Abstract
AIM: To investigate the expression of miR-29a in rat acute pancreatitis and its functional role in AR42J cell apoptosis.
METHODS: Twelve SD rats were divided into a control group and an acute edematous pancreatitis (AEP) group randomly. AEP was induced by intraperitoneal injection of L-arginine (150 mg/kg) in the AEP group and equal volume of 0.9% NaCl was injected in the control group. The apoptosis of acinar cells in pancreatic tissue was determined by TUNEL assay. miRNA chip assay was performed to examine the expression of miRNAs in two groups. Besides, to further explore the role of miR-29a in apoptosis in vitro, recombinant rat TNF-α (50 ng/mL) was administered to treat the rat pancreatic acinar cell line AR42J for inducing AR42J cell apoptosis. Quantitative real-time PCR (qRT-PCR) was adopted to measure miR-29a expression. Then, miRNA mimic, miRNA antisense oligonucleotide (AMO) and control vector were used to transfect AR42J cells. The expression of miR-29a was confirmed by qRT-PCR and the apoptosis rate of AR42J cells was detected by flow cytometry analysis. Western blot was used to detect the expression of activated caspase3. Moreover, we used bioinformatics software and luciferase assay to test whether TNFRSF1A was the target gene of miR-29a. After transfection, qRT-PCR and Western blot was used to detect the expression of TNFRSF1A in AR42J cells after transfection.
RESULTS: The expression of miR-29a was much higher in the AEP group compared with the control group as displayed by the miRNA chip assay. After inducing apoptosis of AR42J cells in vitro, the expression of miR-29a was significantly increased by 1.49 ± 0.04 times in comparison with the control group. As revealed by qRT-PCR assay, the expression of miR-29a was 2.68 ± 0.56 times higher in the miR-29a mimic group relative to the control vector group, accompanied with an obviously increased acinar cell apoptosis rate (42.83 ± 1.25 vs 24.97 ± 0.15, P < 0.05). Moreover, the expression of miR-29a in the miRNA AMO group was 0.46 ± 0.05 times lower than the control vector group, and the cell apoptosis rate was much lower accordingly (17.27 ± 1.36 vs 24.97 ± 0.15, P < 0.05). The results of bioinformatics software and luciferase assay showed that TNFRSF1A might be a target gene of miR-29a. TNFRSF1A expression was up-regulated in the miR-29a mimic group, while the miR-29a AMO group showed the reverse trend.
CONCLUSION: miR-29a might promote the apoptosis of AR42J cells via up-regulating the expression of its target gene TNFRSF1A.
Keywords: Acute edematous pancreatitis, miR-29a, Apoptosis, AR42J, Target gene, TNFRSF1A
Core tip: Apoptosis is a self-protection mechanism in acute pancreatitis. miRNAs are short non-coding RNAs and play important roles in regulating gene expression in multiple cellular processes, such as apoptosis. Here, our group studied the role of miR-29a in pancreatic acinar cell apoptosis. The pancreatic acinar cells showed a tendency to apoptosis when the expression of miR-29a elevated, while the apoptosis rate exhibited the opposite trend by down-regulating the expression of miR-29a. Moreover, we found TNFRSF1A, which encode TNFR1 protein, was a target gene of miR-29a. Our results demonstrated that miR-29a could promote the apoptosis of pancreatic acinar cells via up-regulating the expression of TNFRSF1A gene in acute pancreatitis.
INTRODUCTION
Tumor necrosis factor (TNF)-α is a pleiotropic cytokine that plays a crucial role in angiogenesis, inflammation, proliferation and apoptotic cell death[1]. TNF-α is involved in the development of pancreatitis, and mediates apoptosis in acinar cell suspensions in vitro as well as in an in vivo model of pancreatitis. Pancreatic acinar cells produce, release, and respond to TNF-α[2], which acts by binding to its two receptors, TNF-R1 and TNF-R2, on the cell surface. TNF-R1, which is the major signaling receptor for TNF-α, is expressed on all cell types. Both soluble and membrane-bound forms of the cytokine can activate TNFR1. The binding of TNF to TNFR1 results in immediate nuclear factor-κB (NF-κB) activation and subsequent apoptosis[3,4].
MicroRNAs (miRNAs) are short non-coding RNAs involved in multiple cellular processes including development, proliferation, differentiation, apoptosis and metabolism[5]. Most studies on miRNAs have focused on the repression of their target genes by binding to complementary sites, causing target mRNAs degradation and/or translational repression[6-9]. In recent years, it had been demonstrated that miRNAs could up-regulate the expression of their target genes. Vasudevan et al[10] demonstrated that human miRNA369-3 directs association of the proteins with AREs (AU-rich elements) to activate translation, while let-7 and the synthetic miRNA miRNAcxcr4 induced upregulation of target genes. miR-29a has been extensively demonstrated to play an important role in apoptosis[11-15]. Using miRNA microarray analysis, we found that miR-29a was elevated in a rat model of AEP in vivo. TNFRSF1A, which encodes the TNFR1 protein, was predicted to be the target gene of miR-29a with the aid of three online programs (TargetScan, miRanda and TarBase). Our results demonstrated that miR-29a is elevated significantly in AEP in vivo. The rate of apoptosis and TNFRSF1A expression increased significantly in AR42J cells following upregulation of miR-29a expression.
