Abstract
AIM: To improve the clinical diagnosis and recognition of hepatic epithelioid angiomyolipoma (HEAML).
METHODS: Four cases of primary HEAML were confirmed based on the pathology archive system in our hospital from January 2009 to November 2015. The general state, clinical symptoms, imaging manifestations, histological results and immunohistochemistry of these patients were retrospectively reviewed and analyzed. Studies of HEAML published in the last 15 years were collected from PubMed and MEDLINE to summarize the clinical symptoms, imaging characteristics, pathological features and management of HEAML.
RESULTS: Four cases of primary HEAML were retrieved from our archives. These included three female patients and one male patient, with a mean age of 41.8 ± 11.5 years (ranging from 31 to 56 years). The mean tumor size was 7.3 ± 5.5 cm (ranging from 3.0 to 15 cm). In the contrast-enhanced imaging, the tumor was obviously enhanced in the arterial phase, but enhanced continuously or exhibited a slow-density masse during the venous and delayed phases. Histologically, the tumors mainly consisted of epithelioid cells that comprised approximately 95% of the total neoplastic mass. Although no metastases occurred in our patients, pathological studies revealed necrosis, mitotic figures and liver invasion in two patients, which indicates aggressive behavior. Immunohistochemical staining revealed that human melanoma black 45 (HMB-45) and Melan-A were positive in 4 cases. We only identified 81 cases with primary HEAML, including our present patients, from 26 articles available from PubMed and MEDLINE. The majority of the papers were published as case reports. Only 5 (5/75, 6%) cases were associated with tuberous sclerosis complex (TSC). More than half (35/66) were discovered incidentally upon physical examination. Approximately 65% (22/34) of the patients were misdiagnosed with HCC or other tumors before surgery. Approximately 10% (8/81) of the patients with HEAML had recurrence or metastasis after surgery, which was a very high and alarming rate.
CONCLUSION: HEAML is a very rare primary hepatic tumor that is often misdiagnosed before surgery. Patients should be followed closely after surgery because of its malignant potential.
Keywords: Epithelioid angiomyolipoma, Imaging, Liver, Immunohistochemical staining, Human melanoma black 45
Core tip: Hepatic epithelioid angiomyolipoma (HEAML) is very rare tumor that is often misdiagnosed because of atypical symptoms and imaging manifestation. The diagnosis can be made based upon characteristic pathological and immunohistochemical criteria. Traditionally, it is thought to be a benign tumor and is therefore largely ignored. Thus, it is important to improve the recognition of HEAML. This is the first article to analyze the clinicopathological data and imaging results of HEAML comprehensively by combining our four patients with other cases reported worldwide. In fact, HEAML has malignant potential and should be followed closely after surgery.
INTRODUCTION
Angiomyolipoma (AML) is a kind of solid tumor that contains varying proportions of fat, smooth muscle cells, and blood vessels. Depending upon the dominant cell type, AML can be subcategorized into epithelioid, spindle, and intermediate forms. The former are perivascular epithelioid cells (PECs), including AML, lymphangiomyomatosis, and clear cell “sugar” tumor of the lungs[1]. Epithelioid angiomyolipoma (EAML) is a rare and special type of angiomyolipoma that most commonly occurs in the kidney, lung, heart, mediastinum, retroperitoneum, and vagina. Hepatic EAML (HEAML) was first reported by Yamasaki et al[2], and so far no more than 80 cases have been reported worldwide. HEAML, which was generally considered benign in the past, has malignant potential according to these reports[3]. There are no unique clinical symptoms of HEAML, which leads to confusion with other types of hepatic tumors easily[4]. Therefore, it has a very high rate of misdiagnosis. Here, we retrospectively reviewed the clinicopathological features of HEAML patients based on PubMed and MEDLINE data, including our four patients, who were finally diagnosed with HEAML through pathology and immunohistochemistry. The aim of our study was to improve the rate of accurate preoperative diagnosis and the degree of recognition of this rare tumor.
MATERIALS AND METHODS
Four cases of primary HEAML were confirmed based on the pathology archive system in our hospital from January 2009 to November 2015. The general state, clinical symptoms, imaging manifestations, histological results and immunohistochemistry of these patients were retrospectively reviewed. Important histological parameters including the diameter of the main tumor, the number of tumor nodules, cytological atypia, coagulative necrosis, mitotic count, liver invasion and vascular invasion were collected. Immunohistochemical staining for HMB-45, Melan-A, smooth muscle actin (SMA), HepPar-1, S-100, CK and fetoprotein (AFP) was repeated to verify the diagnosis. Follow-up data were obtained from the clinical records.
Articles about primary HEAML (excluding PEComa) were collected from January 2000 to November 2015 from PubMed and MEDLINE, and non-English publications were included. Clinical data were retrieved from the articles including sex, tumor size, case number, location of tumor, clinical presentation, preoperative diagnosis, association with TSC, treatment and prognosis.
SPSS (version 15.0 for Windows) software was used for statistical analyses. The results are presented as the mean ± SD or median.
