Abstract
We have synthesized and characterized a novel phosphorothioate CpG oligodeoxynucleotide (CpG ODN)-Ficoll conjugated nanoparticulate adjuvant, termed DV230-Ficoll. This adjuvant was constructed from an amine-functionalized-Ficoll, a heterobifunctional linker (succinimidyl-[(N-maleimidopropionamido)-hexaethylene glycol] ester) and the CpG-ODN DV230. Herein, we describe the evaluation of the purity and reactivity of linkers of different lengths for CpG-ODN-Ficoll conjugation, optimization of linker coupling, and conjugation of thiol-functionalized CpG to maleimide-functionalized Ficoll and process scale-up. Physicochemical characterization of independently produced lots of DV230-Ficoll reveal a bioconjugate with a particle size of approximately 50 nm and covalent attachment of more than 100 molecules of CpG per Ficoll. Solutions of purified DV230-Ficoll were stable for at least 12 months at frozen and refrigerated temperatures and stability was further enhanced in lyophilized form. Compared to nonconjugated monomeric DV230, the DV230-Ficoll conjugate demonstrated improved in vitro potency for induction of IFN-α from human peripheral blood mononuclear cells and induced higher titer neutralizing antibody responses against coadministered anthrax recombinant protective antigen in mice. The processes described here establish a reproducible and robust process for the synthesis of a novel, size-controlled, and stable CpG-ODN nanoparticle adjuvant suitable for manufacture and use in vaccines.
Introduction
The discovery and characterization of Toll-like receptors (TLRs) and their ligands have played a critical role in our understanding of the interaction between innate and acquired immunity1,2 and have been a major driving force in the development of new vaccine adjuvants over the last two decades.3,4 The CpG motif containing oligodeoxynucleotide (CpG-ODN) agonists of Toll-like receptor 9 (TLR9) are an extremely well-characterized family of molecules5 with an extensive history of clinical study as both vaccine adjuvants6,7 and cancer immunotherapeutic agents7 when administered in soluble forms. Immunologic adjuvants are substances that generate stronger and/or long-lasting immune responses against co-inoculated antigens and continue to be essential for use with a wide array of purified and recombinant antigens that are otherwise poorly immunogenic.8
Heplisav B, a prophylactic vaccine currently in late-stage clinical development, combining the Hepatitis B surface antigen (HBsAg) with the CpG adjuvant 1018 ISS, is one such example of a product containing a TLR9 agonist. Heplisav B has demonstrated superiority over Engerix B, a currently licensed HBV vaccine combining HBsAg with the long-approved adjuvant aluminum hydroxide in Phase III clinical trials.9,10
Since the distribution of many molecules injected parenterally, including CpG-ODN, is significantly influenced by molecular size,11 we evaluated a CpG-ODN conjugated to a cross-linked polysaccharide, thereby significantly increasing its size and changing other properties such as shape, charge, stability, and adjuvant potency. We and others have shown that parenterally delivered CpG-ODNs, rapidly distribute systemically away from the site of injection and therefore likely not the most efficient means of administering CpG-ODN adjuvants, thus requiring higher and more expensive doses and the consequent possibility of increased incidence of adverse events in vaccines.12 Accordingly, delivering CpG-ODN in a nanoparticulate form is an attractive approach both for enhancing CpG-ODN adjuvant activity and for reducing potential systemic toxicity effects13,14 by increasing retention of the adjuvant at injection-site draining lymph nodes.15 In recent years, a number of approaches combining CpG-ODN with nanoparticulate systems have been described. These include the IC31 peptide/CpG coacervate,16 CpG covalently conjugated to polypropylene sulfide nanospheres,17 cationic PLG and emulsion/CpG nanoparticles, CAPO4 nanoparticles,18 CpG-loaded VLP,19 and CpG-liposomes.20
Herein, we describe the synthesis and formulation of a CpG-ODN-Ficoll nanoparticle adjuvant (DV230-Ficoll) based upon covalent conjugation of a sulfhydryl terminated CpG sequence, with B-class CpG activity21 to the cross-linked sucrose polymer Ficoll,22 which has been used previously as a carrier for polyvalent antigen constructs.23 Ficoll (Ficoll PM400) has many desirable features as a carrier for CpG adjuvants including size (a key determinant for delivery to the macrophage phagocytic system), high aqueous solubility, capacity for diverse surface chemistry, expected low immunogenicity and toxicity, and ease of lyophilization. Notably, Ficoll has also been previously administered to humans.24,25
This adjuvant was developed with the intent of maximizing retention of CpG-ODN at the injection site and draining lymph nodes, and for enhancing induction of innate immune responses. The design elements include the following: Ficoll with size distribution in the range identified for optimal delivery to lymph nodes and uptake by dendritic cells,21 well characterized yet flexible conjugation chemistry, a phosphorothioate oligonucleotide with active mouse and human CpG motifs, a nonimmunogenic scaffold (Ficoll), and long-term stability either as a liquid/frozen formulation or as an easily reconstituted lyophilized trehalose cake. Such a CpG-ODN-Ficoll adjuvant will be useful for human administration in both infectious disease and cancer applications.
Results and Discussion
Synthesis of DV230-Ficoll
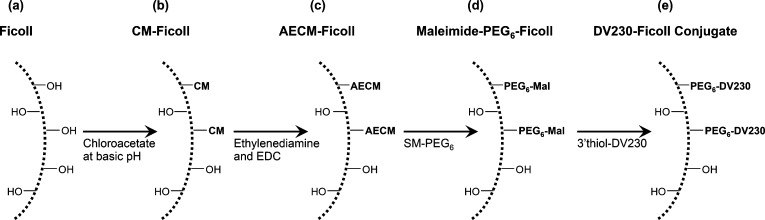
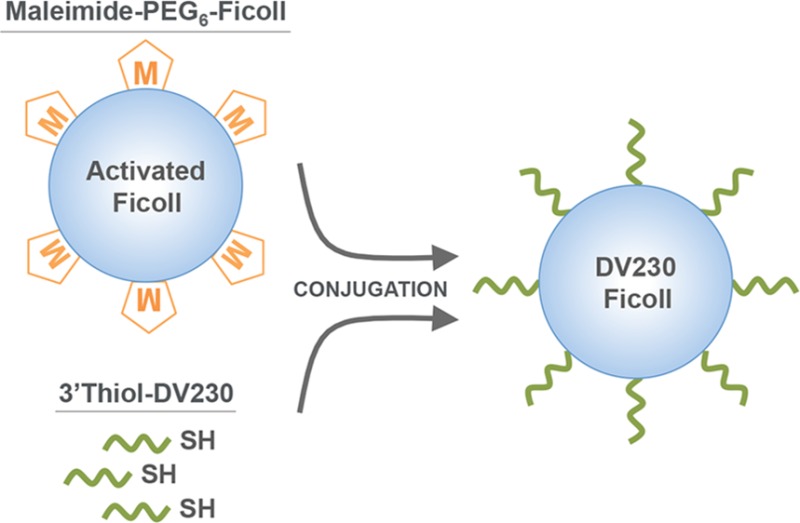
Starting with a solution of Ficoll PM400, a synthetic, neutral, and highly cross-linked polymer of sucrose with an average molecular weight of 400,000 Da (Da) (Figure 1a), carboxymethylated (CM)-Ficoll (Figure 1b), and aminoethylcarbamylmethylated, (AECM)-Ficoll (Figure 1c) intermediates were synthesized following the method of Inman23 (Figure 1). Subsequently, AECM-Ficoll was reacted with a defined amount of a heterobifunctional cross-linker, SM-PEG6, containing a maleimide group (thiol reactive) at one end, and N-hydroxysuccinimidyl (NHS) group (amine reactive) at the other end yielding multifunctionalized maleimide-PEG6-Ficoll (Figure 1d). This mal-PEG6-Ficoll intermediate is reacted with thiol-modified CpG (3′thiol-DV230) yielding DV230-Ficoll, a covalent conjugate, with multiple copies of CpG linked to Ficoll (Figure 1e).
Figure 1.
Synthetic scheme for production of DV230-Ficoll, a TLR9 ligand–polysaccharide conjugate. Ficoll (a) is chemically modified, yielding a series of three Ficoll-intermediates (b, c, and d) resulting in mal-PEG6-Ficoll which is conjugated with 3′ thiol-DV230 (CpG), yielding DV230-Ficoll (e).
The CpG sequence termed DV230, specifically 3′disulfide-DV230, was manufactured by solid phase synthesis using phosphoramidite chemistry with oxidative sulfurization, and was purified and isolated according to the manufacturer’s protocols (see Experimental Procedures). DV230 is a 21-monomer-unit phosphorothioate oligonucleotide with a C6 disulfide linker attached to the 3′ end to enable conjugation to various chemical moieties. DV230 is composed of two heptameric CpG motifs mediating human activity and one heptameric motif mediating mouse activity, each separated by a hexaethylene glycol (HEG) spacer.21 The 3′disulfide-DV230 is reduced with TCEP yielding 3′thiol-DV230 and purified by G25 desalting. This 3′thiol-DV230 intermediate typically isolated at a concentration of 11–12 mg/mL and with a thiol:CpG oligo ratio of ∼1.0 (by Ellman’s assay), is the conjugation partner for mal-PEG6-Ficoll. Finally, the DV230-Ficoll production process uses four separate tangential flow filtration (TFF) steps to separate Ficoll intermediates (Figure 1b,c,d) from added reagents and reaction byproducts. TFF is also a final step to purify DV230-Ficoll (Figure 1e). The process also includes chemical capping and quenching steps.
