Abstract
Ketamine, an N-methyl-d-aspartate antagonist, blunts central pain sensitization at sub-anesthetic doses (0.3 mg/kg or less) and has been studied extensively as an adjunct for perioperative analgesia. At sub-anesthetic doses, ketamine has a minimal physiologic impact though it is associated with a low incidence of mild psychomimetic symptoms as well as nystagmus and double vision. Contraindications to its use do exist and due to ketamine's metabolism, caution should be exercised in patients with renal or hepatic dysfunction. Sub-anesthetic ketamine improves pain scores and reduces perioperative opioid consumption in a broad range of surgical procedures. In addition, there is evidence that ketamine may be useful in patients with opioid tolerance and for preventing chronic postsurgical pain.
Keywords: Ketamine, N-methyl-d-aspartate, pain
Introduction
Ketamine, a phencyclidine derivative originally known as CI-581, was synthesized in the early 1960s as an anesthetic agent with unique properties including minimal negative effects on the cardiorespiratory system.[1,2] Clinical use in anesthesia was first reported in 1966 and since that time ketamine has become a standard anesthetic drug primarily used for induction in hemodynamically unstable patients or as an adjunct for analgesia or sedation.[3] Ketamine is a N-methyl-d-aspartate (NMDA) receptor antagonist which, at anesthetic dose (≥1.0 mg/kg intravenous [IV]), has broad effects in the central nervous system that cause a dissociative anesthetic state.[1,4]
Since the 1980s, investigations have revealed a critical role of the NMDA receptor in pain processing and ketamine has received considerable interest as an analgesic.[5] At sub-anesthetic doses (≤0.3 mg/kg IV), ketamine possesses centrally mediated analgesic properties with minimal effects on consciousness and cognition. Numerous clinical studies support the role of sub-anesthetic ketamine as an analgesic, particularly for acute pain in the perioperative setting.[6] In this review, we discuss the mechanisms, pharmacology, and clinical applications of IV sub-anesthetic ketamine for perioperative pain management.
Mechanisms
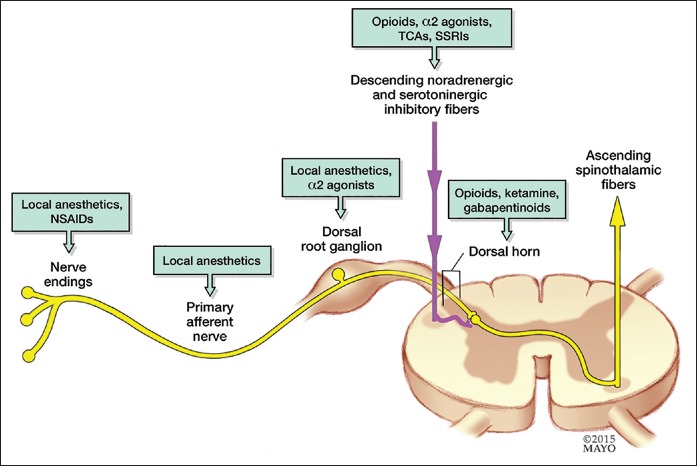
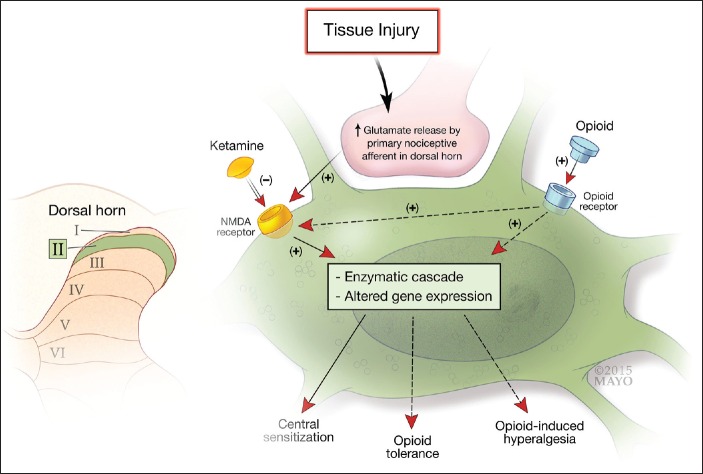
The current model [Figure 1] of pain processing consists of 3 primary sites of molecular and neural modulation including the peripheral nociceptor, nerve and dorsal root ganglion, the dorsal horn of the spinal cord, and the brain and brainstem. The NMDA receptor probably exerts much of its pain processing effect in the dorsal horn of the spinal cord.[7] In response to tissue injury or trauma, the primary nociceptive neuron triggers a release of glutamate in the dorsal horn of the spinal cord, which binds to NMDA receptors on second-order neurons [Figure 2]. Once activated, the NMDA receptor triggers a cascade of intracellular processes that culminate in the altered behavior and expression of NMDA receptors as well as neuronal synaptic plasticity that lies behind the development of central sensitization.