RESULTS
The clinicopathological features of the 4 cases are summarized in Table 1. All 4 cases received surgical resection. The pathological and histological diagnosis of HEAML was verified in all 4 cases. Three patients were female and one patient was male. The mean age was 41.8 ± 11.5 years (ranging from 31 to 56 years). The male patient was rushed to the emergency room because of upper abdominal pain and fever, whereas the other 3 patients presented with liver masses on ultrasound but without symptoms or signs upon physical examination. The medical histories of all four patients were normal. Tests for the hepatitis C virus antibody and hepatitis B virus surface antigen were all negative, and there was no evidence of tuberous sclerosis. The results of the liver function test, routine blood test, serum AFP and carbohydrate antigen199 (CA199) were normal for our 4 patients with the exception of the male patient, who had elevated alkaline phosphatase; however, this patient tested negative for bacterial infection, and a fever caused by the tumor was considered first. All of the cases presented with intraparenchymal tumors, and the mean tumor diameter was 7.3 ± 5.5 cm (ranging from 3.0 to 15 cm). In the three female patients, the tumor presented as a single lesion. One tumor was located in the right lobe, while the other two tumors were located in the left lobe. In contrast, the male patient presented with three tumor lesions in bilateral liver lobes. Detailed imaging results were available for the four patients. Dynamic contrast-enhanced imaging showed two different presentations. The computed tomography scan of the male patient showed multiple low-density masses in the plain phase (Figure 1A), with the biggest lesion, measuring 15.0 cm × 12.0 cm × 10.0 cm in size, in the left hepatic lobe. In the arterial phase, strong contrast enhancement with low density in the center was observed (Figure 1B). The tumors had almost washed out the contrast agent except for a weak contrast-enhanced effect in the center that was perhaps induced by arterioportal venous shunting in the portal phase (Figure 1C). In the delayed phase, the tumors exhibited low density in comparison with the liver tissue (Figure 1D). The imaging of patients 2 and 3 showed similar manifestations. However, for patient 4, the foci had showed boundaries and high signal in T2WI (Figure 2A) but low signal in the plain phase (Figure 2B). A significantly and uniformly enhanced mass during the arterial phase was observed (Figure 2C). However, different from other cases, this mass was enhanced continuously during the venous and delayed phases (Figure 2D). Based on the available clinical findings, a preoperative diagnosis of hepatocellular adenoma (HCA) or HCC was made. None of the cases presented with extrahepatic metastasis, and they were diagnosed as primary liver tumors with no evidence of other organ involvement. Extended left lobectomy and laparoscopic hepatectomy were performed, respectively, because of the uncertain nature.
Table 1.
Clinicopathologic data of 4 cases of hepatic epithelioid angiomyolipoma
| Case | Sex/age | Size (cm) | Location of tumor in the liver | Clinical presentation | Fat | Details of imaging findings | Preoperative diagnosis |
Pathologic features |
|||||
| Satellite tumor | Cytologic atypia | Mitotic count | Coagulative necrosis | LI | VI | ||||||||
| 1 | M/34 | 15.0 | L | Abdominal pain and fever | No | Arterial phase enhancement and washout at portovenous phase (CT) | HCC | + | + | + | + | + | - |
| 2 | F/46 | 3.5 | L | None | No | Arterial phase enhancement and washout at portovenous phase (CT) | HCC | - | - | - | - | - | |
| 3 | F/31 | 3.0 | R | None | No | Arterial phase enhancement and washout at portovenous phase (CT) | HCC | - | + | + | + | + | - |
| 4 | F/56 | 7.5 | L | None | No | Arterial phase enhancement and no delayed washout at portovenous phase (MRI) | HCA | - | - | - | - | - | - |
LI: Liver invasion; VI: Vascular invasion; HCC: Hepatocellular cancer; HCA: Hepatocellular adenoma. Size of the main tumor nodule.
Figure 1.
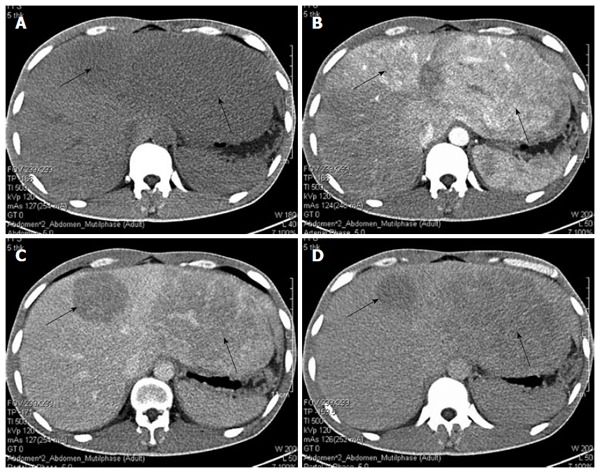
Computed tomography manifestation of the male patient. A: Low-density masses in the plain phase (black arrows); B: In the arterial phase, a strong contrast-enhancing effect with low-density in center was observed; C: In the portal phase, tumors had almost washed out the contrast agent, but a weak contrast-enhancing effect was sustained; D: Low-density masses in the delayed phase.