MATERIALS AND METHODS
Animal care
All animal experiments were approved by the Animal Research Ethics Committee of People’s Hospital of Zhengzhou University, Zhengzhou, China. Surgery was performed under chloral hydrate anesthesia and all efforts were made to minimize animal suffering.
In vivo AEP model
Twelve male SD rats (250-300 g) were divided into two groups randomly (n = 6). All rats were anesthetized with 10% chloral hydrate (300 mg/kg, i.p.). 150 mg/kg L-arginine (Sigma, United States) was injected intraperitoneally to establish the AEP model in vivo. The rats in the control group were injected with equivalent saline. After 12 h the pancreas of the rats was removed.
TUNEL assay
The apoptosis of acinar cells in pancreatic tissue was determined by terminal-deoxynucleotidyl-transferase-mediated dUTP nick-end labeling (TUNEL) assay by using the in situ cell death detection kit (Promega, China). According to the manufacturer’s instructions, the tissue was fixed in 10% buffered formaldehyde, embedded in paraffin, and 4-μm sections were adhered to glass slides. After dewaxing and rehydration, the sections were incubated with TUNEL reaction mixture at 37 °C for 1 h. Finally, the sections were analyzed under a fluorescence microscope (Olympus, Tokyo, Japan). TUNEL-positive cells displayed brown fluorescence.
miRNA microarray
Total RNA of pancreas tissue was extracted using TRIzol. miRNA microarray analysis was applied to detect the differential expression of miRNAs in pancreas tissue between the two groups.
miR-29a mimic and antisense oligonucleotide construct
The mimic and antisense oligonucleotide of miR-29a, which were designed and synthesized chemically with the help of Genechem Bio Company (Shanghai, China), were inserted into a lentiviral vector carrying the green fluorescent protein (GFP) gene. The sequences of miR-29a mimic and AMO are presented in Table 1.
Table 1.
Sequences of miR-29a mimic and antisense oligonucleotide
| Base sequence | |
| miR-29a mimic | ACCCCTTAGAGGATGACTGATTTCTTTTGGTGTTCAGAGTCAATAGAATTTTCTAGCACCATCTGAAATCGGTTATAATGATTGGGGA |
| miR-29a AMO | ACTGATTTCTTTTGGTGTTCAG |
Cell culture and lentiviral transfection
The AR42J cell line (rat pancreatic acinar cell, Institute of Shanghai Cell Biology, Shanghai, China) was cultured in DMEM-F12 medium (Gibco, United States) containing 20% fetal bovine serum (FBS) (Gibco, United States) in a humidified incubator at 37 °C with an atmosphere of 5% CO2. The AR42J cells (1 × 106 cells/well) were seeded in 6-well plates 24 h before transfection and infected at an MOI of 50 with 10 μg/mL of polybrene for 12 h. After 12 h, the transfection liquid was removed and 2 mL normal medium was added for continued culturing. Seventy-two hours after transfection, green fluorescence was observed under a fluorescence microscope.
Induction of apoptosis and amylase assay
AR42J cells at 1 × 106/well were seeded into 6-well plates. After 24 h, the medium was removed and DMEM-F12 medium containing 50 ng/mL recombinant rat TNF-α (Peprotech, United States) was added. Twelve hours later, the supernatant was collected and centrifuged at 1000 rpm for 5 min and then used to detect the level of amylase using an amylase kit (Jiancheng Bio, Nanjing, China) according to the manufacturer’s instructions. The control group was incubated with DMEM-F12 medium only.
Western blot analysis
Total protein from the cultured AR42J cells was extracted with RIPA lysis buffer (150 mmol/L NaCl, 50 mmol/L Tris–HCl (pH 7.4), 1% NP-40, 1 μg/mL leupeptin, 1 mmol/L deoxycholic acid and 1 mmol/L EDTA) containing 1 mmol/L phenylmethylsulfonyl fluoride, according to the manufacturer’s instructions (Beyotime Bio, Wuhan, China). Proteins (40 μg) from each sample were loaded and separated on a 12% SDS polyacrylamide gel. Proteins were then electrophoretically transferred onto PVDF membranes (Millipore, Bedford, MA, United States), which were then incubated with diluted anti-rat monoclonal activated caspase3 antibody at 1/1000 (CST, United States), anti-rat TNFR1 at 1/200 (SANTA CRUZ Bio, United States) or anti-β-actin at 1/1000 (CST, United States) at 4 °C overnight. On the following day, membranes were incubated with an HRP secondary antibody (1:5000) at 37 °C for 2 h and then signals were visualized with an electrochemiluminescence kit (Pierce, Rockford, IL, United States).