[6,7] In addition, the NMDA receptor is intimately involved in the development of opioid tolerance and opioid-induced hyperalgesia, processes which are related to and can occur in parallel with central sensitization when patients are being treated with opioids.[6,8] In addition to being a natural component of acute pain (secondary hyperalgesia and wind-up), central sensitization in response to trauma or surgery can evolve into a pathologic chronic pain condition. By blocking the NMDA receptor, ketamine holds obvious promise for attenuating these centrally mediated pain processes, thereby reducing acute pain and potentially preventing chronic pain. It should be noted that the NMDA receptor is present throughout the central nervous system and that ketamine, in addition to its actions in the spinal cord, probably has multiple effects on pain processing.[4] Functional magnetic resonance imaging data as well as recent studies demonstrating ketamine's efficacy for treating intractable depression suggests that, in addition to spinal actions, the NMDA receptor and ketamine modulate the affective components of pain.[9,10]
Figure 1.
A variety of drugs act at different anatomic locations along the pain signaling pathway. Ketamine modulates pain at the dorsal horn of the spinal cord
Figure 2.
The activated primary nociceptive afferent from the periphery releases glutamate at the second order sensory neuron in the dorsal horn of the spinal cord which binds to N-methyl-d-aspartate receptors. Ketamine blocks the N-methyl-d-aspartate receptor which attenuates the development of central sensitization as well as opioid tolerance and hyperalgesia
Physiologic Effects
Psychiatric
Ketamine is well-known to cause psychomimetic effects such as hallucinations, out-of-body sensations, vivid dreams, and dysphoria. The incidence of psychomimetic effects is dose-dependent and when given at anesthetic doses without benzodiazepines or other hypnotic/amnestic agents, will likely cause these problems. Patients receiving sub-anesthetic ketamine are also at risk for psychomimetic effects. Two large systematic reviews of perioperative sub-anesthetic ketamine reported a 7.4% incidence of psychomimetic effects compared with 3.7-5% in the placebo groups.[11,12] The risk of psychomimetic effects increases with ketamine dose though there is no firm data to suggest there is any “cut-off” dose. Clinical experience suggests that a bolus dose of <0.5 mg/kg typically does not cause major psychomimetic disturbances. Studies with 24-72 h inpatient ketamine infusions show that infusions of 0.12-0.2 mg/kg/h have no increased incidence of psychomimetic effects.[13,14,15,16,17,18,19,20]
Ketamine may reactivate psychosis in schizophrenic patients even at sub-anesthetic doses as low as 0.3 mg/kg.[21,22] It is not known whether other psychiatric illnesses predispose to psychomimetic side effects. Psychosis was an explicit exclusion criterion in some but not all of the clinical studies on sub-anesthetic ketamine, and there are insufficient data to clarify its impact on patients with psychotic disorders.
