Abstract
AIM: To investigate killer inhibitory and activating receptor expression by natural killer (NK), natural killer T-like (NKT-like) and CD8+ T lymphocytes in patients with chronic hepatitis C virus (HCV) infection with elevated and with persistently normal alanine aminotransferase (PNALT).
METHODS: The percentage of peripheral blood Treg cells, KIR2DL3, ILT-2, KIR3DL1, CD160, NKG2D, NKG2C expressing NK, T and NKT-like cells, cytokine production and NK cytotoxicity were determined by flow cytometry. Twenty-one patients with chronic HCV infection with elevated alanine aminotransferase, 11 HCV carriers with persistently normal alanine aminotransferase and 15 healthy volunteers were enrolled.
RESULTS: No significant differences were observed in the percentage of total T, NK or NKT-like cells between study groups. Comparing the activating and inhibitory receptor expression by NK cells obtained from HCV carriers with PNALT and chronic HCV hepatitis patients with elevated alanine aminotransferase, NKG2D activating receptor expression was the only receptor showing a significant difference. NKG2D expression of NK cells was significantly lower in patients with elevated alanine aminotransferase. The expression of CD160, NKG2D and NKG2C activating receptor by CD8+ T cells were significantly lower in patients with chronic HCV hepatitis than in healthy controls and in HCV carriers with PNALT. Plasma TGF-β1 levels inversely correlated with NKG2D expression by NK cells. In vitroTGF-β1 treatment inhibited NK cells cytotoxic activity and downregulated NKG2D expression. CD8+ T cells from HCV carriers with PNALT showed significantly elevated expression of CD160, NKG2D and NKG2C activating receptors compared to chronic HCV patients with elevated alanine aminotransferase. Enhanced expression of inhibitory KIR2DL3 receptor, and decreased ILT-2 expression on NK cells were also found in chronic hepatitis C patients compared to healthy controls.
CONCLUSION: Our study demonstrated a complex dysregulation of activating and inhibitory receptor expression, such as decreased NKG2D and CD160 activating receptor expression and increased KIR2DL3 inhibitory receptor expression by NK and cytotoxic T cells and may provide further mechanism contributing to defective cellular immune functions in chronic hepatitis C. Increased NKG2D receptor expression in HCV patients with persistently normal ALT suggests an important pathway for sustaining NK and CD8 T cell function and a protective role against disease progression.
Keywords: Hepatitis C, Natural killer cell, NKG2D, Cytotoxicity, Cytokine
Core tip: The host immune response to hepatitis C virus (HCV) involves both innate and adaptive arms of the immune system. Natural killer (NK) cells are key components of the innate antiviral immune response. To better characterize the immune defects underlying chronic viral persistence, we focus our analysis on killer inhibitory and activating receptor expression in patients with chronic HCV infection with elevated alanine aminotransferase (ALT) and also in patients with HCV carriers with persistently normal ALT. Decreased NKG2D and CD160 activating receptor expression and increased KIR2DL3 inhibitory receptor expression by NK and cytotoxic T cells in patients with chronic hepatitis C contributing to defective cellular immune functions.
INTRODUCTION
More than 170 million people worldwide are chronically infected by hepatitis C virus (HCV)[1]. Approximately 20% of HCV infected patients resolves acute hepatitis and clears the virus, but most develop life-long infection, making HCV a leading cause of chronic liver disease, cirrhosis and hepatocellular carcinoma[2]. No vaccine is currently available to prevent hepatitis C[3]. The mechanisms favoring persistent infection are still poorly understood.
Approximately 30% of patients with chronic HCV (CHC) infection show persistently normal alanine aminotransferase (ALT) levels and are considered CHC carriers[4].
The host immune response to HCV involves both innate and adaptive arms of the immune system. Natural killer (NK) cells are key components of the innate antiviral immune response. NK cells, a subset of lymphocytes, represent between 5% and 15% of mononuclear cells in the peripheral blood and up to 45% in some organs, such as the liver. The major functional role of NK cells is the defense against tumor cells and lyses of virus-infected cells[5]. However, given their potent cytotoxic and cytokine secretion potential, their activity needs to be tightly regulated. The activation state of an NK cell is determined partly by the integration of activating and inhibitory signals after interaction of surface NK cell receptors, including the highly diverse killer Ig-like receptor (KIR) family, with ligands found on target cells.
Previous results from our laboratory showed an impaired NK activity in chronic hepatitis[6]. Therefore, altered function of NK cells might be one of the mechanisms by which viruses escape the immune system. Activating receptors on NK and T cells might provide not only the machinery to induce proliferation and fight off infection, but also to support maintenance of the cells critically needed under conditions of extended viral infections during CHC.
There is new evidence that NK cells also can be activated to negatively regulate T cell responses because they can produce interleukin-10 (IL-10)[7,8]. Under conditions of continued stimulation - like CHC infection - IL-10 response by NK cells could limit the magnitude of CD8 T cell response and protect from T cell-mediated disease. If the NK cells are not regulated properly, the regulation is lost with detrimental consequences.
Natural killer T-like (NKT-like) cells are a sublineage of T cells that share characteristics of conventional T cells and NK cells and bridge innate and adaptive immunity[9]. The most characteristic immunoregulatory function of NKT cells is their ability to promptly secrete large amounts of Th1 and Th2 cytokines including interferon-γ (IFN-γ) and IL-4, respectively, upon stimulation[10]. Downstream, this culminates in the activation of different cell types of the innate immune system such as macrophages, NK cells, and dendritic cells as well as effector T cells of the adaptive immune system.
Although shown to mediate immunity against a wide range of pathogenic microbes, including bacteria, fungi, parasites, and viruses, the mechanism(s) by which NKT cells are activated during infection is still unclear. NKT-like cells are abundant in the liver; however, their role in the control of hepatitis C virus infection remains to be determined[11].
CD8+ T cells are important in viral elimination by using direct killing of infected cells and non-cytotoxic mechanisms such as the secretion of antiviral cytokines [IFN-γ or tumor necrosis factor (ΤΝF)-α][12]. Despite the detection of HCV-specific CD8+ T cells in the peripheral blood and the intrahepatic lymphocytic infiltrate in patients with chronic hepatitis C, the virus can persist. This persistence in spite of the presence of these cytotoxic cells is still unexplained and suggests that cell killing is not sufficient to eliminate the virus. Studies in humans have revealed that even strong CD8+ T cell responses in the acute phase of infection may not be adequate to prevent progression to chronicity[13-15]. Several investigators have clearly shown that HCV-specific CD8+ T cells have functional defects during chronic infection, as indicated by impaired IFN-γ production, cytotoxic effector functions, and in vitro proliferation[16,17]. While the mechanisms responsible for the dysfunctions of HCV-specific T cells in chronically infected patients remain unclear, recent studies suggest a major contribution of regulatory T cells.