Flow cytometry analysis of apoptosis
AR42J cells were seeded into 6-well plates (5 × 105/well) and incubated with DMEM-F12 medium containing 50 ng/mL recombinant rat TNF-α for 24 h. Cells were harvested, washed twice with 1 × PBS and then stained using an annexin V-APC apoptosis kit (KeyGEN Bio, Nanjing, China) according to the manufacturer’s instructions. All flow cytometric analyses were carried using a FACS Caliber flow cytometer (San Jose, CA, United States).
Target prediction
Three online programs, TargetScan (http://www.targetscan.org), miRanda (http://www.microrna.org/microrna/home.do), and TarBase (http://diana.cslab.ece.ntua.gr/tarbase), were used in combination for predicting the target genes of miR-29a.
Luciferase reporter assay
HEK 293T cells (Institute of Shanghai Cell Biology, Shanghai, China) were cultured in DMEM medium (Gibco, United States) containing 10% FBS (Gibco, United States) in a humidified incubator at 37 °C under 5% CO2. HEK 293T cells were seeded in 24-well plates 24 h before transfection with 100 ng psiCHECKTM-2 vector (RiboBio, Guangzhou, China) containing the wild-type TNFRSF1A 3′UTR (designated TNFRSF1A 3′UTR-WT) or the TNFRSF1A mutant (designated TNFRSF1A 3′UTR -Mut) together with 50 nmol/L miR-29a miRNA mimic; a non-target control was used in the control group. Luciferase activity was measured 48 h after transfection. Lipofectamine 2000 transfection reagent (Invitrogen, United States) was used for co-transfection of RNA oligonucleotides and plasmids.
Quantitative real-time RT-PCR
Total RNA was isolated using Trizol (Invitrogen, United States) and then reverse-transcribed into cDNA with PrimeScript RT Master Mix (Takara, Japan) according to the manufacturer’s instructions. Quantitative real-time PCR was performed using the SYBR Premix Ex TaqTM kit (Takara, Japan). Specific primer sequences are listed as follows: 5’-GTGCTGTTGCCTCTGGTTATCT-3’ (forward) and 5’-GAGACAGGATGACTGAAGCGTG-3’ (reverse) for TNFRSF1A; 5’-TTCAACGGCACAGTCAAGG-3’ (forward) and 5’-CTCAGCACCAGCATCACC-3’ (reverse) for GAPDH. The primers for miR-29a and U6 were synthesized by Guangzhou RiboBio Co. Ltd. (China). Expression of TNFRSF1A, relative to GAPDH and expression of miR-29a, relative to U6, were determined using the 2-ΔΔCT method.
Statistical analysis
The results are expressed as mean ± SD from at least three separate experiments. Statistical analyses were performed using SPSS 13.0 software and comparisons were made using Student’s t-test and one-way ANOVA. P < 0.05 was considered statistically significant.
RESULTS
TUNEL assay
Apoptosis of pancreatic acinar cells was determined by TUNEL assay (Figure 1). The results of TUNEL assays showed that the apoptosis of pancreatic acinar cells increased significantly in the L-arginine group compared with that in the control group (P < 0.05).
Figure 1.
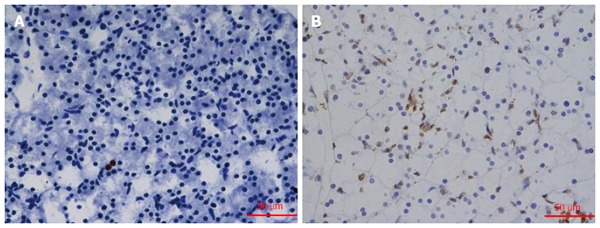
TUNEL staining of pancreatic tissue (× 400). TUNEL-positive cells displayed brown fluorescence. A: TUNEL staining was detected in control rats; B: The tissue of the L-arginine treated rats. The apoptosis increased significantly in Figure 1B.
miR-29a expression in the AEP model in vivo
miRNA-microarray analysis of the miRNAs in the rat pancreas was performed to compare the expression of miRNAs between the control and AEP groups. Numerous miRNAs were significantly different in the AEP group compared with the control group (Figure 2). The expression level of miR-29a was much higher in the AEP group compared with the control group (P < 0.01).
Figure 2.