Preparation of Carboxymethylated-Ficoll (CM-Ficoll) and Aminoethylcarbamylmethylated, (AECM)-Ficoll Intermediates
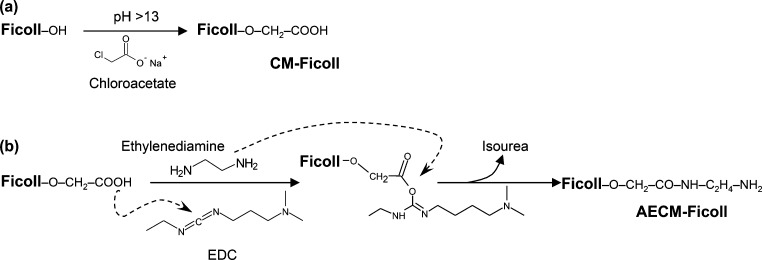
The scheme for synthesizing CM-Ficoll and AECM-Ficoll intermediates is shown in Figure 2.
Figure 2.
Derivatization of Ficoll with aminoethyl groups. (a) Ficoll is reacted with chloroacetate sodium salt at basic pH at 40 °C for 2.5 h, followed by rapid cooling and pH neutralization with chloroacetic acid in order to stop the derivatization reaction, resulting in formation of CM-Ficoll. (b) Excess ethylenediamine is added to CM-Ficoll at pH 4.5, followed by slow addition of excess of EDC, leading to formation of a short-lived but highly reactive o-acylisourea intermediate. This reactive ester reacts with ethylenediamine to form a stable amide bond leading to formation of AECM-Ficoll, with release of isourea as byproduct.
The production and characterization of three independently produced multigram scale lots of AECM-Ficoll are summarized (Table 1). Briefly, Ficoll was reacted with sodium chloroacetate under basic conditions yielding CM-Ficoll (Figure 2a). Since Ficoll PM400 is a polymer with a broad molecular weight distribution (∼300–500 kDa), a 100 kDa MWCO ultrafiltration (UF) membrane was used for diafiltration (by TFF) to remove small molecules and to isolate a defined size range (>100 kDa) of CM-Ficoll. In order to achieve a consistent concentration (∼30 mg/mL) and step yield for this intermediate, it was essential to run this step in the presence of 0.2 M sodium chloride (NaCl).
Table 1. Characterization of AECM-Ficoll Intermediates Isolated by Tangential Flow Filtration.
| step | lot 1 | lot 2 | lot 3 | average (n = 3) |
|---|---|---|---|---|
| CM-Ficoll intermediate concentration (mg/mL) | 28.8 | 31.0 | 31.2 | 30.3 |
| amount of CM-Ficoll used for reaction (g) with Ethylenediamine and EDC | 6.5 | 7.0 | 7.5 | 7.0 |
| ACEM-Ficoll intermediate concentration (mg/mL) | 31.3 | 32.0 | 36.4 | 33.2 |
| AECM-Ficoll intermediate recovered (g) | 5.4 | 5.9 | 6.9 | 6.1 |
| calculated Amine/Ficoll molar ratios for AECM-Ficoll intermediate | 221.0 | 218.0 | 224.0 | 221.0 |
AECM-Ficoll was produced by reacting CM-Ficoll with an excess of ethylenediamine and a water-soluble carbodiimide (EDC) (Figure 2b). This intermediate was also isolated by TFF into 100 mM sodium phosphate, 150 mM sodium chloride, pH 7.5. The Ficoll and amine contents of the AECM-Ficoll were determined and averaged 33.2 mg/mL and 18.4 mM, respectively. On average, there were 221 mol of aminoethyl “reactive amines” per mole of Ficoll. These results demonstrate consistent production of multigram quantities (average = 6.1 g) of AECM-Ficoll with an average step yield of 87% (Table 1). AECM-Ficoll intermediates can be used immediately or stored at −80 °C until needed for coupling with various linkers (e.g., SM-PEG6).
Assessment of Heterobifunctional Cross-Linkers: SM-PEGn for Coupling to AECM-Ficoll
Our laboratory has previously described using sulfo-SMCC, a heterobifunctional linker containing a cyclohexyl moiety, to conjugate a thiol-functionalized CpG-ODN to maleimide-activated-Ficoll and characterized the physical and biological properties of the resultant CpG-Ficoll bioconjugates.21 The resultant maleimide-functionalized Ficoll from this earlier work displayed turbidity upon freezing and thawing suggesting limited solubility (data not shown). As a result, in the work described here, we shifted toward the more hydrophilic polyethylene glycol (PEG) containing heterobifunctional linkers, SM-PEGn. Hydrophilic PEG of increasing length were predicted to improve the aqueous solubility of mal-PEGn-Ficoll intermediates allowing for more consistent downstream processing, i.e., conjugation with thiol-modified CpGs. Results are summarized in Table 2. SM-PEGn linkers with shorter-chain-length PEGs, i.e., SM-PEG6 and SM-PEG24 were viscous and pasty and could not be accurately weighed out. These linkers were dissolved with anhydrous dimethyl sulfoxide (DMSO) directly in the vessel supplied. The SM-PEG6 and SM-PEG24 linkers were 94–100% pure by both RP-HPLC and NMR. Longer-chain-length PEG linkers such as SM-PEG45 and SM-PEG70 were easily handled powders and of high purity by RP-HPLC and NMR. Mass spectrometry indicated that PEG6 and PEG24 linkers had near-perfect agreement between their theoretical molecular weights (MWs), as reported by the supplier, and those subsequently confirmed by an external contract laboratory. These discrete (dPEG) linkers (PEG6 and PEG24) were monodisperse. In contrast, longer PEG chain linkers (PEG45 and PEG70) were more heterogeneous and, as a result, differed somewhat from their theoretical MWs. Also, in our hands, SM-PEG6 and PEG24 linkers from the same supplier (supplier A) had near-fully reactive maleimides. In contrast, the SM-PEG45 linker from a different supplier (supplier B) contained only 64% reactive maleimide, likely due to hydrolysis. A SM-PEG70 linker from yet another supplier (supplier C) was 97% pure by qNMR containing 87% reactive maleimide. The estimated release rates or half-lives (T50) of the NHS ester end of these linkers were determined using an in-process spectrophotometric assay and was expectedly fast, ranging from 6 to 34 min (Table 2).
Table 2. Properties of Various SM-PEGn Linkers from Different Suppliersa.
| linker | physical appearance | MW from supplier | MW by mass spectrometryb | purity by RP-HPLC | purity by qNMR | maleimide reactivity | NHS release T50c |
|---|---|---|---|---|---|---|---|
| SM-PEG6 | viscous, pasty | 601.6 | 601.0 | 100% | 97–99% | ND | 26 min |
| SM-PEG24 | viscous, pasty | 1395.0 | 1395.0 | 97% | 94–100% | 100% | 34 min |
| SM-PEG45 | powder, white | 2294.0 | 2324.0 | ND | 100% | 64% | 6 min |
| SM-PEG70 | powder, white | 3380.0 | 3601.0 | ND | 97% | 87% | ND |
SM-PEG6 and SM-PEG24 from Thermo/Quanta (supplier A) SM-PEG45 from NOF Corp. (supplier B) and SM-PEG70 from Nanocs (supplier C).
Mass spectrometry performed by HT Laboratories Inc., San Diego, CA.
Release rate of 50% of total NHS present in the linker reagent. (ND = not determined).
In a separate experiment, we measured the rate of hydrolysis of the NHS ester end of a representative lot of SM-PEG6 linker by UV spectroscopy at various pHs, times, and temperatures. Results showed that the half-life (T50) of the NHS ester group was ∼30 min at pH 7.5. Also, as expected, the rate of hydrolysis was faster at a higher pH (T50 ∼ 3 min at pH 9.2), and slower at a lower pH (T50 > 76 min at pH 6). In contrast, the half-life of the maleimide group on the opposite end of SM-PEG6 linker was ∼10 h at pH 7.5 as determined using a modified Ellman’s assay (see Methods) and much longer than the 30 min observed for the NHS ester at the same pH. Overall, these data emphasize the need for a reliable supplier of pure and reactive linker, in-process methods for assessing the chemical reactivity of the linker, and well-defined reagent handling and reaction conditions (i.e., solvent, concentration, time, temperature, and pH).