Figure 2.
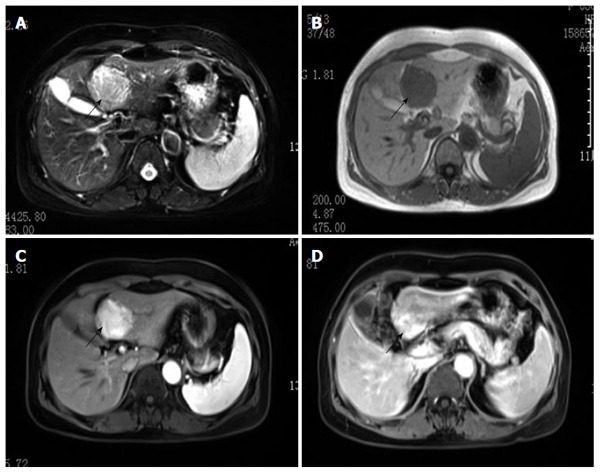
Magnetic resonance imaging manifestation of the female patient. The tumor had clear boundaries and showed high signal on T2WI (A, black arrow) and low signal in the plain phase (B); Hyperenhancement in the arterial phase (C) and delayed phase (D).
With respect to the pathological findings, gross examination revealed that the external surface of the mass was smooth and brownish in color. Sections revealed well-circumscribed, non-encapsulated tumors. The largest tumor was multiloculated with amorphous necrotic tissue and hemorrhagic fluid. The male patient had the other two satellite tumor nodules whose size was 1 cm and 2 cm, respectively. Microscopically, the tumor was comprised of sheets of large polygonal cells with abundant granular eosinophilic cytoplasm in areas with typical features of HEAML (Figure 3A). A radial arrangement around blood vessels was observed (Figure 3B). Microscopic features including cytologic atypia (Figure 3C), coagulative necrosis (Figure 3D) and increased mitotic count (Figure 3E) were observed in two cases, which indicates aggressive behavior. All tumors mainly consisted of epithelioid cells that comprised approximately 95% of the total neoplastic mass (Figure 3F). Tumors also contained a few spindle myoid cells, mature fat, and thick-walled vasculature. Immunohistochemical analysis revealed that 4 patients were positive for the melanocytic markers HMB-45 (Figure 4A), Melan-A (Figure 4B), SMA (Figure 4C) and VIM (Figure 4D), but negative for S-100 (Figure 4E), CK (Figure 4F), AFP (Figure 4G) and HepPar-1 (Figure 4H). The diagnosis was corrected to HEAML based on the above evidence. The median follow-up period was 30 months (ranging from 2 to 72 mo). All patients were alive with no evidence of recurrence at the time of review.
Figure 3.
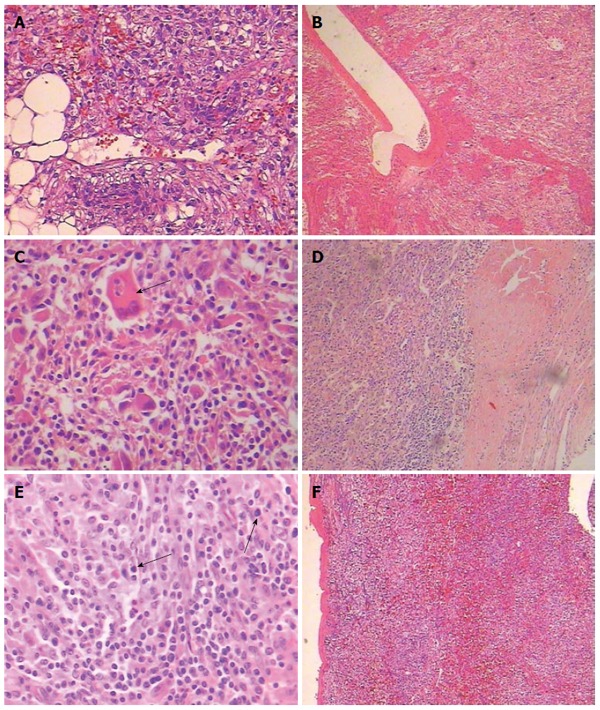
Histology of hepatic epithelioid angiomyolipoma. A: The tumor comprised of sheets of large polygonal cells with abundant granular eosinophilic cytoplasm (HE, × 200); B: The tumor cells were arranged radial around blood vessels (HE, × 40); Microscopic features signifying aggressive behavior were observed: C: Cytologic atypia (black arrow) (HE, × 200); D: Coagulative necrosis (HE, × 40); E: Increased mitotic count (black arrow) (HE, × 200); F: Tumors mainly consisted of epitheli¬oid cells that comprised approximately 95% of the total neoplastic mass (HE, × 40).
Figure 4.