Neurologic
At anesthetic doses (≥1 mg/kg), ketamine induces a “dissociative” anesthetic state resembling catatonia due to its effects on a variety of cortical and subcortical processes. At sub-anesthetic doses (≤0.3 mg/kg), consciousness and arousal are not typically affected to a significant degree. Systematic reviews demonstrate that sub-anesthetic ketamine given perioperatively does not appear to cause increased levels of sedation and in fact, ketamine may decrease sedation possibly due to opioid-sparing effects.[12,23] Ketamine does appear to cause a small increase in intracranial pressure (ICP) probably due to its sympathomimetic effects and a rise in intra-arterial CO2 .[24] The effects of sub-anesthetic doses of ketamine are unknown but are likely to be small. Finally, studies are conflicting and inconclusive about ketamine's effects on seizure thresholds.[21]
Cardiovascular
At anesthetic doses, ketamine causes transient increases in blood pressure, heart rate and cardiac index, presumably secondary to a central sympathomimetic effect.[21,25] Ketamine is also known to exert a direct myocardial depressant effect particularly in patients with already depressed ventricular function though this effect is usually more than offset by the sympathomimetic effects discussed above.[26] This myocardial depressant effect may become relevant in patients who are in shock and have exhausted their adrenergic neurotransmitters. Multiple studies demonstrate that there are no measurable cardiovascular effects with sub-anesthetic ketamine.[14,17,18,27,28,29,30,31,32,33,34,35,36,37,38,39,40]
Respiratory
At anesthetic doses, ketamine is well-known to preserve pharyngeal and laryngeal reflexes as well as respiratory drive, a property, which has made it an agent of choice in situations where maintenance of spontaneous ventilation is desirable. At high doses, ketamine increases oral secretions and might cause a small increase of the incidence of laryngospasm.[21] At sub-anesthetic doses, ketamine does not appear to have any significant effect on the respiratory system.[14,17,18,27,34] Co-administration of benzodiazepines to mitigate psychomimetic symptoms may increase the risk of respiratory depression in postoperative patients, particularly those also receiving opioids.
Gastrointestinal
At anesthetic doses, ketamine is emetogenic when compared with propofol or thiopental.[21] However, according to a systematic review of perioperative sub-anesthetic ketamine, there is not an increased risk of nausea and vomiting at lower doses.[13] In fact, a Cochrane review of perioperative sub-anesthetic ketamine showed that ketamine was associated with a statistically significant reduction in nausea and vomiting, possibly secondary to its opioid-sparing effects.[23]
Ocular
Similar to its effects on ICP at anesthetic doses, ketamine causes a small and probably clinically insignificant increase in intraocular pressure that lasts for 15 min following a bolus dose.[21] It is unknown what effect, if any, sub-anesthetic dose ketamine has on intraocular pressure though it is likely very minimal. Ketamine is also well known to cause both nystagmus and diplopia at high doses as well as sub-anesthetic doses. In a systematic review of perioperative sub-anesthetic ketamine, the authors reported a statistically significant increase in the incidence of nystagmus or diplopia in patients receiving ketamine versus controls (6.2% vs. 2.6%).[12]
Basic Pharmacology
Formulations
Ketamine was originally a racemic mixture of S(+) and R(−) enantiomers in equal parts. Recently, a S(+) ketamine formulation has become available, though not in the United States.[6] S(+) ketamine is more potent, has a shorter duration of action and more rapid clearance than racemic ketamine.[21,35] Racemic ketamine remains the formulation used most commonly in clinical settings.
Metabolism
Ketamine is metabolized in the liver by microsomal cytochrome P450 enzymes, principally CY3A4. Three days following a single dose of ketamine, only 2.3% is excreted in the urine unchanged, 1.6% as norketamine, 16.2% as dehydronorketamine, and 80% as conjugated hydroxylated derivatives.[1] Norketamine and dehydronorketamine have one-third and 1/100th the potency of ketamine, respectively, and the rest of the metabolites are thought to be inactive.[21] In theory, patients with liver disease may have prolonged clearance of the drug; however, studies including patients with liver insufficiency have not shown that these patients metabolize ketamine differently to any clinically significant degree.[25]
Pharmacokinetics
Ketamine clearance is described by a two-compartment model with a rapid distribution phase (distribution half-life of 11-16 min). Elimination half-life is 2-3 h and the clearance is 12-17 mL/kg/min.[36] Ketamine is highly lipid soluble and has a large volume of distribution (3 L/kg).[4] Ketamine will accumulate during prolonged infusions, and the context sensitive half-time is similar to that of propofol.[4,37] Most of the pharmacokinetic modeling of ketamine infusions has been done using anesthetic or sedation doses, and there are little data about the pharmacokinetic implications of prolonged sub-anesthetic ketamine infusions. Stubhaug et al. studied a low-dose ketamine protocol consisting of an initial bolus of 0.5 mg/kg followed by a 72-h continuous infusion (first 24 h at 2 mcg/kg/min followed by 48 h at 1 mcg/kg/min) and measured serum concentrations of ketamine and norketamine at 1, 24, and 72 h. Their results suggest that after 72 h, the serum concentrations of ketamine are still below that 1 h following a 0.5 mg/kg bolus. Norketamine levels do rise, however not significantly.[13]
Renal dysfunction could cause prolonged clearance of ketamine metabolites, though this is probably not clinically significant as the vast majority is metabolized into inactive metabolites.[38] There are no data to suggest that sub-anesthetic ketamine is unsafe in patients with renal dysfunction.