To better characterize the immune defects underlying chronic viral persistence, in this study we focus our analysis on killer inhibitory and activating receptor expression in patients with chronic hepatitis C virus infection with elevated ALT and also in patients with CHC carriers with persistently normal ALT (PNALT) by NK, NKT-like and CD8+ T lymphocytes, given the central role played by these cells in the control of viral infections. Progress in the understanding of antiviral immune responses in CHC carriers with PNALT could elucidate key mechanisms playing a role in the control of viral infection.
MATERIALS AND METHODS
Patients
Persistently normal ALT was defined as ALT < 30 IU/L in men, ALT < 19 IU/L in women measured every 3 mo over an 18-mo period. Patients with Fibroscan result suggesting > F1 liver fibrosis (LS > 7.0 kPa) were excluded from the CHC with PNALT group. Eleven age-matched healthy blood donors served as controls.
All HCV subjects were seronegative for anti-HIV 1, 2 antibodies (ELISA 2.0, Abbott, Wiesbaden, Germany), and HBsAg (Hepanostica Uniform II, Organon Teknika, Oss, The Netherlands), and were positive for both anti-HCV antibody and HCV-RNA. Diagnosis of chronic hepatitis C was established by means of histology in all symptomatic patients, but liver biopsy was not performed in CHC carriers with PNALT.
HCV markers
Anti-HCV antibody was examined using enzyme-linked immunoabsorbent assay (ELISA) (Detect-HCV Ab, Biochem Immunosystem, ITC, Canada). Serum HCV RNA detection and quantification were performed with Roche Cobas Amplicor HCV 2.0 assay (lower limit of detection < 50 IU/mL) and Cobas Amplicor HCV Monitor Assay (Roche Diagnostics) according to the manufacturer’s instructions.
Sample preparation
Venous blood samples were collected in heparanized tubes and peripheral blood mononuclear cells (PBMC) were prepared by Ficoll-Paque density gradient centrifugation.
Antibodies and flow cytometry
Separated cells were washed in PBS and incubated for 30 min at room temperature with the monoclonal antibodies. The following monoclonal antibodies were used for these studies: FITC-conjugated anti-CD3, anti-CD8, anti-CD4, PE-conjugated anti-CD25, anti-KIR2DL3 (CD158b), anti-ILT-2 (CD85), anti-NKG2C, anti-CD160, anti-NKG2D, anti-KIR3DL1 (CD158e) and APC-conjugated anti-CD56. After washing the cells in PBS, cells were fixed with 4% paraformaldehyde, stored at 4 °C, in dark, to be processed for FACS analysis. At least 10000 cells were analyzed on the FACS Calibur flow-cytometer (Becton Dickinson Immunocytometry Systems, Erembodegen, Belgium) after single gating on lymphoid cells for all mAb combinations. The percentage of positive cells was calculated using Cellquest software (Becton Dickinson, San Diego, CA, United States). Figure 1 shows the gating technique used to detect different lymphocyte subpopulations with representative flow cytometric dot plots. The effect of TGF-β1 treatment on NKG2D, CD160 and KIR2DL3 expression by NK cells.
Figure 1.
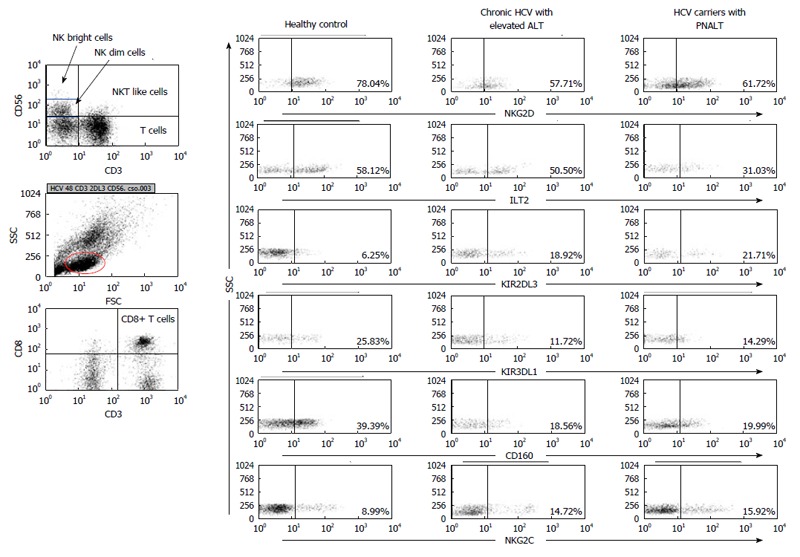
Gating strategy and representative flow cytometric dot plots. Figure 1 shows the gating technique used to detect different lymphocyte subpopulations. For analysis of NK, NKdim, NKbright, NKT-like and CD8+ T cells cells lymphogate was created based on physical characteristics typical of lymphoid cells using forward and side scatter parameters. Representative dot plots show the expression of NKG2D, ILT-2, KIR2DL3, KIR3DL1, CD160 and NKG2C by NK cells in the peripheral blood from patients with chronic HCV with elevated ALT or PNALT and in healthy individuals. HCV: Hepatitis C virus; ALT: Alanine aminotransferase; PNALT: Persistently normal ALT; NK: Natural killer cell.
PBMC were separated from heparinized venous blood on Ficoll-Paque gradient. One million cells were treated with recombinant active TGF-β1 protein (1 ng/mL) for 48 h at 37 °C in a tissue culture incubator. NKG2D, CD160 and KIR2DL3 expression by NK cells was determined by flow cytometry.
NK and CD8+ T separation and cytometric bead array
Natural killer and CD8+ T cells were separated by MACS Cell Separation Technology (all reagents and instruments from Miltenyi Biotec, Frank Diagnosztika Kft., Budapest, Hungary). PBMCs were first magnetically labeled with CD56 or CD8 MicroBeads according to the manufacturer’s instructions and CD56+ or CD8+ T cells were positively selected on the cell separation column. In the next step, the magnetic beads bound to the cell surface were enzymatically released from the CD56+ cells, which were then magnetically labeled with CD3 MicroBeads and the CD3+ subpopulation positively selected to compose the CD3+CD56+ T cell population. The remaining fraction of the CD56+ cells, which did not bind the CD3 beads composed the CD3-CD56+ NK cell population. Purity was greater than 95%. CD8+ and CD3-CD56+ cell populations were stimulated with 1 µg/mL of ionomycin and 25 ng/mL of PMA (Sigma-Aldrich, Sigma-Aldrich Kft., Budapest, Hungary) in RPMI 1640 Medium containing 10% fetal bovine serum, penicillin and streptomycin (all from Invitrogen, Csertex Kft., Budapest, Hungary) overnight for cytokine production. The levels of IL-2, IL-4, IL-5, IL-10, IFN-γ and TNF-α were determined from the culture supernatants with cytometric bead array (CBA) (#550749, BD Biosciences, Soft Flow Hungary Kft., Pécs, Hungary) using different capture beads according to the manufacturer’s instructions to detect the respective cytokines. Samples were analyzed right after the experiment on a FACS calibur flow cytometer (BD Immunocytometry Systems, Erembodegen, Belgium) calculating the amount of cytokines with CBA Software (BD Biosciences, San Diego, CA, United States).