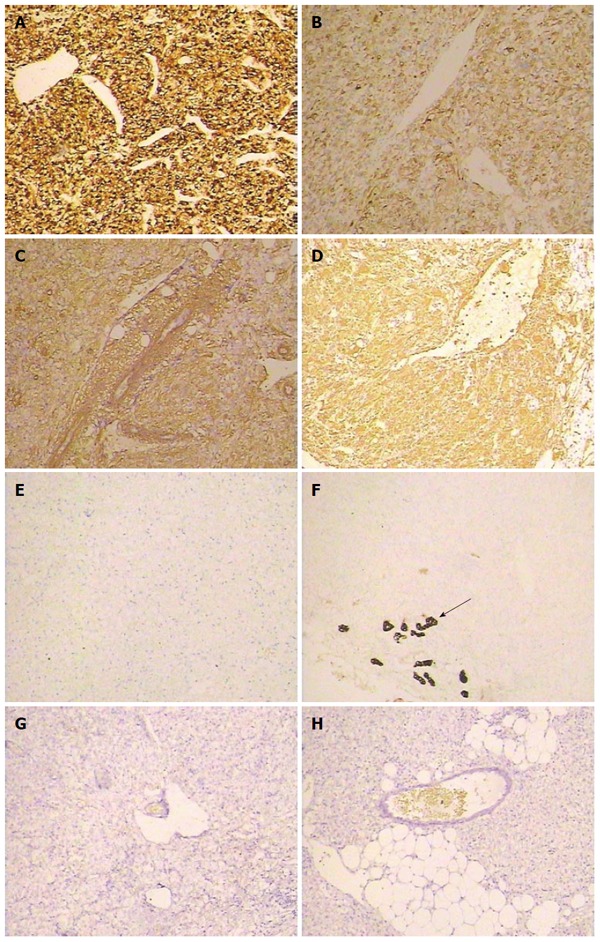
Immunohistochemical staining of hepatic epithelioid angiomyolipoma. The tumor cells were positive for melanocytic markers HMB-45 (A, × 40), Melan-A (B, × 40), SMA (C, × 40), and VIM (D, × 40), but negative for S-100 (E, × 40), CK (the arrow indicates the bile duct epithelium) (F, × 40), AFP (G, × 40), and Herpar-1 (H, × 40).
Our search of PubMed and MEDLINE confirmed a total of 81 cases (including our four cases) with primary HEAML from 26 articles[2,3,5-27] (Table 2). The majority of the cases were single case reports with the exception of 6 publications that reported more than 5 cases per article. Even so, those articles only focused on the pathological or imaging presentations. These limited results reflect the overall poor recognition of HEAML. Summarizing these available reports is necessary to further promote the diagnosis of HEAML.
Table 2.
Summary of available reports of hepatic epithelioid angiomyolipoma from PubMed and MEDLINE
| Year | Authors | Number (F/M) | Size (cm) | Location (L/R) | Clinical symptoms (n) | Diagnosis before surgery (n) | TSC (n) | Treatment | Recurrence/metastasis (n) |
| 2015 | Our study | 3/1 | 3.0-15.0 | 3/1 | Abdominal pain and fever (1) | HCC (3) | None | Surgery | None |
| No symptoms (3) | HCA (1) | ||||||||
| 2014 | Dai et al[24] | 3/2 | 2.5-7.0 | 2/3 | Abdominal pain (2) | HCC (4) | None | Surgery | None |
| None (3) | FNH (1) | ||||||||
| 2014 | Tajima et al[25] | 0/1 | 10.5 | 0/1 | Abdominal pain | Hepatic AML | None | Surgery | None |
| 2014 | Zhou et al[27] | 1/0 | 30.0 | 1/0 | Abdominal discomfort | NA | None | Surgery | None |
| 2014 | Xu et al[26] | 22/3 | 3.0-20.0 | 12/13 | Abdominal pain (10) | HCC or other tumor | 1 | Surgery | 2 |
| Abdominal distention (3) | |||||||||
| No symptoms (15) | |||||||||
| 2013 | Occhionorelli et al[22] | 1/0 | 8.0 | 1/0 | Abdominal pain | NA | NA | Surgery | None |
| 2013 | Zhao et al[23] | 3/2 | 0.6-9.7 | 2/3 | No symptoms | NA | None | Surgery | NA |
| 2013 | Saito et al[21] | 0/1 | 1.2 | 1/0 | No symptoms | HCC | None | Surgery | None |
| 2013 | Lo et al[20] | 5/0 | 1.2-25.0 | 1/4 | Abdominal distention (1) | HCC (1) | None | Surgery | None |
| Abdominal discomfort (1) | HCA (2) | ||||||||
| Epigastralgia (1) | AML | ||||||||
| None (2) | Liver tumor with uncertain nature | ||||||||
| 2013 | Ji et al[19] | 6/0 | 5.0-9.5 | 5/1 | None (5) | AML | None | Surgery and biopsy | NA |
| Abdominal pain (1) | |||||||||
| 2012 | Limaiem et al[18] | 0/1 | 8.2 | 1/0 | Abdominal pain | HCC | None | Surgery | None |
| 2012 | Agaimy et al[16] | 1/0 | 2.0 | 1/0 | Nausea | Metastatic adenocarcinoma or carcinoid | None | Surgery | None |
| 2012 | Xie et al[17] | 1/0 | 3.4 | 0/1 | Dyspnea | HCC | 1 | Biopsy | None |
| 2010 | Wen et al[15] | 0/1 | 4.1 | 1/0 | None | HCC | NA | Surgery | NA |
| 2009 | Leenman et al[13] | 1/0 | 6.0 | 1/0 | Abdominal pain | NA | NA | Surgery | NA |
| 2009 | Xu et al[14] | 10/0 | 1.5-10.0 | 6/6 | NA | NA | 1 | Surgery | 2 |
| 2009 | Alatasis et al[12] | 1/0 | 11.0 | Multiple | None | HCC | 1 | Biopsy | NA |
| 2008 | Deng et al[11] | 0/1 | 18.0 | 0/1 | Abdominal pain | AML | None | Surgery | 1 |
| 2007 | Khalbuss et al[10] | 1/0 | 12.0 | 1/0 | Abdominal pain | Adenoma or hamartoma | 1 | Surgery | None |
| 2006 | Rouquie et al[9] | 1/0 | 7.0 | 1/0 | None | NA | None | Surgery | None |
| 2004 | Tryggvason et al[8] | 1/0 | 6.0 | 1/0 | Abdominal pain | NA | None | Surgery | None |
| 2004 | Mizuguchi et al[7] | 1/0 | NA | 0/1 | None | AML | NA | Surgery | 1 |
| 2000 | Savastano et al[6] | 1/0 | 1.2 | 1/0 | NA | NA | NA | Surgery | None |
| 2000 | Flemming et al[5] | 3/0 | 1.0-20.0 | 2/2 | NA | HCC | None | Surgery (2) | 1 |