Impact of Length of SM-PEGn Linkers on the Particle Size of DV230-Ficoll Conjugates
The strategy of covalent attachment or association of CpG-ODN and/or antigens to nanoparticles (NPs) is a sound approach for targeting draining lymph nodes insofar as the synthetic compound or molecular conjugate mimics the immune stimulatory nature of natural viral and/or microbial agents, i.e., size, shape, and surface chemistry.26,27 Reddy et al. showed that small NPs composed of polystyrene (<45 nm in diameter) drain to lymph nodes and engage dendritic cells (DCs).13 Similarly, Manolova et al. showed that 20–200 nm particles also drain to lymph nodes and target distinct dendritic cells whereas larger particles 500 to 2000 nm were associated with migratory DCs within the draining lymph nodes of mice.28
Our main objective was to synthesize a CpG-ODN nanoparticle adjuvant in the 20–200 nm size range with the aim of targeting antigen presenting cells (APCs) and enhancing immune response. Therefore, we evaluated the effect of various length SM-PEGn linkers on the physical size (particle diameter) of DV230-Ficoll conjugates. Briefly, mal-PEG6,24,45,70-Ficoll intermediates with maleimide:Ficoll molar ratios ranging from 199-to-227 were each conjugated with between 0.6 and 0.75 mol equiv of 3′thiol-DV230 with the goal of producing DV230-Ficoll conjugates with CpG-ODN loadings of ∼120 ± 20 mol of CpG per mole of Ficoll, a range suitable for assessing in vitro activity. Results show a trend of increased mean particle diameter, from 55 to 91 nm (nm), by dynamic light scattering (DLS), for conjugates synthesized using a series of longer PEGs as part of the heterobifunctional linker (Table 3). Also, all four purified DV230-Ficoll conjugates were >99% pure and had fairly consistent CpG:Ficoll molar ratios, ranging from 108 to 116. These results demonstrate a simple approach for modulating the hydrodynamic size of DV230-Ficoll nanoparticles between ∼50 and 100 nm in diameter, an optimal size for dendritic cell uptake. This particle size range is not typically achieved using other CpG carriers such as alum29 or polylactide–coglycolide (PLG),30 both microparticles.
Table 3. Production of DV230-Ficoll Using Different PEG Length SM-PEGn heterobifunctional linkers.
| attribute | DV230-Ficoll conjugates | |||
|---|---|---|---|---|
| # PEG units in SM-PEGn linker | 6 | 24 | 45 | 70 |
| Maleimide:Ficoll molar ratio | 215 | 199 | 227 | 225 |
| DV230:Ficoll molar ratio | 109 | 116 | 110 | 108 |
| Particle sizea (diameter) by DLS | 55 nm | 77 nm | 78 nm | 91 nm |
Mean particle diameter (nm) values based on intensity distribution measurements.
In Vitro Potency of CpG-Ficoll (DV230-Ficoll) Adjuvants Produced with Various SM-PEGn Linkers
CpG-containing ODN are potent inducers of plasmacytoid dendritic cell (pDC)-derived IFN-α production, which exerts a strong adjuvant effect, enhancing Th1 priming.31 Thus, DV230-Ficoll adjuvants produced using SM-PEG6,24,45,70 PEG subunit linkers were evaluated for their ability to stimulate IFN-α from human pDC-enriched PBMC. Results from this assay are shown in Figure 3. Compared to nonconjugated DV230, combined data for multiple lots of DV230-Ficoll incorporating a SM-PEG6 linker showed an approximate 5-fold increase in potency (lower EC50) for IFN-α induction from PDC-enriched human PBMCs. These data demonstrate a key advantage of DV230-Ficoll over nonconjugated, monomeric DV230 for induction of innate immune responses. In comparing potency among DV230-Ficoll conjugates constructed with different length PEG linkers, longer length linkers (PEG24,45,70) were associated with slightly lower IFN-α EC50 values compared with the PEG6-containing construct. However, all EC50 values were within a 2-fold range and differences within this limited range were not considered biologically significant.
Figure 3.
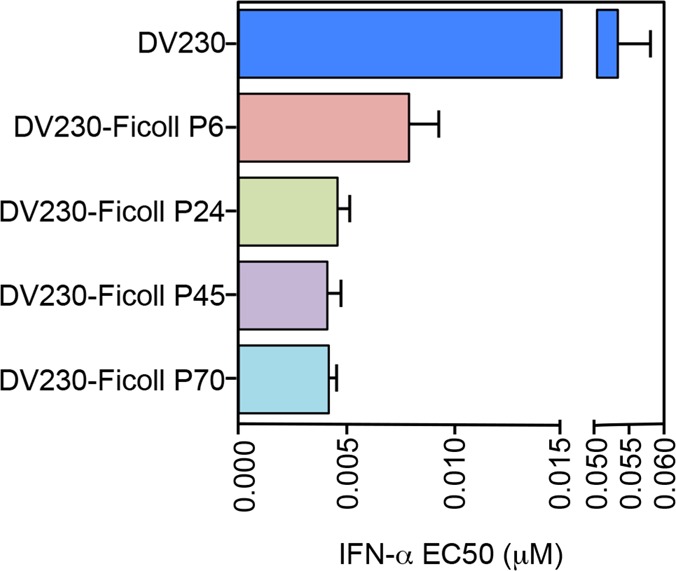
Induction of IFN-α from human pDC-enriched PBMC by DV230-Ficoll conjugates produced with various SM-PEGn linkers. Human pDC-enriched PBMC from six donors were incubated with nonconjugated DV230 or DV230-Ficoll conjugates made with various SM-PEGn linkers (P6, P24, P45, and P70) for 20 to 24 h, with IFN-α levels from supernatants determined by ELISA. Potency for secreted IFN-α was determined (mean and SEM shown).
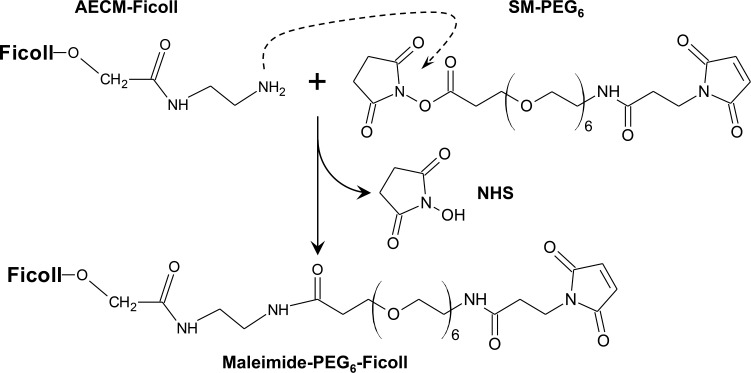
SM-PEG6 Linker Coupling to AECM-Ficoll Yielding Maleimide-PEG6-Ficoll
After preliminary physical and biological evaluation of various SM-PEGn linkers, and DV230-Ficoll conjugates derived from them, the SM-PEG6 linker emerged as the linker for further characterization and use. This choice was based on a number of criteria including the following: a reliable supplier, its monodispersity, purity, predictable reactivity, and demonstrated multimeric coupling to AECM-Ficoll, as well as demonstrated immune activity (i.e., IFN-α induction from pDC-enriched human PBMC) of the purified conjugate, DV230-Ficoll. The scheme for coupling the NHS ester end of the SM-PEG6 heterobifunctional linker with AECM-Ficoll via an amide bond yielding maleimide-PEG6-Ficoll is shown in Figure 4.
Figure 4.
Synthetic scheme for coupling SM-PEG6 to AECM-Ficoll yielding Maleimide-PEG6-Ficoll.
Modulation of the Extent of Functionalization of Maleimide-PEG6-Ficoll Intermediates
We explored production of differentially activated mal-PEG6-Ficoll intermediates by varying the amount of SM-PEG6 linker added to “linker coupling reactions” (Figure 1d and Figure 4) with a fixed amount of AECM-Ficoll characterized as having 218 amines/Ficoll. As such, six different preparations of mal-PEG6-Ficoll were prepared, purified (95–99% pure by SEC-HPLC), and their maleimide content determined. Adding increasing molar ratios of SM-PEG6 (0.25, 0.5, 0.75, 1.0, 1.7, and 2.0) to AECM-Ficoll resulted in mal-PEG6-Ficoll intermediates with 8 to 185 maleimides per mole of AECM-Ficoll (Figure 5). These data illustrate how easily the linker coupling step can be controlled to generate a wide range of maleimide-Ficoll intermediates for conjugation with thiolated ligands, in our case, 3′thiol-DV230.
Figure 5.
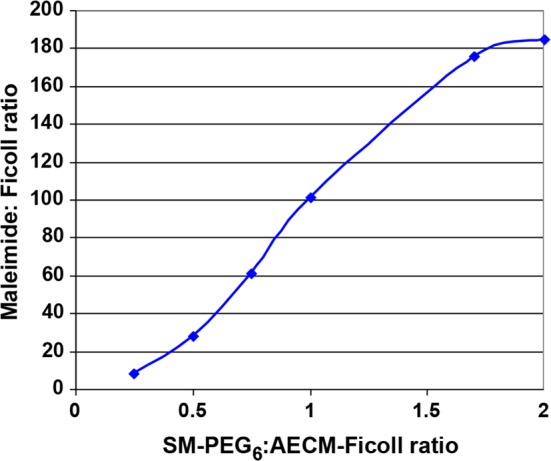
Differentially activated Maleimide-PEG6-Ficoll intermediates derived from varied linker coupling conditions.