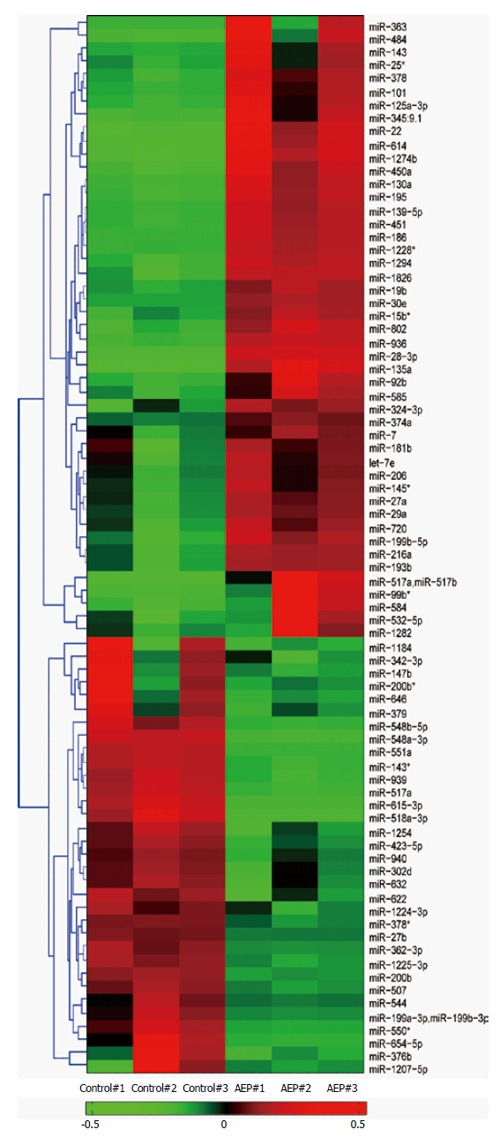
Hierarchically clustered heat map illustrating the changes in miRNA expression profiles between the acute edematous pancreatitis groups and control groups. The significantly expressed miRNA clusters were identified using the Student’s t-test. The red and green sections represent an increase and a decrease in miRNA expression, respectively, between control group and AEP group. The expression of miR-29a was significantly up-regulated in the AEP group compared with the control group. AEP: Acute edematous pancreatitis.
AR42J cell apoptosis in vitro
As shown in Figure 3A, the level of amylase in the experimental group increased significantly compared with that in the control group at 12 h after exposure of AR42J cells to the TNF-α (P = 0.042). Activated caspase 3 was detected by Western blot analysis as described. Caspase 3 expression increased obviously after the AR42J cells were exposed to TNF-α for 12 h. The apoptosis rate was significantly higher (6.26-fold) in the experimental group compared with that in the control group (P = 0.026; Figure 3C). These results demonstrated that the AEP model was successfully established in vitro.
Figure 3.
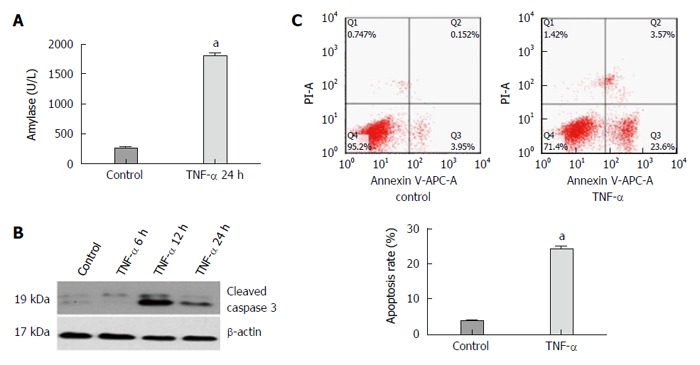
Expression of amylase, activated caspase 3 protein, apoptosis rate of AR42J cells and miR-29a level increase in the experimental group compared with the control group. A: The expression of amylase analysis in the supernatant; B: Western blot analysis of activated caspase 3 in AR42J cells; C: The apoptosis rate of AR42J cells after the treatment with TNF-α for 24 h. Date were obtained from three independent experiments in triplicate and are shown as the mean ± SD. aP < 0.05 vs control group.
Expression of miR-29a is increased in vitro
The expression level of miR-29a was confirmed by quantitative real-time RT-PCR. As shown in Figure 4, the level of miR-29a was significantly higher (1.49-fold) in the experimental group compared with that in the control group after AR42J cells were exposed to TNF-α for 3 h (P = 0.034).
Figure 4.
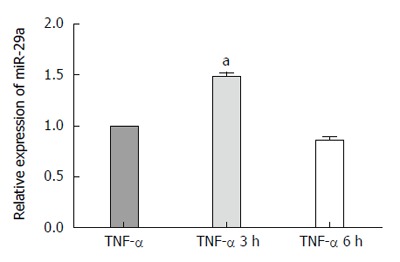
Quantitative real-time PCR analysis of miR-29a in AR42J cells at 3 h and 6 h. The expression of miR-29a was normalized to U6 expression using 2-ΔΔct. Data were obtained from three independent experiments in triplicate and are shown as the mean ± SD. aP < 0.05 vs control group.
miR-29a expression after lentiviral transfection
The lentiviral vector carrying the miR-29a mimic or AMO was transfected into the AR42J cells as described. After 72 h, the cells were evaluated for expression of green fluorescence under a fluorescence microscope. As shown in Figure 5A, most of the AR42J cells expressed green fluorescence, which indicated that the AR42J cells were successfully transfected by the lentivirus, from which protein was successfully expressed. miR-29a expression by lentivirus-transfected AR42J cells was analyzed by qRT-PCR. Cells transfected with the miR-2a mimic expressed significantly increased miR-29a levels (P = 0.018; Figure 5B), while cells transfected with the miR-29a AMO expressed lower miR-29a levels (P = 0.020; Figure 5B) compared with the vehicle groups.