| Biopsy (1) | |||||||||
| 2000 | Yamasaki et al[2] | 1/0 | 2.0 | 0/1 | None | NA | None | Surgery | None |
| 2000 | Dalle et al[3] | 1/0 | 15.0 | 0/1 | Nausea and loss of appetite | HCC | NA | Biopsy | 1 |
F/M: Female/male; L/R: Left/right; TSC: Tuberous sclerosis complex; HCC: Hepatocellular cancer; HCA: Hepatocellular adenoma; FNH: Focal nodular hyperplasia; AML: Angiomyolipoma; NA: Not available.
DISCUSSION
In all series of HEAML, more women suffered from HEAML than men, at a ratio of 5:1. Although some patients with HEAML had atypical gastrointestinal symptoms, including abdominal pain or distension, discomfort and vomiting, more than half (35/66, 53%) of the cases were discovered incidentally upon physical examination based on the available data and were similar to three female patients in our research. One of our patients had a fever due to the central necrosis in the large size of the tumor. Rupture and hemorrhage were reported as the first symptoms in a few cases[22,25]. Abnormal liver function was frequently observed in patients with larger tumor size. Tumor size varied considerably, with lesions ranging from a few millimeters to as large as 30 cm having been reported. In general, bigger tumors have greater malignant potential. There was no difference regarding the location of the tumor in either the left or right lobe (44:39). Some reports showed that 26%-32% of AML patients had associated TSC[28,29]; however, interestingly, this ratio was less than 5% in China[26]. Among 75 patients who had valid data, only 5 (5/75, 6%) were associated with TSC, which was similar to the domestic study. All four cases in our report were solitary tumors without TSC. It was confirmed that approximately 50% of TSC patients had AML, but approximately 80% of the patients with AML were sporadic cases, which were not related to TSC.
HEAML always presents with less typical imaging manifestations, especially when smooth muscle and vascular components dominated in tumor as seen in the less fat types, which leads to confusion with other hepatic tumors easily and making a correct diagnosis very difficult before surgery. In dynamic enhanced CT or magnetic resonance imaging, multiple manifestations of HEAML were observed[21,23,24]. Most were obviously enhanced in the early arterial phase but showed low density in the portal venous phase and delayed phase, which has been confirmed by our research and by previous reports in the literature. Similar imaging signs could be observed in other hypervascular hepatic lesions, just like HCC. It is very difficult to discriminate between HEAML and HCC; 60% of patients with HEAML were misdiagnosed with HCC before surgery in 81 cases. According to the pathology, the so-called “false capsule” of HEAML, different from the real capsule of HCC, was just formed by the compression of the surrounding liver tissues, and had no histological structure in fact. Although the excretion of the contrast medium was relatively slow on the imaging in HEAML, this difference was not enough to distinguish the HCC or HEAML, especially in large tumors with central necrosis or hemorrhage. Focal nodular hyperplasia (FNH) always showed a central scar, which is a characteristic sign and could be an important basis in the differential diagnosis. In addition, FNH presented delayed enhancement on enhancement scans, which was also different from most HEAML. One patient in our study was diagnosed with hepatic adenoma during hospitalization because of enhancement in all phases on enhancement scans. Other fat rich tumors such as lipoma show almost no enhancement on imaging scans because of poor blood supply.