Cytotoxic assay for NK cell activity
Cytotoxicity was determined as described earlier[18]. Cytotoxic activity of NK cells was evaluated by FACS analysis. Target cells (1 × 106) were pre-stained with the green fluorescent membrane dye PKH67 (Sigma, Hungary) and effector cells were added to 25 × 104 target cells to yield effector to target (E:T) ratios of 12.5:1, 25:1, and 50:1. The tubes were centrifuged for 30 s at 1200 rpm to pellet the effector and target cells together. These cell mixtures were incubated for 4 h at 37 °C in 5% CO2. After incubation the cell mixture was centrifuged at 1200 rpm and stained with propidium iodide (PI, 5 μg/mL, Sigma, Hungary). Dead target cells were identified by simultaneous PKH67 and PI-positivity. Target cells incubated without effector cells were used to assess spontaneous cell death. The percentage of lysed target cells was calculated by subtracting background (spontaneous cell death) expression from experimental samples. Cytotoxicity was expressed as the percentage of lysed target cells in each effector-to-target ratio.
Effect of TGF-β1 on NK cytotoxicity
PBMC were separated from heparinized venous blood on Ficoll-Paque gradient. One million cells were treated with recombinant active TGF-β1 protein (1 ng/mL) for 48 h at 37 °C. Following incubation we determined cytotoxicity of NK cells by flow cytometry.
Statistical analysis
Statistical analysis was performed using non-parametric Mann-Whitney U-test with statistical software SPSS version 11.0 package (SPSS, Inc. Chicago, IL, United States) (Figures 2, 3, 4, 5). Results are expressed as mean value ± standard error of the mean (SEM). Statistical comparisons were made by using one-way ANOVA with Bonferroni correction (Figures 6 and 7). The results were expressed as the mean value ± SEM. Differences were considered significant if the P value was equal to or less than 0.05. Correlation between variables was assessed by calculating Spearman rank correlation coefficient. Differences were accepted as significant at a level of P < 0.05.
Figure 2.
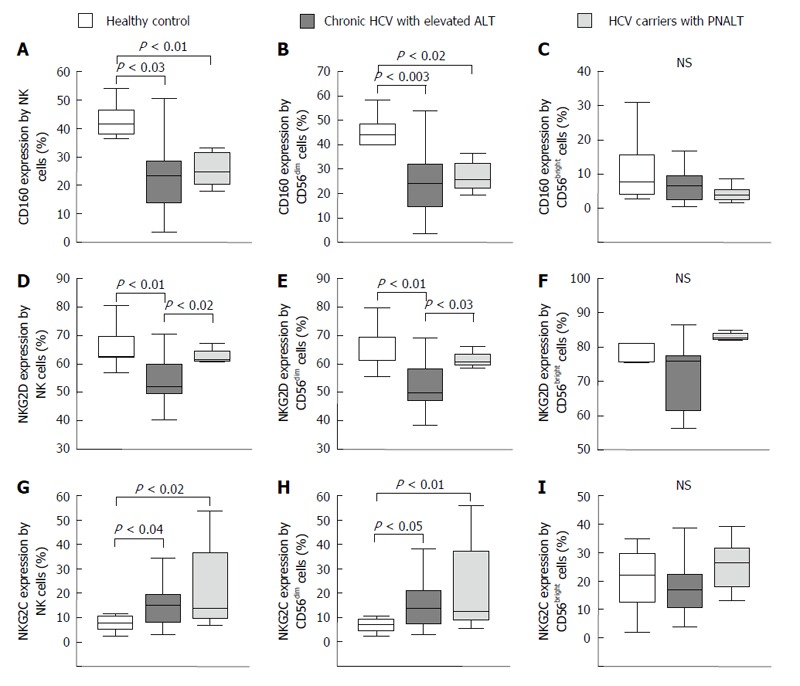
Activating natural killer cell receptor expression by natural killer cells in the peripheral blood from patients with chronic hepatitis C virus with elevated alanine aminotransferase or persistently normal alanine aminotransferase and in healthy individuals. The expression of CD160 (A-C), NKG2D (D-F) and NKG2C (G-I) by NK cells and NK cell subsets in the peripheral blood from patients with chronic HCV with elevated ALT or PNALT and in healthy individuals. The solid bars represent medians; the boxes indicate the interquartile ranges and the lines show the most extreme observations. Differences were considered statistically significant for P values ≤ 0.05. HCV: Hepatitis C virus; ALT: Alanine aminotransferase; PNALT: Persistently normal ALT; NK: Natural killer cell.
Figure 3.
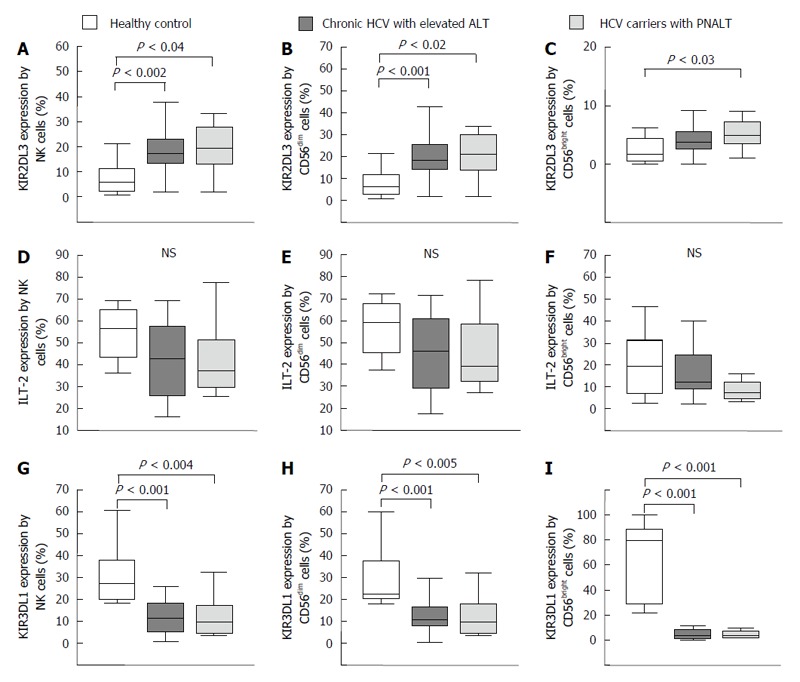
Inhibitory natural killer cell receptor expression by natural killer cells in the peripheral blood from patients with chronic hepatitis C virus with elevated alanine aminotransferase or persistently normal alanine aminotransferase and in healthy individuals. The expression of KIR2DL3 (A-C), ILT-2 (D-F) and KIR3DL1 (G-I) by NK cells and NK cell subsets in the peripheral blood from patients with chronic HCV with elevated ALT or PNALT and in healthy individuals. The solid bars represent medians; the boxes indicate the interquartile ranges and the lines show the most extreme observations. Differences were considered statistically significant for P values ≤ 0.05. HCV: Hepatitis C virus; ALT: Alanine aminotransferase; PNALT: Persistently normal ALT; NK: Natural killer cell.