Toxicity and Contraindications
Ketamine has been shown to cause a mild and transient increase in liver enzymes in after sub-anesthetic infusions.[39,40] Clinical liver dysfunction was not observed in any of these cases, and transaminase levels returned to normal after ketamine cessation. Nonetheless, it is possible that certain patients may be at risk for developing abnormal liver function tests during prolonged low-dose ketamine infusions.
Bladder complaints ranging from urinary frequency to ulcerative cystitis have been reported in patients with prolonged exposure to ketamine either from recreational or medicinal use.[35,41] This has not been reported following short-term administration of ketamine in the perioperative period.
Contraindications to sub-anesthetic ketamine
Relative contraindications to sub-anesthetic ketamine are listed in Table 1. Sub-anesthetic ketamine for perioperative analgesia is an elective treatment, and a risk-benefit assessment should be done in all cases where the patient may have a relative contraindication.
Table 1.
Contraindications to sub-anesthetic ketamine

Clinical Applications
There is ample evidence that perioperative ketamine is effective for postsurgical pain management. In 2005, Elia and Tramèr performed a systematic review of 53 randomized trials of perioperative ketamine in adults and children and reported that, in a sub-group of ten trials that examined visual analog scale (VAS), perioperative ketamine was associated with a statistically significant reduction in pain scores at 6, 12, 24, and 48 h postoperation.[12] In a Cochrane review from 2010, Bell et al. reviewed 37 randomized controlled trials of adult surgical patients who received perioperative ketamine or placebo and found that 27 of the 37 trials demonstrated that ketamine reduced analgesic requirements and/or pain scores.[23] In a sub-group of ten of these studies that measured 24-h patient-controlled analgesia (PCA) morphine, the authors concluded that perioperative ketamine reduces 24-h morphine consumption by roughly 16 mg (Elia and Tramèr report a similar number). It should be noted that the studies in both of these large systematic reviews were heterogenous with respect to ketamine dose, timing of administration, and importantly, route of administration (epidural and IV). Though there are conflicting data, neuraxial ketamine is potentially neurotoxic and is not currently recommended for the treatment of noncancer pain.[6] Furthermore, epidural and regional anesthesias are probably confounders in ketamine studies because they are typically independently effective for postoperative analgesia. In 2011, Laskowski et al. published a systematic review of 70 studies that looked at only IV ketamine for perioperative analgesia (importantly excluding all studies that used neuraxial or regional anesthesia).[11] Using a random effects statistical model, the authors found that perioperative IV ketamine significantly reduced postoperative opioid consumption and increased the time to first postoperative analgesic requirement.
