Abstract
Japanese eel endothelial cells-infecting virus (JEECV) has spread in eel farms and caused serious economic loss. In this study, we examined the prevalence of JEECV infection in 100 wild Japanese eel (Anguilla japonica) elvers caught from Yamaguchi prefecture, Japan, using quantitative PCR and conventional PCR. Total genomic DNA was obtained from the cranial quarter of the body in 70 of 100 eels and from the gill in the remaining. Of 30 gill samples, 20 were analyzed after pooling with other samples, and the remaining 10 were analyzed separately. A single positive result for JEECV was detected following analysis of the 10 separately analyzed samples. This result constitutes the first report of JEECV infection in wild A. japonica elvers.
Keywords: Anguilla japonica, elver, Japanese eel endothelial cells-infecting virus
Temperate eel stocks, including European eel [Anguilla anguilla (Linnaeus)], American eel [A. rostrate (Lesueur)] and Japanese eel [A. japonica (Temminck & Schlegel)], have decreased drastically by 90–99% since the 1980s [16]. Consequently, the International Union for Conservation of Nature added these eels to its Red List. The exact cause of this phenomenon is still unknown, however, some anthropogenic reasons, including overfishing, habitat loss, contamination and diseases, have been proposed [1, 5].
Recently, many researchers have shown interest in the severely pathogenic agents, including parasites, viruses and bacteria, that have potentially adverse effects on fish migration [5]. Jørgensen et al. [7] and van Beurden et al. [18] reported that severely pathogenic viruses in the European eel also infect wild A. anguilla elvers [7, 8], although no studies have attempted to detect viruses in this species.
Viral endothelial cells necrosis (VECNE) is caused by Japanese eel endothelial cells-infecting virus (JEECV) [9]. VECNE has caused serious economic losses for eel farms since the 1980s [4, 8, 9, 12] in that VECNE has a mortality rate of ~60% in experimental infection [11] with dilatation of the central venous sinus in the gills and abnormalities in systemic vasculature [6]. Having an open reading frame (ORF) referred to as polyomavirus large T like protein (LTLG) homologous for the large T antigen gene is characteristic of the polyomavirus. Interestingly, JEECV can be classified as a member of the polyomavirus family, although it has at least putative 14 ORFs of uncertain function other than LTLG [9]. Previously, we showed that JEECV infection was present in yellow-staged A. japonica caught in their natural habitat [10]. Given these findings, it can be presumed that JEECV is capable of infecting in A. japonica at other developmental stages. Hence, the present study focused on detecting JEECV in A. japonica elvers.
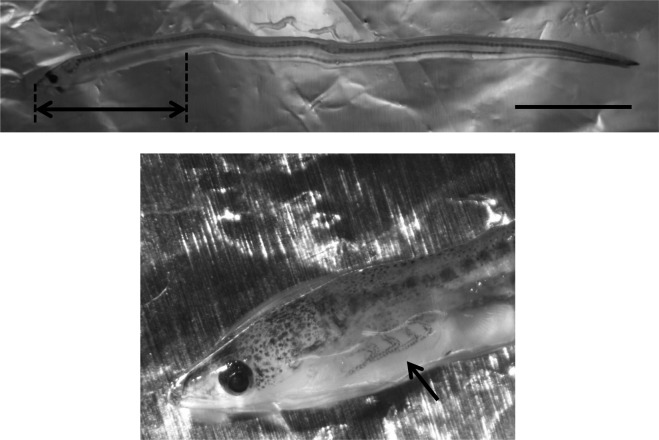
In March 2015, 100 A. japonica elvers (glass eel) were caught in the Asagawa River, Yamaguchi, Japan. They were anesthetized with Ethyl 3-aminnobenzoate methanesulfonate salt (MS-222) purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.) in concentration of 1,000 parts per million (ppm) and then stored individually at −80°C until use. The eels exhibited no apparent abnormalities. Total genomic DNA was obtained from the cranial quarter of the body in 70 of 100 eels and from only the gill in the remaining (Fig. 1). Of the 30 gill samples, 20 were analyzed after being pooled with other samples, and 10 of the remaining samples were analyzed separately. These samples were homogenized using a sterilized homogenizer (Nippi Inc., Tokyo, Japan), and total DNA was extracted using a High Pure Viral Nucleic Acid Kit (Roche Diagnostics Deutschland GmbH, Mannheim, Germany).
Fig. 1.
Sampling site in Anguilla japonica elver. Upper: arrow marks indicate the cranial quarter of the body. Lower: arrow marks indicate the gills (Scale bar=1 cm).
To detect JEECV, quantitative PCR (qPCR) and conventional PCR were conducted according to Mizutani el al. [9]. Briefly, the qPCR was conducted using TaqMan Gene Expression Master Mix with a 7300 Real-time PCR System (Applied Biosystems, Grand Island, NY, U.S.A.) with a minimum detection limit of 100 copies/reaction. Primers and probe were designed for amplification of the LTLG region. Conventional PCR was performed using AmpliTaq Gold PCR Master Mix (Applied Biosystems) using primer sets A, B and C, from the cited study [9]. Primer set A targets the LTLG region, and primer sets B and C target other putative ORFs as described in the previous report [9]. The amplified products were analyzed by electrophoresis on a 1.8% agarose gel stained with ethidium bromide.