Consistent Production of Mal-PEG6-Ficoll Intermediates
After demonstrating the capability to generate a range of maleimide-activated Ficoll we sought to determine how consistently mal-PEG6-Ficoll intermediates could be synthesized using different lots of AECM-Ficoll and SM-PEG6 linker and at different scales. We compared the extent of activation of various mal-PEG6-Ficoll intermediates derived from three different lots of AECM-Ficoll, two different lots of SM-PEG6 linker, and from two different scales of production, i.e., bench (∼15 mg) and pilot scales (∼500 mg and 2.1 g). Coupling the SM-PEG6 linker to AECM-Ficoll under similar conditions across nine different lots of mal-PEG6-Ficoll resulted in from 162 to 221 mol of maleimide per mole of Ficoll (Table 4). Interestingly, pilot lots 4 and 5 derived from SM-PEG6 linker (lot B) and produced at different scales had the highest maleimide:Ficoll molar ratios at 221 and 206, respectively, suggesting that this lot of linker was slightly more reactive than the linker from lot A used to produce all other Mal-PEG6-Ficoll intermediates.
Table 4. Production of Maleimide-PEG6-Ficoll Intermediates at the Bench and Pilot Scales.
| Mal-Ficoll lot no. | SM-PEG6 linker lot no. | Amine/Ficoll molar ratio | Maleimide/Ficoll molar ratio |
|---|---|---|---|
| Bench lot 1 | Lot A | 218 | 174 |
| Bench lot 2 | Lot A | 218 | 162 |
| Bench lot 3 | Lot A | 218 | 176 |
| Bench lot 4 | Lot A | 218 | 181 |
| Pilot lot 1 | Lot A | 221 | 163 |
| Pilot lot 2 | Lot A | 218 | 182 |
| Pilot lot 3 | Lot A | 224 | 187 |
| Pilot lot 4 | Lot B | 224 | 221 |
| Pilot lot 5 | Lot B | 224 | 206 |
Producing mal-PEG6-Ficoll, a critical process intermediate, within a specified range of maleimide:Ficoll molar ratios requires control of the following: (1) preparation of AECM-Ficoll with a defined range for amine:Ficoll molar ratios, (2) use of a highly pure SM-PEG6 linker, and (3) and use of defined linker coupling reaction conditions. Clearly, mal-PEG6-Ficoll intermediates provide a flexible platform for conjugating both wide-ranging and targeted amounts of thiolated-CpG. Furthermore, our laboratory has shown that mal-PEG6-Ficoll intermediates are reactive with thiols on various proteins, peptides, and small molecules, though not the subject of this article.
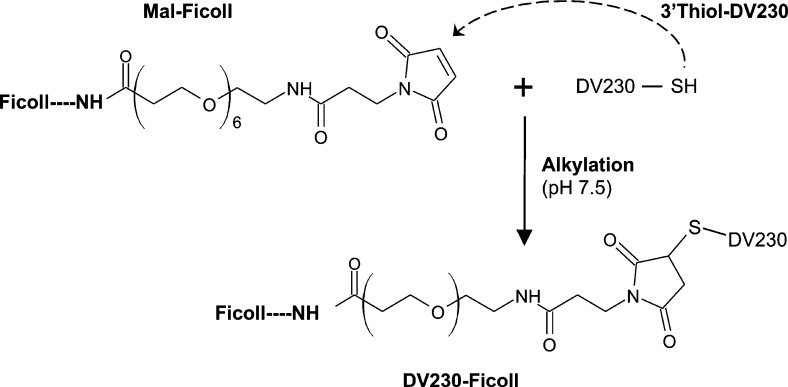
Production of CpG (DV230)-Ficoll Conjugates
DV230-Ficoll conjugates produced at various scales throughout development were synthesized by reacting 3′thiol-DV230 with mal-PEG6-Ficoll, under varied conditions, according to the general scheme in Figure 6. Scaled-up pilot lots of DV230-Ficoll were similarly prepared but used defined conditions (i.e., addition of a calculated amount of 3′thiol-DV230 to mal-PEG6-Ficoll having a predetermined number of reactive maleimides) to ensure consistent production of DV230-Ficoll conjugates.
Figure 6.
Scheme for producing DV230-Ficoll by conjugating 3′thiol-DV230 with mal-PEG6-Ficoll.
Modulation of the Conjugation Reaction Between 3′thiol-DV230 (CpG) and Maleimide-PEG6-Ficoll to Yield DV230-Ficoll with Different CpG Loadings
Four of the six maleimide-PEG6-Ficoll preparations previously described in Figure 5 were each conjugated with ∼1.1 mol equiv of 3′thiol-DV230, yielding four different CpG-Ficoll conjugates with conjugation efficiencies of 81–87%. The CpG loading densities (DV230/Ficoll molar ratios) for these conjugates ranged from 24 to 154 and reveal the ease of synthesizing CpG-ODN-Ficoll conjugates (DV230-Ficoll) with wide-ranging CpG loading simply by reacting excess 3′thiol-DV230 with variably activated mal-PEG6-Ficoll. Also, this set of conjugates showed fairly similar levels of activity for IL-6 induction from human B cells (Table 5).
Table 5. Preparation of DV230-Ficoll with Varied CpG-ODN Loadings Induced Similar Levels of IL-6 from Human B Cells.
| sample | Mal/Ficoll molar ratio | DV30/Ficoll molar ratio | conjugation efficiency (%) | induction of IL6 - EC50 (μM) | induction of IL6 - Geomean max (pg/mL) |
|---|---|---|---|---|---|
| 1 | 28 | 24 | 86 | 0.028 | 745 |
| 2 | 61 | 53 | 87 | 0.022 | 844 |
| 3 | 101 | 82 | 81 | 0.017 | 777 |
| 4 | 185 | 154 | 83 | 0.017 | 984 |
In a separate CpG loading experiment, five DV230-Ficoll conjugates with variable DV230 loadings (i.e., 26, 62, 89, 106, and 113 DV230s per Ficoll) showed similar potency for IL-6 induction by human B cells at the four highest adjuvant loading densities. However, the conjugate with the lowest level (26) of CpG showed decreased levels of IL-6 (data not shown). Also, the potency of IFN-α induction by human PBMC was fairly consistent for the same samples. However, maximum IFN-α production was decreased for conjugates with the three lowest CpG loadings (data not shown). Also, these same DV230-Ficoll samples showed a higher number of nonresponding donors possibly indicating that a threshold level of conjugated CpG may be required for maximal adjuvant activity.
Scale-up, Production, and Characterization of DV230-Ficoll Conjugates
After necessary development and optimization, the DV230-Ficoll process was scaled up from the bench scale, yielding ∼15 mg of product (bench lots 1–3), to an intermediate scale, yielding 129 mg (bench lot 4), to a pilot scale, yielding ∼500 mg (pilot lots 1–4), to pilot lot 5, which yielded 2.1 g of purified DV230-Ficoll after an additional 4-fold scale-up. Hence, pilot lot 5 represents a 140-fold scale-up compared with bench lots 1–3. Significantly, scale-up was achieved while maintaining consistent product yield, purity, particle size distribution, and CpG loading density (Table 6). These lots were >99% pure by SE-HPLC and had <1% residual DV230 oligonucleotide and/or small molecules. DV230 concentrations ranged from 2.9 to 5.7 mg/mL and Ficoll concentrations ranged from 1.2 to 2.3 mg/mL in these CpG-ODN-Ficoll conjugates. Also, the mean particle diameters ranged from 49 to 53 nm by DLS, and the amount of CpG covalently attached to Ficoll ranged from 117 to 140 DV230s per Ficoll. Lastly, 0.2 μm filtered solutions of purified DV230-Ficoll were visually clear, had uniform pH values of 7.2 ± 0.1, and contained low levels of bacterial endotoxin. Overall, these data demonstrate reproducible and robust production of DV230-Ficoll, necessary features of a viable manufacturing process.
Table 6. Physicochemical Characterization of Five Pilot Lots of Purified DV230-Ficoll.
| attributes | pilot 1 | pilot 2 | pilot 3 | pilot 4 | pilot 5 |
|---|---|---|---|---|---|
| Appearance | clear | clear | clear | clear | clear |
| pH | 7.3 | 7.2 | 7.1 | 7.2 | 7.2 |
| Purity by SEC-HPLCa (%) | >99% | >99% | >99% | 100% | 100% |
| Residual CpG by SEC-HPLC (%) | <1% | <1% | <1% | 0% | 0% |
| Ficoll contentb (mg/mL) | 1.3 | 1.2 | 1.3 | 1.56 | 2.34 |
| DV230 contentb (mg/mL) | 2.9 | 3.2 | 3.0 | 3.8 | 5.7 |
| DV230: Ficoll ratio (M) | 117 | 140 | 117 | 126 | 125 |
| Particle size distributions, mean diameterc (nm) | 49 ± 20 | 53 ± 23 | 47 ± 20 | 47 ± 20 | 48 ± 20 |
| Amount of purified DV230-Ficoll (mg) | 518 | 554 | 468 | 494 | 2100 |
| Endotoxind (EU/mg) | 0.097 | 0.088 | <3 | <1 | <1 |
Purity was determined using an SE-HPLC silica-based TSK-Gel G30000SWxl column.