Laskowski et al. performed a sub-group analysis and concluded that ketamine reduced opioid consumption most profoundly in upper abdominal and thoracic procedures. Ketamine was effective, although less so in orthopedic (limb and spine) and lower abdominal surgery. Opioid-sparing in ear, nose, and throat and oral surgery procedures was not significant. Furthermore, the authors found that opioid-sparing was greatest in patients with high VAS scores (70% maximum or greater) and was not beneficial with lower VAS scores (<40% maximum), suggesting that ketamine is more useful for the most painful operations. This is intuitively appealing though there are studies demonstrating the analgesic efficacy of IV ketamine in less traumatic procedures such as outpatient knee arthroscopy. There exists debate as to whether ketamine should be reserved for certain types of procedures.[42]
There is less support in the literature for the use of ketamine for pediatric patients. Pediatric subgroup analysis in the Laskowski article suggests that ketamine is not an effective analgesic adjunct in children though the pediatric subgroup was dominated by tonsillectomy studies which were independently associated with the minimal effect. Furthermore, a meta-analysis of pediatric ketamine studies concluded that, though ketamine was associated with lower postanesthesia care unit (PACU) pain scores and nonopioid requirements, it did not reduce postoperative opioid consumption.[43]
Ketamine has received considerable interest for patients on chronic opioid therapy who are undergoing surgery. Four studies have looked at ketamine in this patient population, and the conclusions have been mixed.[15,20,30,44] Loftus et al. published the largest (101 patients) and methodologically best of these studies looking at intraoperative IV ketamine in opioid-tolerant patients undergoing spine surgery.[30] These authors report an average 37% reduction in morphine consumption over 48 h (309 mg vs. 195 mg). Pain scores were not significantly different in the 48-h postoperative period though interestingly, at the 6-week follow-up, the ketamine group reported 26% lower pain scores (4.2 vs. 3.1).
Because of its unique mechanisms and potential to blunt central sensitization, ketamine may theoretically prevent the development of chronic postsurgical pain. In fact, this has been looked at in several studies, but the results have been mixed. In a recent meta-analysis, McNicol et al. combined the data from 14 studies that compared perioperative IV ketamine with placebo.[45] The authors found that at 3 and 6 months respectively, the ketamine group had 25% and 30% reduction in the risk of persistent postsurgical pain. At 12 months, there was no significant difference between the groups. Though not conclusive, these findings offer some support for the use of perioperative ketamine in operations well-known to cause chronic postsurgical pain such as mastectomy, thoracotomy, and limb amputation.
In summary, the balance of evidence in the current literature suggests that perioperative ketamine improves postoperative analgesia and reduces postoperative opioid consumption in a wide range of surgical procedures. Furthermore, there is emerging evidence that ketamine may be useful for surgical patients on chronic opioid therapy and may play a role in preventing persistent postsurgical pain. Indications for perioperative ketamine are listed in Table 2.
Table 2.
Indications for perioperative sub-anesthetic ketamine

Practical Considerations: How to Use It
There is little consensus on the best way to administer the drug for perioperative analgesia. IV is preferable to epidural due to toxicity concerns mentioned previously, and because patients with epidurals may be less likely to benefit from adjunctive ketamine. A myriad of IV ketamine dosing strategies have been examined including pre- and post-incision bolus, intraoperative infusion, postoperative infusion, and PCA. All have demonstrated efficacy in various trials.
Kwok et al. compared preincision and postoperative sub-anesthetic ketamine and found that pre-emptive ketamine was associated with a small but statistically significant reduction of PACU pain scores and opioid consumption as well as lower oral analgesic consumption over the 1st postoperative week.[29] However, other studies comparing pre-emptive ketamine with postoperative ketamine have not found any benefit.[11,31,46,47] There are many studies demonstrating efficacy and safety of intraoperative and postoperative ketamine infusions as well as the addition of ketamine to opioid in PCA though there are no head-to-head comparisons between these different approaches. In a subgroup analysis of a large systematic review, Laskowski et al. found no difference in the analgesic effect when comparing mode of delivery (i.e., PCA, bolus, infusion) of IV ketamine.[12]
Himmelseher and Durieux lay out a compelling argument that the ideal dosing strategy should include a preincision bolus followed by either a prolonged infusion or serial boluses.[6] These authors argue that because ketamine works by blocking central sensitization, plasma, and central nervous system tissue levels should be maintained throughout the period of painful stimulation which includes not only the surgery but the postoperative period as well. However, despite the theoretical appeal of this approach, there is not substantial literature to support its superiority. From a practical standpoint, infusions are more applicable if the period of administration is going to be longer than 2 or 3 h. In short cases, serial bolus administration is a reasonable approach.