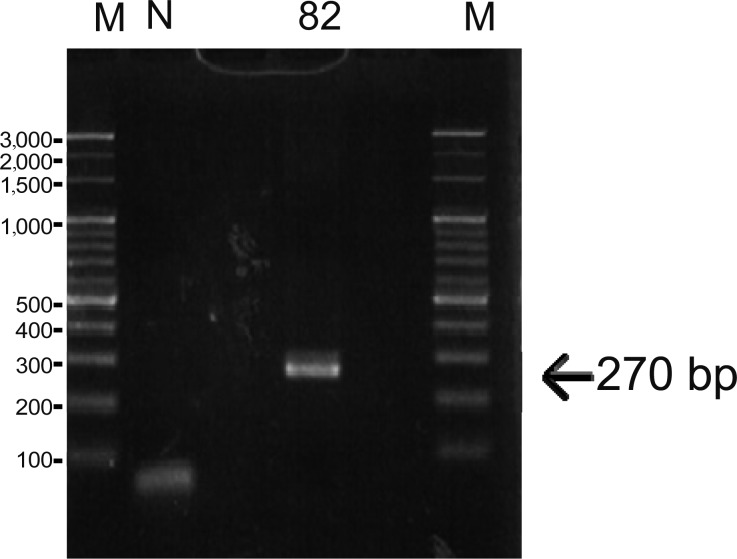
According to the results of qPCR and conventional PCR, JEECV was not detected in samples excited from the cranial quarter or pooled gill samples. However, one (#82) of the 10 gill samples that were analyzed individually returned a positive result using qPCR, and its viral load showed 189 copies per whole gill. Additionally, the amplification product (primer set A) in the same sample (#82) matches the JEECV gene sequence (Fig. 2). Further analysis using blastx algorithm of the NCBI database verified that the amino acid sequences predicted from the base sequences obtained from the amplification product were 100% identical with the LTLG (GenBank accession no.YP009103994) and under 61% identical with other large T antigens. The result of conventional PCR using primer sets B and C was negative in ten gill samples that were analyzed individually (data not shown).
Fig. 2.
Amplification of the JEECV genome from gills by PCR. Lane 82: Identification of sample. M: DNA marker (100 bp ladder). N: Negative control (nuclease-free water).
This study is the first report of JEECV infection of A. japonica elvers in its natural habitat. However, it should be noted that JEECV was detected in 1 of 10 A. japonica elvers whose gills were analyzed individually. This result could be attributed to the small amount of virus in A. japonica elvers. Consequently, the samples containing the pooled DNA from other organs or eels were negative for JEECV. The infection rate in A. japonica elvers corresponded approximately to that in yellow-staged A. japonica [10]. It has been proposed that some polyomaviruses, including BK virus, JC virus, simian virus 40 and murine polyoma virus, are transmitted vertically [2, 3, 13, 14, 17, 19, 20], as well as horizontally [15]. It is, therefore, possible that JEECV could be transmitted vertically from spawner to egg. Consequently, JEECV may keep the infection rate. Further studies regarding whether JEECV infects spawner and egg should be conducted.
Acknowledgments
We would like to express our gratitude to the laboratory members from the Research and Education Center for Prevention of Global Infectious Diseases of Animals and Animal Behavior for their help in sample collection.
REFERENCES
- 1.Amilhat E., Fazio G., Simon G., Manetti M., Paris S., Delahaut L., Farrugio H., Lecomte-Finiger R., Sasal P., Faliex E.2014. Silver European eels health in Mediterranean habitats. Ecol. Freshwat. Fish 23: 49–64. doi: 10.1111/eff.12077 [DOI] [Google Scholar]
- 2.Boldorini R., Allegrini S., Miglio U., Paganotti A., Cocca N., Zaffaroni M., Riboni F., Monga G., Viscidi R.2011. Serological evidence of vertical transmission of JC and BK polyomaviruses in humans. J. Gen. Virol. 92: 1044–1050. doi: 10.1099/vir.0.028571-0 [DOI] [PubMed] [Google Scholar]
- 3.Brandner G., Burger A., Neumann-Haefelin D., Reinke C., Helwig H.1977. Isolation of simian virus 40 from a newborn child. J. Clin. Microbiol. 5: 250–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egusa S., Tanaka M., Ogami H., Oka H.1989. Histopathological Observations on an Intense Congestion of the Gills in Cultured Japanese Eel, Anguilla japonica. Fish Pathol. 24: 51–56. doi: 10.3147/jsfp.24.51 [DOI] [Google Scholar]
- 5.ICES2006. Report of the 2006 session of the Joint EIFAC/ICES Working Group on Eels. FAO European Inland Fisheries Advisory Commission; International Council for the Exploration of the Sea, Rome, 23–27 January 2006. EIFAC Occasional Paper 38: 352.