Figure 5.
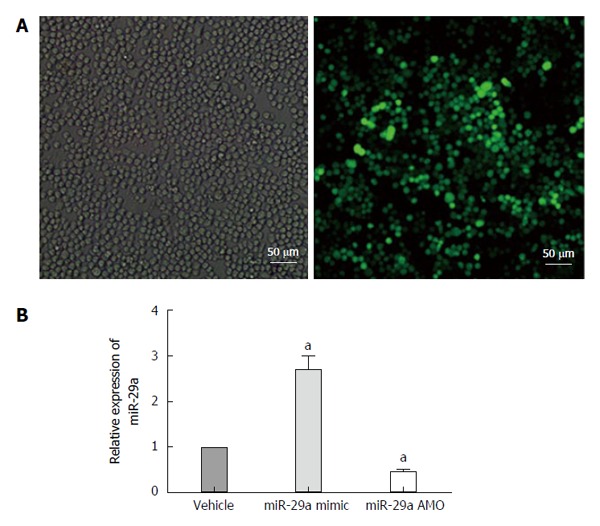
Lentiviral transfection and miRNA expression after transfection. A: Cells were infected with 50 MOI of lentivirus, and imaged 72 h post-transfection. Comparison of bright field filter view to FITC filter view (GFP-expression cells) for the same fields of cells showed about 90% infection efficiency by 72 h; B: Quantitative real-time PCR analysis of miR-29a expression in the AR42J cells after transfection. Date are shown as a ratio of mi-29a mimic and AMO groups to vehicle groups using the 2-ΔΔct. Data are representative of three independent experiments. aP < 0.05 vs vehicle group.
Induction of apoptosis after transfection
After lentiviral transfection, the AR42J cells were exposed to TNF-α and the level of amylase in the supernatant was detected as described, suggesting that the AEP model was established successfully (Figure 6A). To investigate the proapoptotic activity of miR-29a, we detected the rate of apoptosis and activated caspase 3 expression in AR42J cells after the AEP model was established in vitro. Interestingly, compared with the vehicle group, upregulation of miR-29a increased the expression of activated caspase 3, while the miR-29a AMO group showed a lower level of activated caspase 3 (Figure 6B). The apoptosis rate in the miR-29a mimic group (42.83 ± 1.25%) was significantly higher than that in the vehicle group (24.97 ± 0.15%) (P = 0.030; Figure 6C). In contrast, the apoptosis rate in the miR-29a AMO group (17.27 ± 1.36%) was significantly lower than that in the vehicle group (P = 0.025; Figure 6C).
Figure 6.
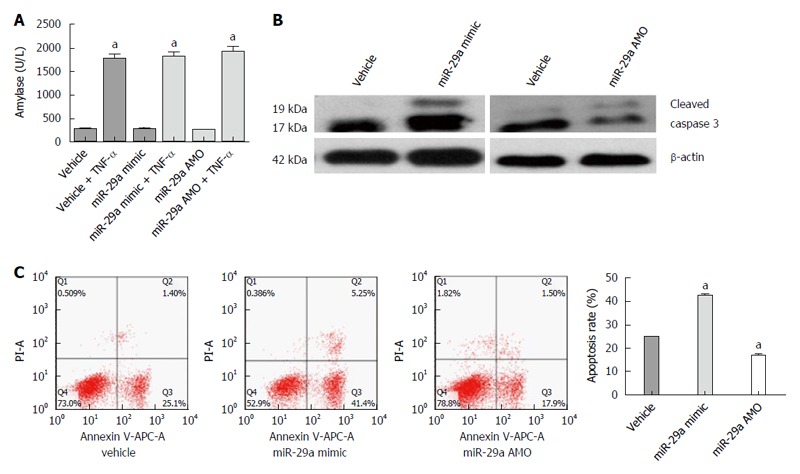
miR-29a promotes the apoptosis of the AR42J cells. A: The amylase analysis in the supernatant increased obviously; B: Western blot analysis of activated caspase 3 in AR42J cells; C: The apoptosis rate of AR42J cells was determined by FACS analysis. Data are representative of mean ± SD from three independent experiments performed in triplicate. aP < 0.05 vs control or vehicle group.