Pathology is the only definite diagnostic criteria. The gross observation of HEAML is not characteristic; most are solitary but multifocal tumors, such as cystic degeneration, have been reported in several case reports. Its morphological features under the microscope were revealed by Xu et al[26] by analyzing 25 cases of HEAML. It was characterized by marked cytological atypia; relatively rare mitotic figures; radial distribution of tumor cells around the thin-walled blood vessels or muscular vessels; and the presence of common multinucleated giant cells and large ganglion-like tumor cells. Although the presence of epithelioid cells is important for the diagnosis of EAML, the ratio is still under debate. Aydin et al[29] thought that it could be defined as an EAML if epithelioid cell components were greater than 10%, but most scholars believed that this standard was too low and should be as high as 50%, or even more than 90%. In our patients, the epithelial cells of EAML reached more than 95%. Apparently, more cases and follow-up results are needed to reach a unanimous conclusion.
Renal EAML has malignant potential and may metastasize to the lymph node, liver, lung, or bone in approximately one-third of cases. Poor outcome is considered when necrosis, mitotic figures, or a plastic nucleus are observed in pathological studies. Nese et al[30] showed that a carcinoma-like growth pattern and extrarenal extension and/or renal vein involvement were significant independent prognostic factors in a multivariate analysis. Brimo et al[31] summarized the pathological characteristics of renal EAML progression: (1) ≥ 2 mitotic figures per 10 high-power field; (2) atypical mitotic figures; (3) ≥ 70% of atypical epithelioid cells; and (4) necrosis. The presence of 3 or more features was highly predictive of malignant behavior. Faraji et al[32] also showed that marked cytological atypia and extensive tumor necrosis were related to the progression of EAML. Although HEAML is considered to be a benign tumor in several series of case reports, 8 cases of malignant HEAML have been reported[3,5,7,11,14,26], and there is a lack of evidence to determine whether the same prognostic parameters of renal EAML are applicable to HEAML.
As a member of the PEComa family, the immunological phenotype of HEAML has the characteristics of bidirectional differentiation of melanoma cells and smooth muscle cells. The tumor cell is positive for the expression of cell markers including MART-1, HMB45, Melan A and SMA, but negative for all epithelial markers including EMA and S-100. This is the most important criterion for the differential diagnosis of HEAML. Those tumor cells are also negative for typical markers of HCC, including AEP, HepPar 1 and canalicular polyclonal CEA. HEAML is sometimes misdiagnosed as malignant melanoma because of the differentiation of melanocytes; however, primary hepatic melanoma is very rare, and the tumor cells are positive for S-100 but negative for smooth muscle cell markers based on immunohistochemical analysis.
Based on previous reports, surgery is the only effective way to cure HEAML; however, biopsies were also used in a few cases with the risk of tumor growth and metastasis. In one case, the tumor volume increased from 760.8 cm3 to 1967.8 cm3, a 2.6-fold increase during the 102 d after HEAML was diagnosed by biopsy[7]. A metastatic mass in the right lower quadrant and portal vein thrombosis were suspected in another biopsy case. Approximately 10% (8/81) of patients with HEAML had recurrence or metastasis after surgery, which was a very high and alarming rate. Although no recurrence or metastasis occurred in our study, pathological studies still show necrosis, mitotic figures and liver invasion in two patients, which indicates aggressive behavior. To be vigilant, although the majority of AMLs always are considered as benign tumors for their biological behavior, the potential risk of malignant changes of HEAML needs to be noticed and should be followed rigorously after surgery.
COMMENTS
Background
Hepatic angiomyolipoma (HAML) is a rare benign tumor that belongs to a family of tumors that have collectively been called “PEComa”. As a specific form, the hepatic epithelioid angiomyolipoma (HEAML) has malignant potential and is often misdiagnosed because of atypical symptoms and imaging manifestations. Characteristic pathological and immunohistochemical features are the diagnostic criteria. Therefore, it is important to improve the recognition of HEAML.
Research frontiers
In the past15 years, there have been scattered reports of HEAML, and the majority of those were case reports. In addition, those articles only focused on the pathology or imaging aspects, respectively. Therefore, more cases need to be collected and summarized, and more attention should be paid to this disease.
Innovations and breakthroughs
This is the first study to summary the clinical symptoms, imaging manifestations and pathological features of HEAML by retrospectively analyzing 81 cases from 26 articles, including our four patients, to improve the recognition of HEAML and reduce the misdiagnosis of this disease.
Applications
By understanding the characteristics of the clinical symptoms, imaging manifestations and pathological features of HEAML, this study could help us to improve confidence in the diagnosis of HEAML, especially in the differential diagnosis with other solid tumors in the liver, such as hepatic cellular cancer and adenoma.
Peer-review
In this manuscript, the authors analyze and summarize the clinical symptoms, imaging manifestations and pathological features of patients with HEAML by collecting all 81 cases to improve the rate of accurate diagnosis. No similar report has been published before. It will be helpful for scholars to obtain knowledge of this disease.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Zhejiang Provincial People’s Hospital Institutional Review Board.
Informed consent statement: The study participant provided informed written consent for this study.