- 6.Inouye K., Miwa S., Aoshima H., Oka H., Sorimachi M.1994. A Histopathological Study on the Etiology of Intense Congestion of the Gills of Japanese Eel, Anguilla japonica. Fish Pathol. 29: 35–41. doi: 10.3147/jsfp.29.35 [DOI] [Google Scholar]
- 7.Jørgensen P., Castric J., Hill B., Ljungberg O., De Kinkelin P.1994. The occurrence of virus infections in elvers and eels (Anguilla anguilla) in Europe with particular reference to VHSV and IHNV. Aquaculture 123: 11–19. doi: 10.1016/0044-8486(94)90115-5 [DOI] [Google Scholar]
- 8.Kusuda R., Isshiki T., Kawai K.1989. Characteristics of a Virus Isolated from Cultured Eel Showing Congestion Of Central Venous Sinus in Gill Filaments. Suisanzoshoku 37: 43–48. [Google Scholar]
- 9.Mizutani T., Sayama Y., Nakanishi A., Ochiai H., Sakai K., Wakabayashi K., Tanaka N., Miura E., Oba M., Kurane I., Saijo M., Morikawa S., Ono S.2011. Novel DNA virus isolated from samples showing endothelial cell necrosis in the Japanese eel, Anguilla japonica. Virology 412: 179–187. doi: 10.1016/j.virol.2010.12.057 [DOI] [PubMed] [Google Scholar]
- 10.Okazaki S., Manabe H., Omatsu T., Tsuchiaka S., Yamamoto T., Chow S., Shibuno T., Watanabe K., Ono S., Kuwada H., Mizutani T.2014. Detection of Japanese eel endothelial cells-infecting virus (JEECV) in the Japanese eel Anguilla japonica (Temminck & Schlegel), living in natural habitats. J. Fish Dis. 13: 12294. [DOI] [PubMed] [Google Scholar]
- 11.Ono S., Wakabayashi K., Nagai A.2003. Sequence of genome segment A of a birnavirus isolated from cultured Japanese eel with virual endothelial cell necrosis of eel. J. Sch. Mar. Sci. Tech. 1: 39–49. [Google Scholar]
- 12.Ono S., Wakabayashi K., Nagai A.2007. Isolation of the Virus Causing Viral Endothelial Cell Necrosis of Eel from Cultured Japanese Eel Anguilla japonica. Fish Pathol. 42: 191–200. doi: 10.3147/jsfp.42.191 [DOI] [Google Scholar]
- 13.Patel N. C., Halvorson S. J., Sroller V., Arrington A. S., Wong C., Smith E. O., Vilchez R. A., Butel J. S.2009. Viral regulatory region effects on vertical transmission of polyomavirus SV40 in hamsters. Virology 386: 94–101. doi: 10.1016/j.virol.2008.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietropaolo V., Di Taranto C., Degener A. M., Jin L., Sinibaldi L., Baiocchini A., Melis M., Orsi N.1998. Transplacental transmission of human polyomavirus BK. J. Med. Virol. 56: 372–376. doi: [DOI] [PubMed] [Google Scholar]
- 15.Potti J., Blanco G., Lemus A. J., Canal D.2007. Infectious offspring: how birds acquire and transmit an avian polyomavirus in the wild. PLoS ONE 2: p.e 1276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Stone R.2003. Ecology. Freshwater eels are slip-sliding away. Science 302: 221–222. doi: 10.1126/science.302.5643.221 [DOI] [PubMed] [Google Scholar]
- 17.Taguchi F., Nagaki D., Saito M., Haruyama C., Iwasaki K., Suzuki T.1975. Transplacental transmission of BK virus in human. Jpn. J. Microbiol. 19: 395–398. doi: 10.1111/j.1348-0421.1975.tb00897.x [DOI] [PubMed] [Google Scholar]
- 18.van Beurden S. J., Engelsma M. Y., Roozenburg I., Voorbergen-Laarman M. A., van Tulden P. W., Kerkhoff S., van Nieuwstadt A. P., Davidse A., Haenen O. L.2012. Viral diseases of wild and farmed European eel Anguilla anguilla with particular reference to the Netherlands. Dis. Aquat. Organ. 101: 69–86. doi: 10.3354/dao02501 [DOI] [PubMed] [Google Scholar]
- 19.Verhagen W., Hubert C. M., Mohtaschem E.1993. Tumour induction by transplacental infection with polyoma virus of the F1 generation of Wistar rats. Arch. Virol. 133: 459–465. doi: 10.1007/BF01313783 [DOI] [PubMed] [Google Scholar]
- 20.Zhang S., McNees A. L., Butel J. S.2005. Quantification of vertical transmission of Murine polyoma virus by real-time quantitative PCR. J. Gen. Virol. 86: 2721–2729. doi: 10.1099/vir.0.81168-0 [DOI] [PubMed] [Google Scholar]