TNFRSF1A is regulated by miR-29a
By using three online programs (TargetScan, miRanda and TarBase), we predicted that TNFRSF1A was the target gene of miR-29a. As shown in Figure 7, the 3′UTR of TNFRSF1A contains a putative target site for miR-29a. To obtain direct evidence in support of this prediction, the 3′UTR of the rat TNFRSF1A gene was cloned into the XbaI-site of the pGL3-luciferase reporter vector, which was then used to test its capacity to serve as the direct functional target of miR-29a; the construct was designated pGL3-TNFRSF1A-WT. In parallel, another luciferase reporter construct was prepared in which the putative miR-29a targeting region was specifically mutated and predicted to abolish miR-29a binding; this construct was designated pGL-TNFRSF1A-Mut. Transient transfection of HEK293T cells with pGL-TNFRSF1A-wt and miR-29a led to a significant decrease in luciferase activity compared to that in the control group (P = 0.0018). The activity of the mutant reporter construct, however, was unaffected by co-transfection with miR-29a (P = 0.626).
Figure 7.
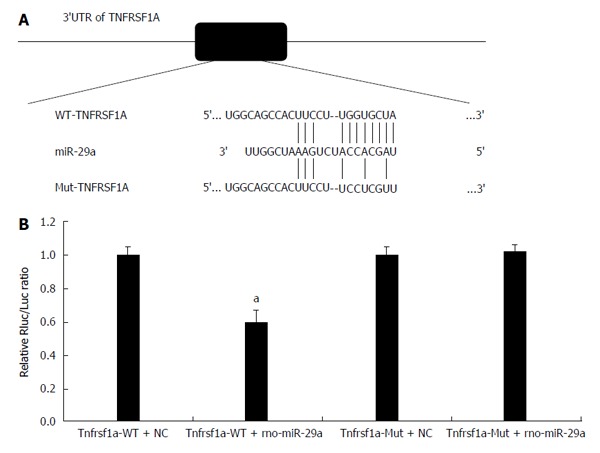
miR-29a targets TNFRSF1A. A: The predicted miR-29a binding sites within the 3’UTR of TNFRSF1A and mutant version generated by site mutagenesis are shown; B: Luciferase activity was determined 48 h after transfection. The ratio of normalized sensor to control luciferase activity is shown. Data are shown as the mean ± SD and were obtained from three independent experiments performed in triplicate (aP < 0.05 vs control miR-transfected cells).
Expression of TNFRSF1A after transfection
To examine the effect of miR-29a on TNFRSF1A expression, AR42J cells were transfected with a miR-29a mimic or an AMO. TNFRSF1A mRNA levels were analyzed by qRT-PCR. As shown in Figure 8A, the expression of TNFRSF1A was significantly higher (1.86-fold) in the miR-29a mimic group compared with the vehicle group (P = 0.022), while the expression was significantly lower (0.61-fold) in the miR-29a AMO group (P = 0.048). In parallel, we analyzed the expression of TNFR1 protein in the miR-29a mimic and AMO groups. The miR-29a mimic group expressed higher levels of TNFR1 protein compared with the vehicle group, while the levels were lower in the miR-29a AMO group (Figure 8B).
Figure 8.
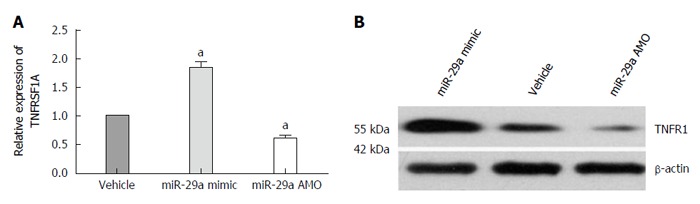
miR-29a promotes TNFRSF1A gene expression. A: Quantitative real-time RT-PCR analysis of TNFRSF1A expression in AR42J cells after transfection. Date are shown as a ratio of miR-29a mimic and AMO groups to vehicle group using the 2-ΔΔct. Data are representative of three independent experiments (aP < 0.05 vs vehicle group); B: Western blot analysis of TNFR1 protein in AR42J cells after transfection.
DISCUSSION
AP is a common clinical condition with high morbidity and mortality, and its incidence increases over recent years[16,17]. There are two patterns of pancreatic acinar cells death: necrosis and apoptosis. Apoptosis is a physiological and programmed form of cell death. The stereotypical and characteristic morphology of apoptosis includes cell shrinkage, retention of organelles and nuclear chromatin condensation, which occurs in response to stimuli[18]. The relationship between apoptosis and AP had been extensively investigated and it has been demonstrated that the severity of AP is inversely related to the rate of apoptosis and correlates directly with the extent of necrosis[19,20]. The feature of the AP model which is induced by L-arginine is reproducible and dose-dependent. In this study, we successfully induced AEP with L-arginine (150 mg/kg) in vivo and the result of TUNEL assays confirmed that apoptosis occurred in pancreatic acinar cells.