Pilot 5 was intentionally delivered at higher DV230 and Ficoll concentrations.
Mean particle diameters based on intensity particle size distributions.
Endotoxin was measured using a Limulus Amoebocyte Lysate (LAL).
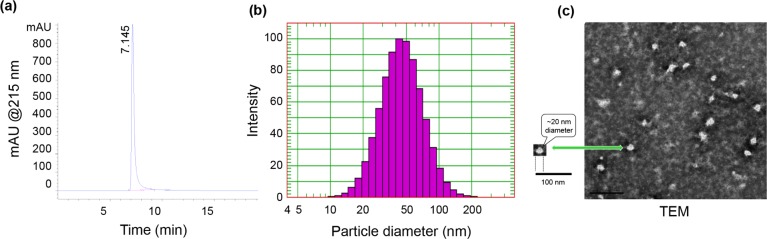
Apart from consistent CpG loading onto Ficoll, the two most defining physical features of purified DV230-Ficoll conjugates are purity and particle size distribution. A representative SE-HPLC chromatogram of a solution of DV230-Ficoll show a single large molecular weight peak (peak retention time of ∼7.2 min) and purity of ∼99%, indicating that the product is free of unreacted DV230 or other UV-absorbing small molecules (Figure 7a). A representative particle size distribution of a solution of DV230-Ficoll by DLS shows a mean particle diameter of 50 nm with a RSD of 22 nm (Figure 7b). Lastly, analysis of DV230-Ficoll by transmission electron microscopy (TEM), after dehydration and negative staining, shows spherical and nonspherical particles of various sizes. A selected particle, indicated by the arrow, had an estimated core particle diameter of ∼20 nm, relative to the 100 nm scale bar (Figure 7c).
Figure 7.
Purity of DV230-Ficoll by SE-HPLC and particle size by DLS and TEM. (a) SE-HPLC analysis of a solution of DV230-Ficoll with UV detection at multiple wavelengths (A215 depicted). (b) Histogram of a representative DV230-Ficoll particle size distribution by DLS. (c) TEM image of DV230-Ficoll.
Stability of Purified DV230-Ficoll
The physicochemical stability of DV230-Ficoll formulated in 10 mM NaPO4, 141 mM NaCl, pH 7.2 (liquid formulation) was evaluated at three different storage temperatures (−80, 5, and 37 °C) at various time points over 12 months. In parallel, the stability of a lyophilized formulation of DV230-Ficoll prepared in 10 mM K2HPO4, 300 mM Trehalose, pH 7.5, was evaluated after storage for 3, 6, and 12 months at 5, 25, and 37 °C. The main stability parameter was percent purity by SE-HPLC analysis, since this method was previously shown to be stability-indicating for samples stored at elevated temperature, i.e., 37 °C. Particle size, pH, and CpG content were also measured. For liquid formulations, sample pH, concentration, purity, and particle size did not change significantly when stored at frozen (−80 °C) or refrigerated (5 °C) conditions for up to 12 months. However, at 37 °C, both purity and pH decreased significantly with longer storage times, while concentration and particle size remained fairly constant. At 37 °C, about a 5% decrease in purity was detected as early as 1 month and purity steadily declined from an initial level of >99% to 77% by the 9 month stability time point (Figure 8). Unfortunately, the 12 month stability sample stored at 37 °C was compromised and not measured.
Figure 8.
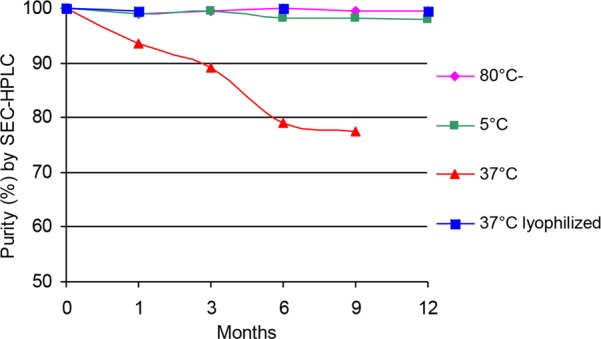
Stability (% purity) of liquid and lyophilized (37 °C only) DV230-Ficoll by SE-HPLC after storage at various temperatures and times.
Interestingly, SE-HPLC showed that the main degradation species formed at 37 °C comigrated with a peak retention time consistent with free DV230 oligo, suggesting that degradation is related to the CpG (DV230) portion of the conjugate. Additionally, this degradation species was isolated by semipreparative SEC and analyzed by LC/MS, revealing a major component with a mass of 8218.2 Da, approximately the mass of CpG (DV230) plus most of the SM-PEG6 linker.
In contrast, all lyophilized stability samples maintained their original high levels of purity at ≥98% and showed no product degradation, even at 37 °C. The percent purity of lyophilized DV230-Ficoll stored at 37 °C for 1, 6, and 12 months was compared with liquid formulations stored at −80, 5, and 37 °C for 1, 3, 6, 9, and 12 months (Figure 8). These data show good stability for liquid product (DV230-Ficoll) either frozen or refrigerated and potential for long-term cold-chain independent storage of an easily reconstituted lyophilized product, an advantageous feature for a vaccine adjuvant. It should be noted that other CpG-ODN delivery options, including adsorption to alum or encapsulation in liposomes, are not amenable to lyophilization.
Immunogenicity of Anthrax Recombinant Protective Antigen (rPA) Co-Administered with DV230-Ficoll Nanoparticle Adjuvant in Mice
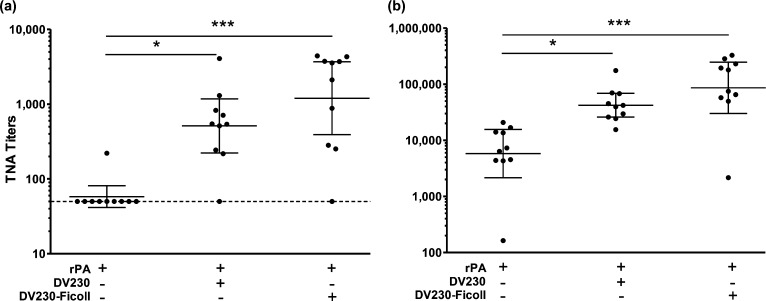
To evaluate the relative adjuvant activity of DV230-Ficoll and nonconjugated DV230, we immunized mice with 5 μg (μg) of anthrax recombinant protective antigen (rPA) mixed with either 10 μg (CpG-ODN-based doses) of DV230-Ficoll or DV230. Toxin neutralizing antibody (TNA) titers, a recognized correlate of protection32 were measured. At 4 weeks post first immunization, DV230-Ficoll + rPA induced a geomean TNA titers >1000 (a level predictive of protection to anthrax challenge33), whereas the geomean titer induce by DV230 + rPA was 512 (rPA alone induced minimal titers) (Figure 9a). Titers were increased >70-fold following booster immunizations with highest titers in DV230-Ficoll + rPA-immunized mice (Figure 9b). These data demonstrate an advantage of DV230-Ficoll over DV230 for rapid induction of high titer neutralizing antibody responses.
Figure 9.
Comparison of the TNA titers in mice following immunization with the following compounds, combinations, and dose levels: rPA (5 μg) ± DV230 (10 μg) or DV230-Ficoll (10 μg DV230) (A) 4 weeks post first immunization, and (B) 2 weeks post second immunization. Kruskai-Wallis with Dunn’s post test, * p < 0.05, *** p < 0.001. The data is depicted as geomean +95% CI.
This enhancement in the immune response by DV230-Ficoll demonstrates the potential of a Ficoll-based platform for the development of next-generation vaccine adjuvants, and possibly for other drug delivery applications such as cancer immunotherapy. Further extensive evaluations concerning the biology and immunology of the DV230-Ficoll conjugate compared with free CpG-ODN were also conducted by our laboratory; including protection of monkeys treated with DV230-Ficoll + rPA from lethal exposure to anthrax spores.15
Conclusions
We have demonstrated that chemically reactive sucrose polymers, specifically, multiamine AECM-Ficoll and multimaleimide functionalized (mal-PEG6-Ficoll) intermediates were flexible platforms for both tunable and consistent coupling of linkers to AECM-Ficoll and for adaptable and consistent covalent linkage of 3′thiol-DV230 (CpG) to mal-PEG6-Ficoll, yielding DV230-Ficoll. In order to reproducibly synthesize DV230-Ficoll conjugates it was critical to control the purity and reactivity of the SM-PEG6 heterobifunctional linker, as well as the concentration, purity, and reactivity of two key process intermediates, mal-PEG6-Ficoll and 3′thiol-DV230. It was also important to establish well-defined conjugation and purification conditions which facilitated efficient process scale-up. At the pilot scale, five consecutive batches of DV230-Ficoll were manufactured in high yield and delivered a stable product of consistent purity, particle size, and CpG-ODN loading. The in vitro (human) and in vivo activity (mice) of DV230-Ficoll was either elevated or significantly enhanced compared to nonconjugated monomeric CpG-ODN-DV230. Overall, we have developed a well-defined, reproducible, and scalable process for the production of DV230-Ficoll, a stable and immunologically active CpG-based nanoparticle adjuvant. The conjugation processes described herein represent an adaptable platform not only for the production of DV230-Ficoll, but also as a carrier for use with proteins, peptides, and small molecules and combinations thereof for applications in infectious diseases and cancer immunotherapy.