Figure 4.
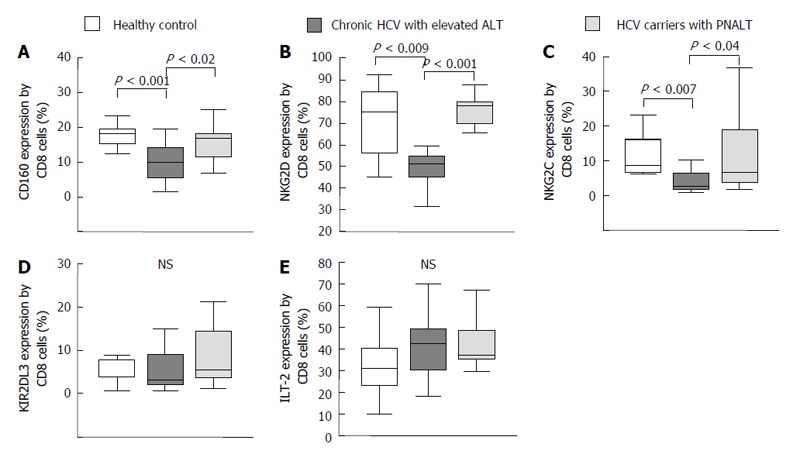
Activating and inhibitory natural killer cell receptor expression by CD8+ T cells in the peripheral blood from patients with chronic hepatitis C virus with elevated alanine aminotransferase or persistently normal alanine aminotransferase and in healthy individuals. The expression of CD160 (A), NKG2D (B), NKG2C (C), KIR2DL3 (D) and ILT-2 (E) by CD8+ T cells in the peripheral blood from patients with chronic HCV with elevated ALT or PNALT and in healthy individuals. The solid bars represent medians; the boxes indicate the interquartile ranges and the lines show the most extreme observations. Differences were considered statistically significant for P values ≤ 0.05. HCV: Hepatitis C virus; ALT: Alanine aminotransferase; PNALT: Persistently normal ALT; NK: Natural killer cell.
Figure 5.
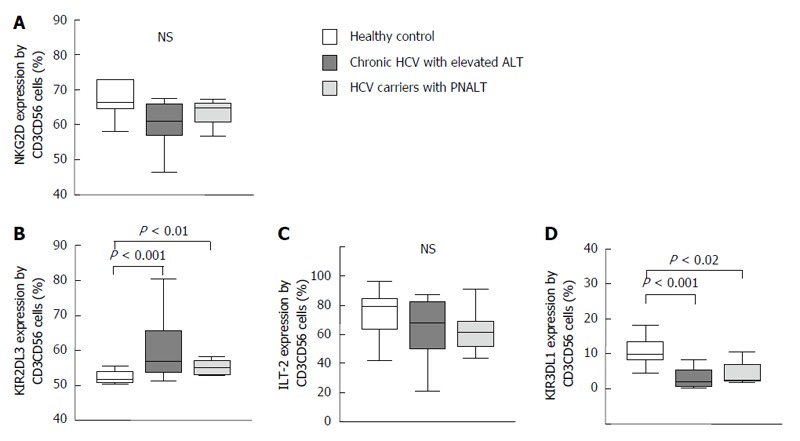
Activating and inhibitory natural killer cell receptor expression by NKT-like cells in the peripheral blood from patients with chronic hepatitis C virus with elevated alanine aminotransferase or persistently normal alanine aminotransferase and in healthy individuals. The expression of NKG2D (A), KIR2DL3 (B), ILT-2 (C) and KIR3DL1 (D) by NKT-like cells in the peripheral blood from patients with chronic HCV with elevated ALT or PNALT and in healthy individuals. The solid bars represent medians; the boxes indicate the interquartile ranges and the lines show the most extreme observations. Differences were considered statistically significant for P values ≤ 0.05. HCV: Hepatitis C virus; ALT: Alanine aminotransferase; PNALT: Persistently normal ALT; NK: Natural killer cell.
Figure 6.
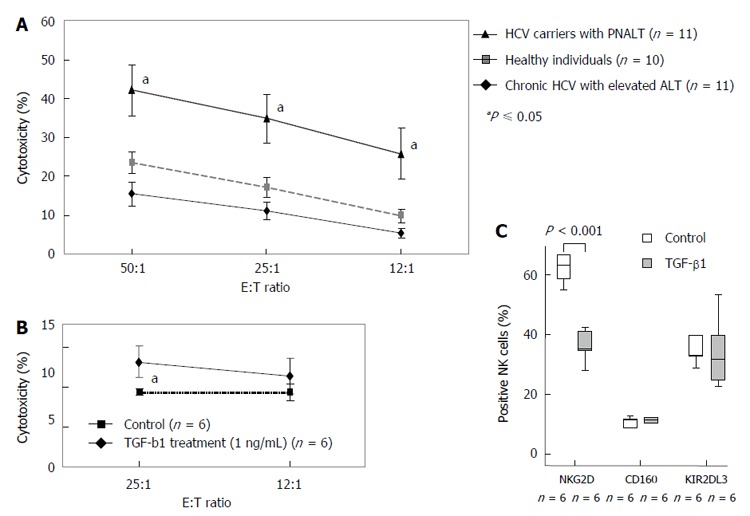
Natural killer cell cytotoxicity against K562 cells in patients with chronic hepatitis C virus with elevated alanine aminotransferase or persistently normal alanine aminotransferase and in healthy individuals and the effect of in vitro TGF-β1 treatment on the cytotoxicity and natural killer cell receptor expression of freshly isolated natural killer cells. A: Cytotoxicity of NK cells isolated from healthy individuals, HCV carriers with PNALT and chronic HCV with elevated ALT. Cytotoxic activity of NK cells as a percentage of lysed cells is indicated in patients with chronic HCV with elevated ALT or PNALT and in healthy individuals at different effector and target cell ratios. Statistical comparisons were made by using one-way ANOVA with Bonferroni correction. The results were expressed as the mean value ± standard error of the mean (SEM). aP ≤ 0.05, significant from patients with chronic HCV with elevated ALT and healthy individuals. B: Cytotoxicity of TGF-β treated NK cells isolated from healthy individuals. Cytotoxic activity of NK cells as a percentage of lysed cells is indicated after TGFβ1 treatment (1 ng/mL) at different effector and target cell ratios. C: Expression of NKG2D, KIR2DL3 and CD160 receptors by TGF-β-treated NK cells. Different NK cell receptor expression by NK cells after TGFβ1 treatment (1 ng/mL). Statistical comparisons were made by one-way ANOVA with Bonferroni correction. The solid bars represent medians; the boxes indicate the interquartile ranges and the lines show the most extreme observations. Differences were considered statistically significant for P values ≤ 0.05. E: Effector cell; T: Target cell; HCV: Hepatitis C virus; ALT: Alanine aminotransferase; PNALT: Persistently normal ALT; NK: Natural killer cell.