The AR42J cell line used in this study possessed the properties of exocrine digestive enzymes[21]. There are many advantages of AR42J cells for the investigation of signaling mechanism in the pancreas, such as the ease of cell line culture maintenance, high transduction efficiency and responsiveness to many agonists[22]. The AR42J cell line had been used extensively to establish the AP model in vitro. Chanthaphavong et al[23] demonstrated that TNF-α induced apoptosis of several cell lines. Furthermore, a recent study showed that TNF-α activated the NF-κB pathway and induced the expression of proinflammatory mediators in pancreatic acinar cells[24]. The results of our study demonstrated that rat recombinant TNF-α cytokine was used successfully to establish the AEP model in vitro.
miRNAs are short non-coding RNAs that play an important role in regulating gene expression in multiple cellular processes including apoptosis, metabolism, proliferation, differentiation and development[5]. To date, over 15000 mature miRNAs have been identified in 133 species[25]. In recent years, it has been demonstrated that miRNAs can upregulate the target genes. Vasudevan et al[10] showed that human miRNA369-3 directed association of the proteins with AREs to activate translation, and let-7 and the synthetic miRNA miRNAcxcr4 could induce up-regulation of target genes.
miR-29a has been extensively demonstrated to promote cell apoptosis via suppressing survival genes. Direct repression of CDC42 and p85α by miR-29a can result in the activation of p53 and induction of apoptosis[11]. MCL1, which encodes an anti-apoptotic Bcl-2 family protein, is also the target gene of miR-29a. By repressing MCL-1, miR-29a sensitizes cholangicarcinoma and ALT+ ALCL cells to apoptosis[14]. The results of our miRNA microarray and qRT-PCR analyses revealed that miR-29a is elevated in the AEP model in vivo and in vitro. miR-29a mimic and AMO were designed to investigate the function of miR-29a. This technology utilizes non-natural synthetic nucleic acids, which bind to the unique sequence of the target mRNAs in a gene-specific manner and has the same effects as the endogenous miRNAs[26,27]. Our result showed that AR42J cells showed a tendency to apoptosis in the miR-29 mimic group, while the apoptosis rate was significantly decreased in the miR-29a AMO group in the AEP model in vivo. TNFRSF1A, which encodes the TNFR1 protein, was predicted to be the target gene of miR-29a with the help of three online programs (TargetScan, miRanda and TarBase). TNF-R1, which is the major signaling receptor for TNF-α, is expressed on all cell types. Both soluble and membrane-bound forms of the cytokine can activate TNFR1. The binding of TNF-α to TNFR1 results in immediate NF-κB activation and subsequent apoptosis[3,4]. Our study demonstrated that miR-29a mediated up-regulation of the TNFRSF1A gene. AR42J cells transfected with the miR-29a mimic expressed higher levels of TNFRSF1A mRNA and TNFR1 protein, while the miR-29a AMO group exhibited the opposite trend. miR-29a promotes the expression of TNFRSF1A gene and its regulation mechanism is not binding to and degrading TNFRSF1A gene as the the result of luciferase assay in HEK 293T cells. We hypothesized that the combination of miR-29a and TNFRSF1A gene might guide some protein factors, which promote the transcription and translation of genes in pancreatic acinar cells, bind to the target gene. Further investigations need to be performed.
In summary, miR-29a is elevated significantly in AEP, indicating that miR-29a might promote pancreatic acinar cell apoptosis by enhancing expression of TNFRSF1A gene.
COMMENTS
Background
Apoptosis is a self-protective mechanism in acute pancreatitis. miRNAs are short non-coding RNAs involved in multiple cellular processes including development, proliferation, differentiation, metabolism and apoptosis.
Research frontiers
It has been reported that miR-29a plays an important role in apoptosis, but little is known about the effect of miR-29a on apoptosis in acute pancreatitis and its regulatory mechanisms.
Innovations and breakthroughs
In this study, the authors demonstrated that miR-29a is elevated significantly in acute edematous pancreatitis. The rate of apoptosis and TNFRSF1A expression increased significantly in AR42J cells following upregulation of miR-29a expression. miR-29a promotes pancreatic acinar cell apoptosis by enhancing expression of TNFRSF1A gene directly.
Applications
The study results suggest that miR-29a promotes pancreatic acinar cell apoptosis by enhancing expression of TNFRSF1A gene directly in acute pancreatitis, and these findings may provide a theoretical basis for the prevention and treatment of acute pancreatitis.
Terminology
MicroRNAs (miRNAs) are a set of 21- to 24- nucleotide(nt), endogenous, non-coding, regulatory RNA molecules that contribute to modulating the expression levels of specific proteins based on base pairing with their target mRNA molecules.
Peer-review
Authors demonstrated that miR-29a, which has been demonstrated to play an important role in apoptosis, is elevated significantly in acute edematous pancreatitis. The data are interesting and the experiments are well organized. Those findings give novel information on the pathogenesis of acute pancreatitis.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of People’s Hospital of Zhengzhou University.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Ethics Committee of People’s Hospital of Zhengzhou University.