Experimental Procedures
Materials
SM-PEG6 (succinimidyl-((N-maleimidopropionamidol)-hexethylene glycol) ester) was obtained from Thermo Scientific (1 g) or Pierce (100 mg) (Rockford, IL). SM-PEG45 was supplied by Nanocs (New York, NY). SM-PEG70 was supplied by NOF American Corporation. Ficoll PM400 was purchased as a spray-dried powder from GE Healthcare (Pittsburgh, PA). Sulfo-N-hydroxysuccinimide acetate was from Thermo Fisher. All other chemicals were at least A.C.S. grade purchased from Sigma-Aldrich (St. Louis, MO) or USP grade purchased from either Sigma or J.T. Baker (Avantor, Center Valley, PA).
CpG-ODN DV230 (3′disulfide-DV230)
CpG-ODN’s were purchased from Nitto Denko Avecia, Inc. (formerly known as Avecia, Inc., Milford, MA) or TriLink Biotechnologies (San Diego, CA). The 3′disulfide DV230 sequence is 5′-TCGGCGC-3′-HEG-5′-AACGTTC-3′-HEG-5′-TCGGCGC-3′-(CH2)6-SS-(CH2)6-OH. The 3′disulfide DV230 was synthesized on a solid phase synthesizer programmed to add the nucleotide monomers, HEG spacers, and linkers in the desired order, with the synthesis occurring in the 3′ to 5′ direction. The 3′-nucleoside or linker group (e.g., 3′-Thiol-Modifier C6 S–S CPG) was attached to the solid support. After complete synthesis and cleavage from the solid support the compound was purified using anion exchange chromatography, desalted by diafiltration, lyophilized, and stored at −20 °C as lyophilized solids. All lots of 3′disulfide DV230 had the appearance of a white powder, and the found molecular weights were 7780–7789 Da (theoretical 7785 Da). The purity by reversed-phase HPLC ranged 85–89% and the purity by ion exchange HPLC was 85–86%.
rPA Protein
Purified recombinant protective antigen (rPA) was supplied by PharmAthene, Inc. (Annapolis, MD).
Methods
DV230 Assay
The concentrations of 3′disulfide-DV230, 3′thiol-DV230, and DV230-Ficoll were determined using ultraviolet spectrophotometry and the Beer’s law equation. Absorbance of DV230-containing samples was measured at 260 nm, and DV230 concentrations were calculated using the mass extinction coefficient of 22.65 mg/mL–1 cm–1. DV230/Ficoll molar ratio was determined by dividing the molarity of DV230 by the molarity of Ficoll.
Ficoll Assay
The Ficoll content in CM-Ficoll, AECM-Ficoll, Mal-Ficoll intermediates, and DV230-Ficoll were each determined by a modified method of the Pierce Glycoprotein Carbohydrate Estimation kit (Thermo Scientific, Rockford, IL), using Ficoll PM400 as calibration standard. Serial dilutions of Ficoll (25 to 200 μg/mL) and test samples were made in 75 mM sodium phosphate buffer pH 6.3. Standards and test samples were reacted with 0.71 mg/mL sodium meta-periodate for 10 min at room temperature followed by 2.5 mg/mL of Glycoprotein Detection Reagent for 1 h. Absorbance of standards and samples was measured at 550 nm.
Amine Assay
The amine content on AECM-Ficoll was determined using Fluoraldehyde o-Phthaldialdehyde Reagent (Thermo Scientific, Rockford, IL) as detection solution, and glycine as calibration standard. Serial dilutions of glycine (10 to 60 μM) and test samples were made in PBS pH 7.2. Two hundred microliters of standard or sample was mixed with 2 mL of detection solution and reacted for 1 min at room temperature, followed by fluorescence reading at excitation and emission wavelengths of 335 and 445 nm, respectively
Maleimide Assay
The maleimide content of maleimide-Ficoll intermediates was determined using an indirect Cysteine-DTNB assay. DTNB (5,5′-dithio-bis-[2-nitrobenzoic acid]) (Ellman′s Reagent, Thermo Scientific, Rockford, IL) reacts with free sulfhydryls to produce a yellow-colored product TNB (2-nitro-5-thiobenzoic acid), with a specific absorbance at 412 nm. When mixed with cysteine, maleimide on mal-Ficoll reacts with the free thiol of cysteine at a stoichiometric ratio. When a known excess of cysteine is used, unreacted cysteine can be detected using Ellman’s reagent, and compared to a cysteine calibration curve. The derived amount of reacted cysteine (total cysteine used minus unreacted cysteine) is equal to the amount of maleimide on mal-Ficoll. Typically, serial dilutions of mal-Ficoll were reacted with 1.25 mM cysteine for 3 h at room temperature, in PBS pH 7.5. The solutions were then reacted with 80 μg/mL of Ellman’s reagent for 15 min and absorbance was measured at 412 nm. Cysteine calibration standards (0.25 to 1.5 mM) were similarly processed.
Particle Size
Particle size distributions were obtained by dynamic light scattering using either a NiComp 380 ZLS instrument (Particle Sizing Systems, Port Richey, FL) and expressed as average intensity distribution, or a Zetasizer nano S (Malvern Instruments Ltd., Worcestershire, UK) and expressed as z-average. DV230-Ficoll solutions were diluted with 10 mM NaPO4, 141 mM NaCl, pH 7.2 to a final Ficoll concentration of approximately 0.5 mg/mL for analysis.
MALDI-TOF
SM-PEGn samples were dissolved in pure water at 5 mg/mL and analyzed by matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF), performed by HT Laboratories (San Diego, CA). Molecular weight estimate for each SM-PEGn were derived from the mass spectrum expressed in % intensity versus mass-to-charge ratio (m/z).
Transmission Electron Microscopy (TEM)
Negative stains were prepared with carbon coated parlodion-filmed grids and 2% potassium phosphotungstate, pH 6.5 using the drop method. After 15 s, excess fluid was removed, and a drop of 2% potassium phosphotungstate was added to the moist surface for 5–10 s. Excess solution was removed, and the grid was air-dried. Grids were examined at 80 kV in a JEOL 1230 electron microscope (JEOL USA, Inc., Peabody, MA) and photographed with a Gatan Ultrascan USC1000 digital camera (Gatan Inc., Warrendale, PA). TEM was performed by the Gladstone Institute, UCSF, San Francisco, CA.
Purity Analysis by Size Exclusion HPLC (SE-HPLC)
SE-HPLC was performed using an Agilent 1200 HPLC system fitted with a TSK-Gel G3000SWxl column. Samples (10–50 μL injected volume) were run isocratically for 30 min, at a flow rate of 0.75 mL/min with UV detection at 215 and 260 nm. Peak integration at 215 nm was used to calculate the purity of DV230-Ficoll based on the following formula: Purity % = DV230-Ficoll peak area divided by the sum of all peak areas, multiplied by 100. The purity of mal-PEGn-Ficoll and 3′thiol-DV230 intermediates were also determined using this method.
Reduction of 3′Disulfide DV230 and Isolation of 3′Thiol-DV230 by G25 Desalting Chromatography
A solution of 3′disulfide-DV230 at 25 ± 2.5 mg/mL in 100 mM sodium phosphate, 150 mM sodium chloride, 1 mM ethylenediaminetetraacetic acid (EDTA), pH 7.5 was reduced by the addition of 5 equiv of tris(2-carboxyethyl)phosphine hydrochloride (TCEP, Thermo Scientific, Rockford, IL) for 2 h at 40 ± 2 °C. The 3′thiol-DV230 oligonucleotide was isolated by gel filtration (desalting) using Sephadex G-25 Fine, GE Healthcare, Pittsburgh, PA, packed into XK50/30 columns, according to the manufacturer’s recommended procedures and controlled by an AKTA purifier chromatography system (GE Healthcare, Pittsburgh, PA). The 3′thiol-DV230 was loaded onto a G25 column equilibrated with 100 mM NaPO4, 150 mM NaCl, 1 mM EDTA, pH 7.5 at a flow rate of 30 cm/h in a volume of ∼12–16% of the packed column volume. The 3′thiol-DV230 was collected starting when the UV signal (A215 nm) rose above ∼100 mAU and ending when a pool volume of 1.6 to 1.8 times the load volume was collected. The G25 purified 3′thiol-DV230 was stored frozen at −80 °C.