Figure 7.
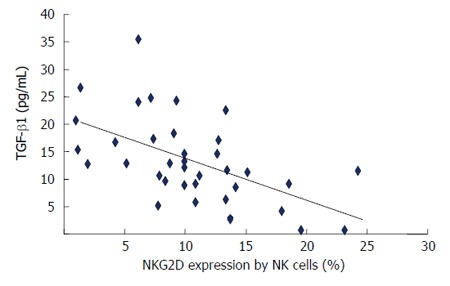
The correlation of plasma transforming growth factor β1 levels with NKG2D expression by natural killer cells in patients with chronic hepatitis C virus hepatitis. Shown is plasma TGF-β1 levels with NKG2D expressed by NK cells. Correlation between variables was assessed by calculating Spearman rank correlation coefficient. NK: Natural killer cell; TGF-β1: Transforming growth factor β1.
RESULTS
Patients
Twenty one patients with CHC infection with elevated ALT > 2 × ULN (11 males, 10 females, mean age: 57 years; range 38-70 years) and 11 (2 males, 9 females, mean age: 56 years; range 41-63 years) HCV carriers with persistently normal ALT were studied.
Lymphocyte frequency
No significant differences were observed in the percentage of helper (CD4+) or cytotoxic (CD8+) T cells, regulatory (CD4+CD25high) T cells, NK (CD3-CD56+) or NKT-like (CD3+CD56+) cells in peripheral blood of patients with CHC hepatitis with elevated ALT compared to CHC patients with PNALT and also to healthy controls (Table 1).
Table 1.
Peripheral blood mononuclear cell phenotype characteristics in patients with chronic hepatitis C virus with elevated alanine aminotransferase or persistently normal alanine aminotransferase and in healthy individuals
| Healthy individuals | Chronic HCV with elevated ALT | HCV carriers with PNALT | |
| Percentage of PBL | |||
| CD3+CD4+ | 29.38 ± 8.11 | 33.15 ± 9.08 | 34.46 ± 6.49 |
| CD3+CD4+CD25+ | 4.27 ± 1.62 | 4.05 ± 1.57 | 5.24 ± 1.43 |
| CD3+CD4+CD25bright+ | 0.52 ± 0.26 | 0.38 ± 0.26 | 0.40 ± 0.13 |
| CD3+CD56+ | 2.99 ± 1.73 | 5.07 ± 4.15 | 3.48 ± 2.72 |
| CD3-CD56+ | 17.34 ± 7.42 | 16.42 ± 5.26 | 17.04 ± 7.71 |
| CD3-CD56dim+ | 16.04 ± 7.36 | 14.58 ± 5.72 | 15.92 ± 7.14 |
| CD3-CD56bright+ | 1.15 ± 0.64 | 1.75 ± 1.54 | 1.27 ± 1.01 |
| CD3+CD8+ | 28.59 ± 4.55 | 25.27 ± 8.77 | 24.66 ± 6.14 |
Statistical comparisons were made by using the ANOVA tests. The results were expressed as the mean value ± SD. HCV: Hepatitis C virus; ALT: Alanine aminotransferase; PNALT: Persistently normal ALT; NK: Natural killer cell.
Activating and inhibitory NK cell receptor expression by NK cells in the peripheral blood from patients with CHC with elevated ALT or PNALT and in healthy individuals
The phenotypes and functional activities of various populations of innate effectors have been reported to be impaired in all stages of HCV infection[6,19-21]. The balance of activating and inhibitory signals through the killer activating and the inhibitory receptors control NK cell activity. Since the role of NK cells determining disease inflammatory activity reflected by ALT elevation in chronic hepatitis C is not clear, we investigated different activating and inhibitory NK cell receptor expression by NK cells in patients with CHC with elevated ALT or PNALT.
CD160 and NKG2D activating NK cell receptor expression was significantly lower in patients with CHC infection than in healthy controls (Figure 2A and D). NKG2D expression by NK cells was significantly lower in CHC patients with elevated ALT than in CHC positive patients with PNALT (Figure 2D). Expression of NKG2C was higher in NK cells from patients with CHC infection as compared to healthy controls but significantly lower than CHC carriers (Figure 2G). NK cells can be divided into two populations based on the intensity of the CD56 marker at the cell surface. CD56dim NK cells are the more cytotoxic subset, whereas CD56bright cells are poorly cytotoxic and preferentially secrete cytokines when activated[22]. The majority of NK cells were of the CD3-CD56dim phenotype and our data shows that this cell population represents the above mentioned alterations in activating receptor expression by NK cells (Figure 2B, E and H). In contrast to CD56dim cells, we found no significant difference in the killer activating NK cell receptor expression by CD56bright cells between the investigated groups (Figure 2C, F and I).
An enhanced expression of inhibitory KIR2DL3 receptor on NK cells was found in peripheral blood of patients with CHC infection and also in CHC carriers with PNALT in comparison to healthy controls (Figure 3A). No significant differences were found between study groups in regarding expression of ILT-2 on NK cells (Figure 3D-F). KIR3DL1 inhibitory NK cell receptor expression was significantly decreased by NK, CD56dim and CD56bright cells in patients with CHC irrespectively of ALT compared to healthy individuals (Figure 3G-I).
Activating and inhibitory receptor expression by CD8+ T cells
Significantly lower percentage of CD160, NKG2D and NKG2C activating receptor expressing CD8+ T cells were found in patients with CHC hepatitis than in healthy controls and in CHC carriers with PNALT. (Figure 4A-C). We found no difference in the percentage of inhibitory receptor (KIR2DL3 and ILT-2) expression by CD8+ T cells in patients with CHC infection compared to healthy controls (Figure 4D and E).
Activating and inhibitory NK cell receptor expression by NKT-like cells
No significant difference was found between healthy controls and patients with HCV infection with respect to expression of NKG2D on NKT-like cells (Figure 5A). KIR2DL3 inhibitory NK cell receptor expression by NKT-like cells revealed an enhanced proportion of KIR2DL3-expressing cells in peripheral blood of patients with CHC in comparison to healthy controls (Figure 5B).