Conflict-of-interest statement: We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any products, service and/ or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “miR-29a up-regulation in the AR42J cells contributes to apoptosis via targeting the TNFRSF1A gene”.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 9, 2016
First decision: February 18, 2016
Article in press: March 18, 2016
P- Reviewer: Shimizu Y, Soria F S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Ghezzi P, Cerami A. Tumor necrosis factor as a pharmacological target. Mol Biotechnol. 2005;31:239–244. doi: 10.1385/MB:31:3:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gukovskaya AS, Gukovsky I, Zaninovic V, Song M, Sandoval D, Gukovsky S, Pandol SJ. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853–1862. doi: 10.1172/JCI119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 4.Borghini S, Fiore M, Di Duca M, Caroli F, Finetti M, Santamaria G, Ferlito F, Bua F, Picco P, Obici L, et al. Candidate genes in patients with autoinflammatory syndrome resembling tumor necrosis factor receptor-associated periodic syndrome without mutations in the TNFRSF1A gene. J Rheumatol. 2011;38:1378–1384. doi: 10.3899/jrheum.101260. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, Enright AJ, Schier AF. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 8.Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci USA. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen CP, Bordeleau ME, Pelletier J, Sharp PA. Short RNAs repress translation after initiation in mammalian cells. Mol Cell. 2006;21:533–542. doi: 10.1016/j.molcel.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 11.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009;16:23–29. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- 12.Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y, Jia WH, Zhuang SM. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology. 2010;51:836–845. doi: 10.1002/hep.23380. [DOI] [PubMed] [Google Scholar]
- 13.Bargaje R, Gupta S, Sarkeshik A, Park R, Xu T, Sarkar M, Halimani M, Roy SS, Yates J, Pillai B. Identification of novel targets for miR-29a using miRNA proteomics. PLoS One. 2012;7:e43243. doi: 10.1371/journal.pone.0043243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desjobert C, Renalier MH, Bergalet J, Dejean E, Joseph N, Kruczynski A, Soulier J, Espinos E, Meggetto F, Cavaillé J, et al. MiR-29a down-regulation in ALK-positive anaplastic large cell lymphomas contributes to apoptosis blockade through MCL-1 overexpression. Blood. 2011;117:6627–6637. doi: 10.1182/blood-2010-09-301994. [DOI] [PubMed] [Google Scholar]
- 15.Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem. 2010;110:1155–1164. doi: 10.1002/jcb.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaz J, Akbarshahi H, Andersson R. Controversial role of toll-like receptors in acute pancreatitis. World J Gastroenterol. 2013;19:616–630. doi: 10.3748/wjg.v19.i5.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kylänpää L, Rakonczay Z, O’Reilly DA. The clinical course of acute pancreatitis and the inflammatory mediators that drive it. Int J Inflam. 2012;2012:360685. doi: 10.1155/2012/360685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mareninova OA, Sung KF, Hong P, Lugea A, Pandol SJ, Gukovsky I, Gukovskaya AS. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem. 2006;281:3370–3381. doi: 10.1074/jbc.M511276200. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2004;286:G189–G196. doi: 10.1152/ajpgi.00304.2003. [DOI] [PubMed] [Google Scholar]
- 20.Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology. 2004;4:567–586. doi: 10.1159/000082182. [DOI] [PubMed] [Google Scholar]
- 21.Sato H, Siow RC, Bartlett S, Taketani S, Ishii T, Bannai S, Mann GE. Expression of stress proteins heme oxygenase-1 and -2 in acute pancreatitis and pancreatic islet betaTC3 and acinar AR42J cells. FEBS Lett. 1997;405:219–223. doi: 10.1016/s0014-5793(97)00191-9. [DOI] [PubMed] [Google Scholar]
- 22.Twait E, Williard DE, Samuel I. Dominant negative p38 mitogen-activated protein kinase expression inhibits NF-kappaB activation in AR42J cells. Pancreatology. 2010;10:119–128. doi: 10.1159/000290656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chanthaphavong RS, Loughran PA, Lee TY, Scott MJ, Billiar TR. A role for cGMP in inducible nitric-oxide synthase (iNOS)-induced tumor necrosis factor (TNF) α-converting enzyme (TACE/ADAM17) activation, translocation, and TNF receptor 1 (TNFR1) shedding in hepatocytes. J Biol Chem. 2012;287:35887–35898. doi: 10.1074/jbc.M112.365171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR, Shimosegawa T, Pandol SJ. PKC-delta and -epsilon regulate NF-kappaB activation induced by cholecystokinin and TNF-alpha in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G582–G591. doi: 10.1152/ajpgi.00087.2004. [DOI] [PubMed] [Google Scholar]
- 25.Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z. The guideline of the design and validation of MiRNA mimics. Methods Mol Biol. 2011;676:211–223. doi: 10.1007/978-1-60761-863-8_15. [DOI] [PubMed] [Google Scholar]
- 27.Hammond SM. MicroRNA therapeutics: a new niche for antisense nucleic acids. Trends Mol Med. 2006;12:99–101. doi: 10.1016/j.molmed.2006.01.004. [DOI] [PubMed] [Google Scholar]