Preparation of CM-Ficoll
A 100 mL solution of Ficoll PM400 was prepared at ∼130 mg/mL in Milli-Q deionized water, then transferred to a jacketed reaction vessel connected to a 40 °C circulating water bath. To this Ficoll solution, 92.5 mL of 2.7 M sodium chloroacetate solution, 50 mL of 10 N sodium hydroxide solution, and 7.5 mL Milli-Q deionized water were added. The reaction proceeded for 2.5 h at 40 °C while stirring. Immediately after, 10 mL of 2 M sodium phosphate buffer pH 4 was added to the reaction solution, and the pH adjusted to 7.0 by addition of 20% chloroacetic acid solution. The crude CM-Ficoll was purified by TFF using a 100 kDa MWCO membrane and diafiltered extensively (15–18 volume exchanges) against 0.2 M NaCl.
Preparation of AECM-Ficoll
In a jacketed reaction vessel connected to a 22 °C circulating water bath, CM-Ficoll solution, containing approximately 7 g of Ficoll, was mixed with 34.4 g of ethylenediamine dihydrochloride (approximately 13 800 mol equiv per Ficoll). Then, 3 g of N-(3-(dimethylamino)propyl)-N′-ethylcarbodiimide hydrochloride (EDC-HCl, ∼835 mol equiv per Ficoll) were added to the mixture over a period of 10 min while stirring. The reaction proceeded for 3.5 h at 22 °C and the pH monitored and adjusted to 4.7. AECM-Ficoll was purified by TFF using a 100 kDa membrane and extensive diafiltration against 100 mM sodium phosphate and 150 mM sodium chloride, pH 7.5 for a total of approximately 15–20 volume exchanges. Purified AECM-Ficoll solutions were 0.22 μm filtered and stored at −80 °C.
Preparation of Maleimide-Ficoll Using SM-PEG6
A 100 mg/mL SM-PEG6 solution was prepared in DMSO, and added gradually to AECM-Ficoll solution containing 20 mg/mL Ficoll, while stirring at room temperature, to a final molar equivalent of 5 SM-PEG6 per amine (∼1100 SM-PEG6 per Ficoll). The reaction was transferred to a 25 °C incubator and proceeded for 40 min. Then, sulfo-N-hydroxysuccinimidyl-acetate (100 mg/mL in DMSO) was added to reach a molar equivalent of 5 Su-NHS-Ac per amine, and reacted for 15 min at room temperature, in order to cap unreacted amines on Ficoll. Unreacted SM-PEG6 and Su-NHS-Ac were quenched with 10 mol equiv of glycine solution (100 mg/mL in 100 mM NaPO4,150 mM NaCl, pH 7.5), for 15 min at room temperature. Crude mal-PEG6-Ficoll was purified by TFF (100 kDa MWCO) against 100 mM sodium phosphate and 150 mM sodium chloride pH 7.5 and stored at −80 °C.
Preparation of DV230-Ficoll
Frozen (−80 °C) solutions of mal-PEG6-Ficoll and 3′thiol-DV230 were each thawed in a 4 °C water bath for 2–3 h. Once thawed, 3′thiol-DV230 was added to mal-PEG6-Ficoll at a molar equivalent of 0.64 to 0.69 DV230 per maleimide (∼141 DV230 per Ficoll). The reaction volume was adjusted with 100 mM sodium phosphate, 150 mM sodium chloride, pH 7.5 to obtain a DV230 concentration of 5 mg/mL. Conjugation reactions proceeded for 1 h at 25 °C with gentle stirring, followed by capping unreacted maleimide groups on Ficoll using a 100 mg/mL solution of cysteine, at 10 mol equiv of cysteine per maleimide for 15 min at room temperature. Crude DV230-Ficoll conjugates were stored overnight at 2–8 °C and purified the next day by either size exclusion chromatography (pilot lots 1–3) or TFF (pilot lots 4 and 5). Purified DV230-Ficoll was 0.22 μm filtered and stored at −80 °C.
Tangential Flow Filtration (TFF)
TFF was set up using MasterFlex L/S pump (Cole Parmer), Pellicon 2 mini stainless steel plate holder (Millipore, XX42P Mini), and Sius-LSn (Novasep, Cat. No. PP100M01L) 100 K MWCO cassette (0.1 m2 surface area). The TFF system and cassette were thoroughly washed with distilled water, sanitized with 0.1 N sodium hydroxide, and equilibrated with diafiltration buffer: 10 mM sodium phosphate, 141 mM sodium chloride pH 7.2. Diafiltration proceeded at a pump flow rate ranging from 200 to 300 mL/min resulting in permeate flux of 50–80 mL/min and transmembrane pressure of 3–15 psi. Absorbance of the permeate solution (waste) was monitored at 215 nm throughout the process, and the step terminated when permeate absorbance dropped to 0.1 AU.
Lyophilization of DV230-Ficoll
We used a Labconco Freezone 6 stoppering tray dryer. The lyophilization cycle consisted of shelf-freezing at approximately −35 °C, followed by 36 h of primary drying at −35 °C (∼60 μbar vacuum), 15 min transition to shelf temperature at 25 °C, and secondary drying for an additional 24 h. The lyophilized product had residual moisture of 2.8–2.9% by Fischer analysis. The cake reconstituted in 1 mL of pure water within 2 min after mixing.
ELISA Assays
IL-6 and IFN-α content was assayed using a commercially available antibody pair (MabTech, Inc.); the limit of minimal detection was 31 and 23 pg/mL, respectively. 96-well Maxisorp Immuno plates were coated with cytokine specific Ab and then blocked with 1% BSA in DPBS. Culture supernatants were added and bound cytokine was detected by addition of biotin-labeled secondary Ab, followed by HRP and a peroxidase-specific colorimetric substrate. Standard curves were generated using recombinant cytokines purchased from R&D Systems (Minneapolis, MN) or MabTech, Inc. for IL-6 and IFN-α, respectively. Absorbance values were determined at 450 nm with background subtraction 650 nm using either a SpectraMax 190 or VersaMax microplate reader (Molecular Devices).
Isolation and Stimulation of Primary Leukocytes
Human blood was obtained with informed consent from healthy human donors. PBMCs were isolated by Ficoll-Paque (GE Healthcare) density gradient centrifugation. Human B cells were isolated by positive selection with anti-CD19 microbeads (Miltenyi Biotec). PDCs were isolated by positive selection with anti-BDCA-4 microbeads (Miltenyi Biotec) and were added back to untouched PBMC resulting in final PDC percentages of 0.5–2.4. All cells were resuspended in RPMI-1640 (BioWhittaker) supplemented with 10% heat-inactivated FBS (Gemini) plus 50 U/mL penicillin, 50 mg/mL streptomycin, 2 mM l-glutamine, 10 mM HEPES, and 1 mM sodium pyruvate (BioWhittaker). For stimulation, B cells were cultured at 0.75 × 106/mL in 96-well round-bottomed plates in duplicate with CpG-ODN at a concentration range of 5.5–0.0054 μM for 90–93 h. PDC-enriched PBMC were cultured at 2.5 × 106/mL in 96-well flat bottomed plates in triplicate with CpG-ODN at a concentration range of 2.5–0.0049 μM for 21–24 h.
Immunogenicity Testing in Mice
Swiss Webster mice, purchased from Harlan Laboratories (Livermore, CA) and used at 8–12 weeks of age, were maintained at Pacific BioLabs (Hercules, CA). Groups of 10 mice were immunized in the quadriceps at 0 and 4 weeks with 5 μg rPA ± 10 μg DV230-Ficoll or DV230 in a total volume of 50 μL. TNA titers were measured as previously described.34
Statistics for Immunogenicity Testing in Mice: TNA Titers
A Kruskal–Wallis test with Dunn post-test was used to determine statistical significance. A p value ≤0.05 was considered significant.
LAL Assay
Bacterial endotoxin was measured using a Limulus Amoebocyte Lysate (LAL) assay; Endosafe Endochrome K assay from Charles River Laboratories.
Acknowledgments
The support of NIAID Contract No. HHSN272200800038C is gratefully acknowledged. We also kindly thank Nelle Cronen for assistance preparing the manuscript.
Glossary
Abbreviations
- AU
absorbance unit
- Da
Dalton
- DMSO
dimethyl sulfoxide
- DV230
3′disulfide-DV230
- DLS
dynamic light scattering
- IEX-HPLC
ion exchange high performance liquid chromatography
- IFN-α
interferon alpha
- kDa
kilodalton
- LAL
Limulus amoebocyte lysate
- LC/MS
liquid chromatography/mass spectrometry
- TLR
Toll like receptor
- LMW
low molecular weight
- μg
microgram
- mL
milliliter
- MW
molecular weight
- MWCO
molecular weight cut off
- mM
millimolar
- nm
nanometer
- PBMC
peripheral blood mononuclear cells
- pDC
plasmacytoid dendritic cells
- rPA
recombinant protective antigen
- RSD
relative standard deviation
- RP-HPLC
Reversed phase high performance liquid chromatography
- SE-HPLC
size exclusion high performance liquid chromatography
- TFF
tangential flow filtration
- μ
micron
- μM
micromolar
- UF
ultrafiltration
- UV
ultraviolet
The authors declare the following competing financial interest(s): B.M, R.K., G.S.O., C.C., M.K., J.D.C., H.K., and R.L.C. are present or former employees of Dynavax Technologies and may hold stock or stock options.