Conflict-of-interest statement: We declare that we have no financial or personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “Primary hepatic epithelioid angiomyolipoma: A malignant potential tumor which should be recognized”.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at zcw1989@sina.com. Participants gave informed consent for data sharing.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 22, 2016
First decision: March 7, 2016
Article in press: March 30, 2016
P- Reviewer: Berkane S, Leber B S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48:75–82. doi: 10.1111/j.1365-2559.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 2.Yamasaki S, Tanaka S, Fujii H, Matsumoto T, Okuda C, Watanabe G, Suda K. Monotypic epithelioid angiomyolipoma of the liver. Histopathology. 2000;36:451–456. doi: 10.1046/j.1365-2559.2000.00848.x. [DOI] [PubMed] [Google Scholar]
- 3.Dalle I, Sciot R, de Vos R, Aerts R, van Damme B, Desmet V, Roskams T. Malignant angiomyolipoma of the liver: a hitherto unreported variant. Histopathology. 2000;36:443–450. doi: 10.1046/j.1365-2559.2000.00891.x. [DOI] [PubMed] [Google Scholar]
- 4.Park HK, Zhang S, Wong MK, Kim HL. Clinical presentation of epithelioid angiomyolipoma. Int J Urol. 2007;14:21–25. doi: 10.1111/j.1442-2042.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- 5.Flemming P, Lehmann U, Becker T, Klempnauer J, Kreipe H. Common and epithelioid variants of hepatic angiomyolipoma exhibit clonal growth and share a distinctive immunophenotype. Hepatology. 2000;32:213–217. doi: 10.1053/jhep.2000.9142. [DOI] [PubMed] [Google Scholar]
- 6.Savastano S, Piotto M, Mencarelli R, Spanio P, Rubaltelli L. [A monotypic variant of hepatic angiomyolipoma completely composed of perivascular epithelioid cells. A case] Radiol Med. 2000;100:79–81. [PubMed] [Google Scholar]
- 7.Mizuguchi T, Katsuramaki T, Nobuoka T, Nishikage A, Oshima H, Kawasaki H, Kimura S, Satoh M, Hirata K. Growth of hepatic angiomyolipoma indicating malignant potential. J Gastroenterol Hepatol. 2004;19:1328–1330. doi: 10.1111/j.1440-1746.2004.03583.x. [DOI] [PubMed] [Google Scholar]
- 8.Tryggvason G, Blöndal S, Goldin RD, Albrechtsen J, Björnsson J, Jónasson JG. Epithelioid angiomyolipoma of the liver: case report and review of the literature. APMIS. 2004;112:612–616. doi: 10.1111/j.1600-0463.2004.apm1120909.x. [DOI] [PubMed] [Google Scholar]
- 9.Rouquie D, Eggenspieler P, Algayres JP, Béchade D, Camparo P, Baranger B. [Malignant-like angiomyolipoma of the liver: report of one case and review of the literature] Ann Chir. 2006;131:338–341. doi: 10.1016/j.anchir.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Khalbuss WE, Fischer G, Bazooband A. Imprint cytology of epithelioid hepatic angiomyolipoma: mimicry of hepatocellular carcinoma. Acta Cytol. 2007;51:670–672. [PubMed] [Google Scholar]
- 11.Deng YF, Lin Q, Zhang SH, Ling YM, He JK, Chen XF. Malignant angiomyolipoma in the liver: a case report with pathological and molecular analysis. Pathol Res Pract. 2008;204:911–918. doi: 10.1016/j.prp.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Alatassi H, Sahoo S. Epithelioid angiomyolipoma of the liver with striking giant cell component: fine-needle aspiration biopsy findings of a rare neoplasm. Diagn Cytopathol. 2009;37:192–194. doi: 10.1002/dc.20979. [DOI] [PubMed] [Google Scholar]
- 13.Leenman EE, Mukhina MS, Nasyrov AR. [Monophasic angiomyolipoma (PEComa) of the liver] Arkh Patol. 2009;71:44–46. [PubMed] [Google Scholar]
- 14.Xu PJ, Shan Y, Yan FH, Ji Y, Ding Y, Zhou ML. Epithelioid angiomyolipoma of the liver: cross-sectional imaging findings of 10 immunohistochemically-verified cases. World J Gastroenterol. 2009;15:4576–4581. doi: 10.3748/wjg.15.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen MC, Jan YJ, Li MC, Wang J, Lin A. Monotypic epithelioid angiomyolipoma of the liver with TFE3 expression. Pathology. 2010;42:300–302. doi: 10.3109/00313021003631254. [DOI] [PubMed] [Google Scholar]
- 16.Agaimy A, Vassos N, Croner RS, Strobel D, Lell M. Hepatic angiomyolipoma: a series of six cases with emphasis on pathological-radiological correlations and unusual variants diagnosed by core needle biopsy. Int J Clin Exp Pathol. 2012;5:512–521. [PMC free article] [PubMed] [Google Scholar]
