09.010 [DOI] [PubMed] [Google Scholar]
- 70.Wolfesberger B., Hoelzl C., Walter I., Reider G. A., Fertl G., Thalhammer J. G., Skalicky M., Egerbacher M.2006. In vitro effects of meloxicam with or without doxorubicin on canine osteosarcoma cells. J. Vet. Pharmacol. Ther. 29: 15–23. doi: 10.1111/j.1365-2885.2006.00704.x [DOI] [PubMed] [Google Scholar]
- 71.Yu L., Wu W. K. K., Li Z. J., Liu Q. C., Li H. T., Wu Y. C., Cho C. H.2009. Enhancement of doxorubicin cytotoxicity on human esophageal squamous cell carcinoma cells by indomethacin and 4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC236) via inhibiting P-glycoprotein activity. Mol. Pharmacol. 75: 1364–1373. doi: 10.1124/mol.108.053546 [DOI] [PubMed] [Google Scholar]
- 72.Zarghi A., Arfaei S.2011. Selective COX-2 Inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. 10: 655–683. [PMC free article] [PubMed] [Google Scholar]
- 73.Zuccari D. A., Santana A. E., Cury P. M., Cordeiro J. A.2004. Immunocytochemical study of Ki-67 as a prognostic marker in canine mammary neoplasia. Vet. Clin. Pathol. 33: 23–28. doi: 10.1111/j.1939-165X.2004.tb00345.x [DOI] [PubMed] [Google Scholar]