References
- Iwasaki A.; Medzhitov R. (2004) Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995. 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S.; Medzhitov R. (2009) Toll-like receptors and cancer. Nat. Rev. Cancer 9, 57–63. 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- Takeda K.; Kaisho T.; Akira S. (2003) Toll-like receptors. Annu. Rev. Immunol. 21, 335–376. 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Steinhagen F.; Kinjo T.; Bode C.; Klinman D. M. (2011) TLR-based immune adjuvants. Vaccine 29, 3341–3355. 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode C.; Zhao G.; Steinhagen F.; Kinjo T.; Klinman D. M. (2011) CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 10, 499–511. 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D.; Marshall J. D.; Traquina P.; Van Nest G.; Livingston B. D. (2007) Immunostimulatory DNA as a vaccine adjuvant. Expert Rev. Vaccines 6, 747–759. 10.1586/14760584.6.5.747. [DOI] [PubMed] [Google Scholar]
- Krieg A. M. (2007) Development of TLR9 agonists for cancer therapy. J. Clin. Invest. 117, 1184–1194. 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar J. C.; Rodriguez E. G. (2007) Vaccine adjuvants revisited. Vaccine 25, 3752–3762. 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- Sablan B. P.; Kim D. J.; Barzaga N. G.; Chow W. C.; Cho M.; Ahn S. H.; Hwang S. G.; Lee J. H.; Namini H.; Heyward W. L. (2012) Demonstration of safety and enhanced seroprotection against hepatitis B with investigational HBsAg-1018 ISS vaccine compared to a licensed hepatitis B vaccine. Vaccine 30, 2689–2696. 10.1016/j.vaccine.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Janssen R. S.; Mangoo-Karim R.; Pergola P. E.; Girndt M.; Namini H.; Rahman S.; Bennett S. R.; Heyward W. L.; Martin J. T. (2013) Immunogenicity and safety of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in patients with chronic kidney disease. Vaccine 31, 5306–5313. 10.1016/j.vaccine.2013.05.067. [DOI] [PubMed] [Google Scholar]
- Joshi V. B.; Geary S. M.; Salem A. K. (2013) Biodegradable particles as vaccine delivery systems: size matters. AAPS J. 15, 85–94. 10.1208/s12248-012-9418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann J.; Klinman D. M. (2014) Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 32, 6377–6389. 10.1016/j.vaccine.2014.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourquin C.; Anz D.; Zwiorek K.; Lanz A. L.; Fuchs S.; Weigel S.; Wurzenberger C.; von der Borch P.; Golic M.; Moder S.; et al. (2008) Targeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity. J. Immunol. 181, 2990–2998. 10.4049/jimmunol.181.5.2990. [DOI] [PubMed] [Google Scholar]
- Tacken P. J.; Zeelenberg I. S.; Cruz L. J.; van Hout-Kuijer M. A.; van de Glind G.; Fokkink R. G.; Lambeck A. J.; Figdor C. G. (2011) Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood 118, 6836–6844. 10.1182/blood-2011-07-367615. [DOI] [PubMed] [Google Scholar]
- Kachura M. A.; Hickle C.; Kell S. A.; Sathe A.; Calacsan C.; Kiwan R.; Hall B.; Milley R.; Ott G.; Coffman R. L.; et al. (2016) A CpG-Ficoll Nanoparticle Adjuvant for Anthrax Protective Antigen Enhances Immunogenicity and Provides Single-Immunization Protection against Inhaled Anthrax in Monkeys. J. Immunol. 196, 284–297. 10.4049/jimmunol.1501903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. T.; Keller S.; Manganiello M. J.; Cheng C.; Lee C. C.; Opara C.; Convertine A.; Stayton P. S. (2013) pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano 7, 3912–3925. 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Titta A.; Ballester M.; Julier Z.; Nembrini C.; Jeanbart L.; van der Vlies A. J.; Swartz M. A.; Hubbell J. A. (2013) Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl. Acad. Sci. U. S. A. 110, 19902–19907. 10.1073/pnas.1313152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.; Ramishetti S.; Tseng Y. C.; Guo S.; Wang Y.; Huang L. (2013) Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. J. Controlled Release 172, 259–265. 10.1016/j.jconrel.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Wilson K. D.; Raney S. G.; Sekirov L.; Chikh G.; deJong S. D.; Cullis P. R.; Tam Y. K. (2007) Effects of intravenous and subcutaneous administration on the pharmacokinetics, biodistribution, cellular uptake and immunostimulatory activity of CpG ODN encapsulated in liposomal nanoparticles. Int. Immunopharmacol. 7, 1064–1075. 10.1016/j.intimp.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Shivahare R.; Vishwakarma P.; Parmar N.; Yadav P. K.; Haq W.; Srivastava M.; Gupta S.; Kar S. (2014) Combination of liposomal CpG oligodeoxynucleotide 2006 and miltefosine induces strong cell-mediated immunity during experimental visceral leishmaniasis. PLoS One 9, e94596. 10.1371/journal.pone.0094596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. D.; Hessel E. M.; Gregorio J.; Abbate C.; Yee P.; Chu M.; Nest G. V.; Coffman R. L.; Fearon K. L. (2003) Novel chimeric immunomodulatory compounds containing short CpG oligodeoxyribonucleotides have differential activities in human cells. Nucleic Acids Res. 31, 5122–5133. 10.1093/nar/gkg700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fissell W. H.; Manley S.; Dubnisheva A.; Glass J.; Magistrelli J.; Eldridge A. N.; Fleischman A. J.; Zydney A. L.; Roy S. (2007) Ficoll is not a rigid sphere. Am. J. Physiol. Renal Physiol. 293, F1209–1213. 10.1152/ajprenal.00097.2007. [DOI] [PubMed] [Google Scholar]
- Inman J. K. (1975) Thymus-independent antigens: the preparation of covalent, hapten-ficoll conjugates. J. Immunol. 114, 704–709. [PubMed] [Google Scholar]
- Amlot P. L.; Hayes A. E.; Gray D.; Gordon-Smith E. C.; Humphrey J. H. (1986) Human immune responses in vivo to protein (KLH) and polysaccharide (DNP-Ficoll) neoantigens: normal subjects compared with bone marrow transplant patients on cyclosporine. Clin. Exp. Immunol. 64, 125–135. [PMC free article] [PubMed] [Google Scholar]
- Schwander S.; Opravil M.; Luthy R.; Hanson D. G.; Schindler J.; Dawson A.; Letwin B.; Dietrich M. (1994) Phase I/II vaccination study of recombinant peptide F46 corresponding to the HIV-1 transmembrane protein coupled with 2.4 dinitrophenyl (DNP) Ficoll. Infection 22, 86–91. 10.1007/BF01739010. [DOI] [PubMed] [Google Scholar]
- Verma A.; Stellacci F. (2010) Effect of surface properties on nanoparticle-cell interactions. Small 6, 12–21. 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- Bachmann M. F.; Jennings G. T. (2010) Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 10, 787–796. 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- Manolova V.; Flace A.; Bauer M.; Schwarz K.; Saudan P.; Bachmann M. F. (2008) Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 38, 1404–1413. 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- Aebig J. A.; Mullen G. E.; Dobrescu G.; Rausch K.; Lambert L.; Ajose-Popoola O.; Long C. A.; Saul A.; Miles A. P. (2007) Formulation of vaccines containing CpG oligonucleotides and alum. J. Immunol. Methods 323, 139–146. 10.1016/j.jim.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H.; Gursel I.; Ivins B. E.; Singh M.; O’Hagan D. T.; Ulmer J. B.; Klinman D. M. (2005) CpG oligodeoxynucleotides adsorbed onto polylactide-co-glycolide microparticles improve the immunogenicity and protective activity of the licensed anthrax vaccine. Infect. Immun. 73, 828–833. 10.1128/IAI.73.2.828-833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A.; Homma T.; Batchelor J.; Hashimoto N.; Imai S.; Wakiguchi H.; Saito H.; Matsumoto K. (2003) Interferon-alpha/beta receptor-mediated selective induction of a gene cluster by CpG oligodeoxynucleotide 2006. BMC Immunol. 4, 8. 10.1186/1471-2172-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay M. P.; Follmann D. A.; Lynn F.; Schiffer J. M.; Stark G. V.; Kohberger R.; Quinn C. P.; Nuzum E. O. (2012) Anthrax vaccine-induced antibodies provide cross-species prediction of survival to aerosol challenge. Sci. Transl. Med. 4, 151ra126. 10.1126/scitranslmed.3004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin S. A. (2010) Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17, 1055–1065. 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering D.; Thompson W.; Hewetson J.; Little S.; Norris S.; Pace-Templeton J. (2004) Validation of the anthrax lethal toxin neutralization assay. Biologicals 32, 17–27. 10.1016/j.biologicals.2003.09.003. [DOI] [PubMed] [Google Scholar]