- 17.Xie L, Jessurun J, Manivel JC, Pambuccian SE. Hepatic epithelioid angiomyolipoma with trabecular growth pattern: a mimic of hepatocellular carcinoma on fine needle aspiration cytology. Diagn Cytopathol. 2012;40:639–650. doi: 10.1002/dc.21703. [DOI] [PubMed] [Google Scholar]
- 18.Limaiem F, Korbi S, Lahmar A, Bouraoui S, Aloui S, Jedidi S, Miloudi N, Mzabi-Regaya S. A misleading hepatic tumour: epithelioid angiomyolipoma. Acta Gastroenterol Belg. 2012;75:443–445. [PubMed] [Google Scholar]
- 19.Ji JS, Lu CY, Wang ZF, Xu M, Song JJ. Epithelioid angiomyolipoma of the liver: CT and MRI features. Abdom Imaging. 2013;38:309–314. doi: 10.1007/s00261-012-9911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo RC. Epithelioid angiomyolipoma of the liver: a clinicopathologic study of 5 cases. Ann Diagn Pathol. 2013;17:412–415. doi: 10.1016/j.anndiagpath.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Saito Y, Shimada M, Utsunomiya T, Morine Y, Imura S, Ikemoto T, Mori H, Hanaoka J, Sugimoto K, Iwahashi S, et al. Hepatic epithelioid angiomyolipoma with arterioportal venous shunting mimicking hepatocellular carcinoma: report of a case. J Med Invest. 2013;60:262–266. doi: 10.2152/jmi.60.262. [DOI] [PubMed] [Google Scholar]
- 22.Occhionorelli S, Dellachiesa L, Stano R, Cappellari L, Tartarini D, Severi S, Palini GM, Pansini GC, Vasquez G. Spontaneous rupture of a hepatic epithelioid angiomyolipoma: damage control surgery. A case report. G Chir. 2013;34:320–322. [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Ouyang H, Wang X, Ye F, Liang J. MRI manifestations of liver epithelioid and nonepithelioid angiomyolipoma. J Magn Reson Imaging. 2014;39:1502–1508. doi: 10.1002/jmri.24291. [DOI] [PubMed] [Google Scholar]
- 24.Dai CL, Xue LP, Li YM. Multi-slice computed tomography manifestations of hepatic epithelioid angiomyolipoma. World J Gastroenterol. 2014;20:3364–3368. doi: 10.3748/wjg.v20.i12.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tajima S, Suzuki A, Suzumura K. Ruptured hepatic epithelioid angiomyolipoma: a case report and literature review. Case Rep Oncol. 2014;7:369–375. doi: 10.1159/000363690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Wang H, Zhang X, Li G. [Hepatic epithelioid angiomyolipoma: a clinicopathologic analysis of 25 cases] Zhonghua Bing Li Xue Zazhi. 2014;43:685–689. [PubMed] [Google Scholar]
- 27.Zhou Y, Chen F, Jiang W, Meng Q, Wang F. Hepatic epithelioid angiomyolipoma with an unusual pathologic appearance: expanding the morphologic spectrum. Int J Clin Exp Pathol. 2014;7:6364–6369. [PMC free article] [PubMed] [Google Scholar]
- 28.Parfitt JR, Bella AJ, Izawa JI, Wehrli BM. Malignant neoplasm of perivascular epithelioid cells of the liver. Arch Pathol Lab Med. 2006;130:1219–1222. doi: 10.5858/2006-130-1219-MNOPEC. [DOI] [PubMed] [Google Scholar]
- 29.Aydin H, Magi-Galluzzi C, Lane BR, Sercia L, Lopez JI, Rini BI, Zhou M. Renal angiomyolipoma: clinicopathologic study of 194 cases with emphasis on the epithelioid histology and tuberous sclerosis association. Am J Surg Pathol. 2009;33:289–297. doi: 10.1097/PAS.0b013e31817ed7a6. [DOI] [PubMed] [Google Scholar]
- 30.Nese N, Martignoni G, Fletcher CD, Gupta R, Pan CC, Kim H, Ro JY, Hwang IS, Sato K, Bonetti F, et al. Pure epithelioid PEComas (so-called epithelioid angiomyolipoma) of the kidney: A clinicopathologic study of 41 cases: detailed assessment of morphology and risk stratification. Am J Surg Pathol. 2011;35:161–176. doi: 10.1097/PAS.0b013e318206f2a9. [DOI] [PubMed] [Google Scholar]
- 31.Brimo F, Robinson B, Guo C, Zhou M, Latour M, Epstein JI. Renal epithelioid angiomyolipoma with atypia: a series of 40 cases with emphasis on clinicopathologic prognostic indicators of malignancy. Am J Surg Pathol. 2010;34:715–722. doi: 10.1097/PAS.0b013e3181d90370. [DOI] [PubMed] [Google Scholar]
- 32.Faraji H, Nguyen BN, Mai KT. Renal epithelioid angiomyolipoma: a study of six cases and a meta-analytic study. Development of criteria for screening the entity with prognostic significance. Histopathology. 2009;55:525–534. doi: 10.1111/j.1365-2559.2009.03420.x. [DOI] [PubMed] [Google Scholar]