Regarding expression of ILT-2 inhibitory receptors, we found no difference in the percentage of ILT-2 receptor expressing NKT-like cells in study groups (Figure 5C). KIR3DL1 inhibitory receptor expression -similarly to NK cells- was significantly decreased on NKT-like cells in patients with CHC irrespective of ALT compared to healthy individuals (Figure 5D).
Alteration of cytokine production by NK and CD8+ T cells in CHC infection
NK cells produced significantly higher IL-10, TNF-α and IFN-γ in patients with CHC compared to healthy individuals (Table 2). In addition, IL-2, IL-4, IL-5, IL-10, TNF-α and IFN-γ production by NK cells isolated from CHC carriers with PNALT was significantly higher compared to healthy controls (Table 2). PNALT was associated with higher level of IL-4 and TNF-α compared to CHC hepatitis group (Table 2). CHC infection was associated with significantly higher IL-4, IL-5, IL-10 and TNF-α levels produced by CD8+ T cells compared to healthy individuals. Only IL-10 differed significantly between PNALT and elevated ALT group. CD8+ cells of CHC carriers with PNALT produced significantly higher amount of IL-10 compared to CHC hepatitis group (Table 2).
Table 2.
Cytokine production by natural killer cell and CD8+ T cells in the peripheral blood from patients with chronic hepatitis C virus with elevated alanine aminotransferase or persistently normal alanine aminotransferase and in healthy individuals
| IL-2 | IL-4 | IL-5 | IL-10 | TNF-α | IFN-γ | |
| NK cells | ||||||
| Healthy individuals | 400.4 ± 357.4 | 32.4 ± 23.5 | 6.3 ± 1.39 | 5.50 ± 0.23 | 1301.7 ± 417.3 | 2739.2 ± 61.2 |
| Chronic HCV with elevated ALT | 817.3 ± 316.2 | 20.2 ± 8.6c | 27.9 ± 15.6 | 9.30 ± 1.63a | 3034.3 ± 649.7ac | 3268.6 ± 140.4a |
| HCV carriers with PNALT | 1182.3 ± 447.7e | 169.5 ± 48.1a | 18.3 ± 5.83e | 9.70 ± 1.22a | 4491.6 ± 148.6b | 3231 ± 77.9b |
| CD8+ | ||||||
| Healthy individuals | 1944.5 ± 303.9 | 26.1 ± 6.9 | 65.5 ± 20.8 | 18.2 ± 4.9 | 1369.0 ± 224.5 | 2608.4 ± 81.2 |
| Chronic HCV with elevated ALT | 2440.5 ± 378.3 | 276.3 ± 98.1a | 461.2 ± 213.9a | 58.3 ± 15.8ac | 3247.4 ± 590.8a | 2721.9 ± 78.7 |
| HCV carriers with PNALT | 2502.1 ± 238.5e | 253.1 ± 95.2a | 374.8 ± 171.6a | 258.3 ± 71.9e | 3895.0 ± 218.3b | 2719.7 ± 127.0 |
Concentrations of cytokines are given in picograms per milliliter (mean ± SD).
P < 0.05,
P < 0.02,
P < 0.001, vs healthy individuals, significantly different;
P < 0.05 vs symptomatic individuals, significantly different. HCV: Hepatitis C virus; ALT: Alanine aminotransferase; PNALT: Persistently normal ALT; NK: Natural killer cell; IL-2: Interleukin-2; TNF-α: Tumor necrosis factor α; IFN-γ: Interferon-γ.
Cytotoxicity of NK cells and the effect of in vitro TGF-β1 treatment
Earlier we demonstrated that TGF-β1 levels significantly higher in patients with chronic hepatitis C with elevated ALT compared to PNALT group and healthy individuals. TGF-β1 levels positively correlated with Knodell histological activity index assessed by liver biopsy. Since impaired NK cell function has been attributed to down-modulation of activating receptors NKG2D via secretion of TGF-β1 in lung and colorectal cancer patients, we investigated the effect of TGF-β1 treatment on NK cell cytotoxicity[23].
To determine whether decreased NKG2D expression by NK cells in CHC patients with elevated ALT is potentially related to their increased TGF-β1 levels, we studied the in vitro effect of TGF-β1 treatment on the cytotoxicity of NK cells in response to co-culture with the classical NK cell target K562 cells. NK cells from CHC carriers with PNALT showed significantly higher cytotoxic activity compared to patients with chronic CHC with elevated ALT or healthy individuals (Figure 6A). Treatment of freshly isolated NK cells with TGF-β1 suppressed NK-dependent lysis of K562 cells (13.21 vs 9.43, P < 0.01) (Figure 6B).
Effect of TGF-β1 on NKG2D expression of freshly isolated NK cells
To investigate if TGF-β1 was responsible for down-modulation of NKG2D, we incubated freshly isolated NK cells obtained from healthy volunteers with 1 ng/mL TGF-β1 for 48 h and analyzed NKG2D expression by FACS. Incubation of NK cells with TGF-β1 significantly down-regulate surface NKG2D expression by NK cells (63.08 vs 36.21, P < 0.01). In contrast, TGF-β1 did not alter the level of other NK receptors, including the activating NK cell receptor, CD160 (37.47 vs 34.04 NS) or the inhibitory NK cell receptor, KIR2DL3 (11.05 vs 11.04 NS). These data suggest that TGF-β1 specifically down-modulates NKG2D without affecting other NK receptors.
Together, our data strongly suggest that secretion of TGF-β1 in CHC patients can down-modulate NKG2D expression by NK cells (Figure 6C). Plasma TGF-β1 levels inversely correlated with NKG2D expression by NK cells in CHC infected individuals (Figure 7).
DISCUSSION
Following acute HCV infection a majority of healthy adults will develop persistent viremia. Effective clearance of an acute viral infection typically requires the coordinated function of multiple arms of the immune system, including the innate immune system (interferons, NK and NKT-like cells), as well as the acquired immune response specific to a given pathogen (CD4+ and CD8+ T cells). A functional impairment of NK, NKT-like and CD8 T cells has been reported in CHC infections by several observations, and different mechanisms have been proposed to explain this defective function[5,19-21,24].
Natural killer cell receptors are important regulators of NK and CD8+ T cell functions. Regarding the fact that impaired activities of CD8+ T[6,20] and NK cells[19,21,24] have been reported in patients with chronic hepatitis C infection, we analyzed whether dysregulation of NK cell receptors on these cell might be involved in the inefficient cellular immune response observed in chronic hepatitis C. In this study we analyzed the expression of different activating and inhibitory NK cell receptors in CHC patients with elevated and with persistently normal ALT.
We found that the percentages of NKG2D or CD160 activating receptor positive NK and CD8+ T cells were significantly decreased in CHC patients with elevated ALT compared to healthy individuals. This discrepancy was not found in CHC infected patients with persistently normal ALT. CD8+ T cells from CHC carriers with PNALT showed significantly elevated expression of CD160, NKG2D and NKG2C activating receptors compared to CHC patients with elevated ALT. Comparing the activating and inhibitory receptor expression by NK cells obtained from CHC carriers with PNALT and CHC hepatitis patients with elevated ALT, NKG2D activating receptor expression was the only receptor showing a significant difference. Investigating CD56dim NK cells, NKG2D receptor expression was significantly elevated in persistently normal ALT group compared to CHC hepatitis patients.
Analyzing inhibitory NK cell receptors expressed by CD8+ T, NK or NKT-like cells, we did not find any difference in the expression of KIR2DL3, ILT-2 or KIR3DL1 between patients with CHC infection with elevated ALT and CHC carriers with PNALT. Interestingly KIR3DL1 expression by NK and NKT-like cells was significantly lower in patients with chronic hepatitis C with elevated ALT compared to healthy individuals. On the other hand KIR2DL3 expression by NK and NKT-like cells were significantly increased in patients with CHC infection with elevated ALT and CHC carriers with PNALT compared to healthy individuals.
The mechanisms by which NK and NKT-like cells in hepatitis express KIR2DL3 at considerable high levels remain elusive. One possibility is that the expression levels of NK receptors may be modified by various types of cytokines released under chronically inflamed conditions. Given previous findings that high level of serum TGF-β production were observed in CHC infected patients[25] and that TGF-β can up regulate the expression of inhibitory receptors on NK cells[26] we hypothesized that TGF-β would contribute to the high expression of KIR2DL3 on NK and NKT-like cells in CHC infection. We found that TGF-β1 significantly down-regulated surface NKG2D expression by NK cells, but did not alter the level of other NK receptors, including the activating NK cell receptor CD160 or the inhibitory NK cell receptor KIR2DL3, suggesting that other factors, including the virus itself, play a role in the modulation of activating or inhibitory receptor expression.
The activation status of NK, NKT-like and CD8+ T cells depends on the balance between activating and inhibitory signals delivered by surface receptors. Thus, our present findings of up-regulated expression of inhibitory receptors combined with the concomitant down-regulation of activating receptors synergistically leads to the dominant delivery of inhibitory signals to NK, NKT cells and KIR receptor-positive T cells. This altered receptor expression is reflected by impaired cytotoxic function of NK cells. Our data agree with other studies[27,28], which reported a significant reduction in NK cytotoxic activity in patients with CHC infection, but disagree with several other studies[24,29].
Our results show that NK cells from CHC carriers with PNALT have significantly higher cytotoxic activity compared to patients with CHC with elevated ALT or healthy individuals. Since NKG2D expression by NK cells in PNALT patients was at normal level we hypothesize that further factors may be involved in the regulation of their activity.
The pathogenesis of impaired CD8 response in CHC infection still remains partially understood. To further clarify this issue, in this study, we looked for activating and inhibitory receptor expression by CD8+ T cells. Our results show that CD8 T cell triggering can be hindered by engagement of inhibitory natural killer cell receptors, which are expressed on previously activated CD8 T cells. Although we found no differences in the expression of inhibitory receptor expression by CD8+ T cells between healthy individuals and HCV infected patients, but we found that activating NK receptors are expressed at significantly lower frequencies on CD8+ T cells during CHC infection. These findings suggest that decreased expression of activating NK receptors may play a role in HCV infection, possibly by inhibiting CD8 T-cell triggering.
Other factors such as cytokines may also play an important role during CHC infection[30-32]. IL-10 has largely been appreciated for its direct and indirect inhibitory effects on several T cell responses. IL-10 has been shown to contribute to regulation of immunopathology. The increased production of IL-10 by CD8+ T cells in CHC patients with persistently normal ALT reported in this paper is supposed to be important for limiting CD8 T cell response and contributing to asymptomatic virus carrier state. The NK cells of CHC carriers with PNALT produced significantly higher level of IL-4 and TNF-α, together with high cytotoxic activity compared to patients with CHC infection. We showed that cytokine pattern of NK cells and CD8+ T cells are shifted towards a virus-permissive profile in patients with CHC infection which may ultimately contribute to HCV chronicity.
A major unresolved issue is the exact role of immune cells in different patient populations with acute and chronic hepatitis C virus infection. Further investigations are needed to clear whether NK, NKT-like and CD8+ T cells expressing various activating and inhibitory receptors could lead to viral clearance.
In conclusion we found complex dysregulation of activating and inhibitory receptor expression, such as decreased NKG2D and CD160 activating receptor expression and increased KIR2DL3 inhibitory receptor expression by NK and cytotoxic T cells in patients with chronic hepatitis C contributing to defective cellular immune functions. NKG2D receptor expression was significantly elevated in CHC infected patients with persistently normal ALT suggesting an important pathway for sustaining NK and CD8 T cell function and its critical role in protection against disease progression.
COMMENTS
Background
The host immune response to hepatitis C virus (HCV) involves both innate and adaptive arms of the immune system. Natural killer (NK) cells are key components of the innate antiviral immune response.
Research frontiers
To better characterize the immune defects underlying chronic viral persistence, the authors focus their analysis on killer inhibitory and activating receptor expression in patients with chronic HCV (CHC) infection with elevated alanine aminotransferase (ALT) and also in patients with CHC carriers with persistently normal ALT.
Innovations and breakthroughs
The authors found complex dysregulation of activating and inhibitory receptor expression by NK and cytotoxic T cells in patients with chronic hepatitis C contributing to defective cellular immune functions.
Applications
The percentage of Treg cells, KIR2DL3, ILT-2, KIR3DL1, CD160, NKG2D, NKG2C expressing NK, T and NKT-like cells, cytokine production and NK cytotoxicity were determined by flow cytometry.
Terminology
Persistently normal ALT was defined as ALT < 30 IU/L in men, ALT < 19 IU/L in women measured every 3 mo over a 18-mo period.
Peer-review
The manuscript described some phenotypical and functional differences in peripheral lymphocyte subsets between CHC patients with high and normal ALT. They describe a different expression in one NK activating receptor that could be related with TGF-β1 regulation. The manuscript is well written and the methodology is properly done.
Footnotes
Supported by Grants from Hungarian National Research Fund (OTKA K81454 and OTKA K104960); Liver Research Foundation (Pécs), United European Gastroenterology Federation; Janos Bolyai Research Scholarship of the Hungarian Academy of Sciences to Szereday L.
Institutional review board statement: Written informed consent was obtained from all patients. The study protocol conforms to ethical guidelines of 1975 Declaration of Helsinki. Approval from the Regional Ethics Committee at the Medical School, University of Pécs, was obtained.
Conflict-of-interest statement: All authors do not have any conflict of interest.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 15, 2016
First decision: February 18, 2016
Article in press: April 7, 2016
P- Reviewer: Larrubia JR, Pandey VN, Picardi A S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
References
- 1.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J. The scientific challenge of hepatitis C. Science. 1999;285:26–30. doi: 10.1126/science.285.5424.26. [DOI] [PubMed] [Google Scholar]
- 3.Stoll-Keller F, Barth H, Fafi-Kremer S, Zeisel MB, Baumert TF. Development of hepatitis C virus vaccines: challenges and progress. Expert Rev Vaccines. 2009;8:333–345. doi: 10.1586/14760584.8.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puoti C, Bellis L, Guarisco R, Dell’ Unto O, Spilabotti L, Costanza OM. HCV carriers with normal alanine aminotransferase levels: healthy persons or severely ill patients? Dealing with an everyday clinical problem. Eur J Intern Med. 2010;21:57–61. doi: 10.1016/j.ejim.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Nattermann J, Nischalke HD, Hofmeister V, Ahlenstiel G, Zimmermann H, Leifeld L, Weiss EH, Sauerbruch T, Spengler U. The HLA-A2 restricted T cell epitope HCV core 35-44 stabilizes HLA-E expression and inhibits cytolysis mediated by natural killer cells. Am J Pathol. 2005;166:443–453. doi: 10.1016/S0002-9440(10)62267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pár G, Rukavina D, Podack ER, Horányi M, Szekeres-Barthó J, Hegedüs G, Paál M, Szereday L, Mózsik G, Pár A. Decrease in CD3-negative-CD8dim(+) and Vdelta2/Vgamma9 TcR+ peripheral blood lymphocyte counts, low perforin expression and the impairment of natural killer cell activity is associated with chronic hepatitis C virus infection. J Hepatol. 2002;37:514–522. doi: 10.1016/s0168-8278(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 7.Maroof A, Beattie L, Zubairi S, Svensson M, Stager S, Kaye PM. Posttranscriptional regulation of II10 gene expression allows natural killer cells to express immunoregulatory function. Immunity. 2008;29:295–305. doi: 10.1016/j.immuni.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le Gall S, Waring MT, Moss K, et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114:346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Exley MA, Koziel MJ. To be or not to be NKT: natural killer T cells in the liver. Hepatology. 2004;40:1033–1040. doi: 10.1002/hep.20433. [DOI] [PubMed] [Google Scholar]
- 10.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 11.Ye L, Wang X, Wang S, Wang Y, Song L, Hou W, Zhou L, Li H, Ho W. CD56+ T cells inhibit hepatitis C virus replication in human hepatocytes. Hepatology. 2009;49:753–762. doi: 10.1002/hep.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C, Zhu H, Tu Z, Xu YL, Nelson DR. CD8+ T-cell interaction with HCV replicon cells: evidence for both cytokine- and cell-mediated antiviral activity. Hepatology. 2003;37:1335–1342. doi: 10.1053/jhep.2003.50207. [DOI] [PubMed] [Google Scholar]
- 13.Cox AL, Mosbruger T, Lauer GM, Pardoll D, Thomas DL, Ray SC. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005;42:104–112. doi: 10.1002/hep.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, Mori C, Missale G, Ferrari C. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126–139. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan DE, Sugimoto K, Newton K, Valiga ME, Ikeda F, Aytaman A, Nunes FA, Lucey MR, Vance BA, Vonderheide RH, et al. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654–666. doi: 10.1053/j.gastro.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 16.Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R, Pape GR, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–5558. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 18.Kane KL, Ashton FA, Schmitz JL, Folds JD. Determination of natural killer cell function by flow cytometry. Clin Diagn Lab Immunol. 1996;3:295–300. doi: 10.1128/cdli.3.3.295-300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golden-Mason L, Rosen HR. Natural killer cells: multifaceted players with key roles in hepatitis C immunity. Immunol Rev. 2013;255:68–81. doi: 10.1111/imr.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sung PS, Racanelli V, Shin EC. CD8(+) T-Cell Responses in Acute Hepatitis C Virus Infection. Front Immunol. 2014;5:266. doi: 10.3389/fimmu.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golden-Mason L, Cox AL, Randall JA, Cheng L, Rosen HR. Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in vitro. Hepatology. 2010;52:1581–1589. doi: 10.1002/hep.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–7340. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 24.Golden-Mason L, Madrigal-Estebas L, McGrath E, Conroy MJ, Ryan EJ, Hegarty JE, O’Farrelly C, Doherty DG. Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut. 2008;57:1121–1128. doi: 10.1136/gut.2007.130963. [DOI] [PubMed] [Google Scholar]
- 25.Nelson DR, Gonzalez-Peralta RP, Qian K, Xu Y, Marousis CG, Davis GL, Lau JY. Transforming growth factor-beta 1 in chronic hepatitis C. J Viral Hepat. 1997;4:29–35. doi: 10.1046/j.1365-2893.1997.00124.x. [DOI] [PubMed] [Google Scholar]
- 26.Bertone S, Schiavetti F, Bellomo R, Vitale C, Ponte M, Moretta L, Mingari MC. Transforming growth factor-beta-induced expression of CD94/NKG2A inhibitory receptors in human T lymphocytes. Eur J Immunol. 1999;29:23–29. doi: 10.1002/(SICI)1521-4141(199901)29:01<23::AID-IMMU23>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez VD, Falconer K, Björkström NK, Blom KG, Weiland O, Ljunggren HG, Alaeus A, Sandberg JK. Expansion of functionally skewed CD56-negative NK cells in chronic hepatitis C virus infection: correlation with outcome of pegylated IFN-alpha and ribavirin treatment. J Immunol. 2009;183:6612–6618. doi: 10.4049/jimmunol.0901437. [DOI] [PubMed] [Google Scholar]
- 28.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–877. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morishima C, Paschal DM, Wang CC, Yoshihara CS, Wood BL, Yeo AE, Emerson SS, Shuhart MC, Gretch DR. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43:573–580. doi: 10.1002/hep.21073. [DOI] [PubMed] [Google Scholar]
- 30.Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71–99. doi: 10.1146/annurev.immunol.25.022106.141602. [DOI] [PubMed] [Google Scholar]
- 31.Nishitsuji H, Funami K, Shimizu Y, Ujino S, Sugiyama K, Seya T, Takaku H, Shimotohno K. Hepatitis C virus infection induces inflammatory cytokines and chemokines mediated by the cross talk between hepatocytes and stellate cells. J Virol. 2013;87:8169–8178. doi: 10.1128/JVI.00974-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yue M, Deng X, Zhai X, Xu K, Kong J, Zhang J, Zhou Z, Yu X, Xu X, Liu Y, et al. Th1 and Th2 cytokine profiles induced by hepatitis C virus F protein in peripheral blood mononuclear cells from chronic hepatitis C patients. Immunol Lett. 2013;152:89–95. doi: 10.1016/j.imlet.2013.05.002. [DOI] [PubMed] [Google Scholar]