Another area of uncertainty is what constitutes the ideal dose of sub-anesthetic ketamine. In most of the published studies, effective intraoperative bolus doses range from 0.15 mg/kg to 0.5 mg/kg and infusions are most commonly in the range of 0.1-0.2 mg/kg/h (2 mcg/kg/min is a common infusion dose as well). Psychosensory effects increase at doses above 0.3 mg/kg, so this can be considered a soft upper limit for bolus doses in awake patients. The effects of a bolus dissipate after 30-45 min, so obviously an anesthetized patient can tolerate higher doses so long as they will not be emerging from anesthesia in the immediate future. Furthermore, during long operations, serial boluses of ketamine every 30-45 min should be considered. There is no consensus on the upper limit of ketamine infusion, but 0.3 mg/kg/h is a reasonable upper limit to be considered in awake patients in a non-Intensive Care Unit setting. In obese patients, ideal body weight should be used for dose calculation.[48]
The ideal duration of postoperative infusion is also not clear from the literature, but 24-72-h infusions are both efficacious and safe. With prolonged infusions of any drug, there is always some concern about drug and metabolite accumulation. As discussed earlier, Stubhaug et al. demonstrated clinically insignificant accumulation of ketamine and norketamine after a 72 h infusion of sub-anesthetic ketamine.[13] There are no studies of postoperative ketamine infusions that suggest prolonged infusions are associated with increased incidence of side effects.
Conclusions
Ketamine, an NMDA antagonist, blunts central pain sensitization at sub-anesthetic doses (0.3 mg/kg or less) and has been studied extensively as an adjunctive analgesic in the perioperative setting. At sub-anesthetic doses, ketamine has a minimal physiologic impact though it is associated with a low incidence of mild psychomimetic symptoms as well as nystagmus and double vision. Relative contraindications to its use do exist and due to ketamine's metabolism, caution should be exercised in patients with renal or hepatic dysfunction.
Sub-anesthetic ketamine improves pain scores and reduces perioperative opioid consumption in a broad range of surgical procedures with a minimal risk of side effects. Sub-anesthetic ketamine has efficacy when given as an intraoperative bolus alone or as an intraoperative dose followed by a postoperative infusion of 24-72 h. The ideal dose of sub-anesthetic ketamine is 0.1-0.3 mg/kg as a bolus and 0.1-0.3 mg/kg/h as an infusion. In Table 3 the authors present their recommended dosing strategy. Further research is needed to define the best indications for perioperative sub-anesthetic ketamine and to further explore the potential long-term implications of perioperative ketamine on the development of persistent postsurgical pain.
Table 3.
How to administer sub-anesthetic ketamine for perioperative analgesia

Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Domino EF. Taming the ketamine tiger 1965. Anesthesiology. 2010;113:678–84. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 2.Domino EF, Chodoff P, Corssen G. Pharmacologic effects of CI-581, a new dissociative anesthetic, in man. Clin Pharmacol Ther. 1965;6:279–91. doi: 10.1002/cpt196563279. [DOI] [PubMed] [Google Scholar]
- 3.Chodoff P, Stella JG. Use of CI-581 a phencyclidine derivative for obstetric anesthesia. Anesth Analg. 1966;45:527–30. [PubMed] [Google Scholar]
- 4.Reves JG, Glass PS, Lubarsky DA, McEvoy MD, Martinez-Ruiz R. Intravenous anesthetics. In: Miller RE, editor. Miller's Anesthesia. 7th ed. Philadelphia: Churchill Livingstone; 2010. pp. 724–6. [Google Scholar]
- 5.Chizh BA. Low dose ketamine: A therapeutic and research tool to explore N-methyl-D-aspartate (NMDA) receptor-mediated plasticity in pain pathways. J Psychopharmacol. 2007;21:259–71. doi: 10.1177/0269881105062484. [DOI] [PubMed] [Google Scholar]
- 6.Himmelseher S, Durieux ME. Ketamine for perioperative pain management. Anesthesiology. 2005;102:211–20. doi: 10.1097/00000542-200501000-00030. [DOI] [PubMed] [Google Scholar]
- 7.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: A current view of their possible interactions. Pain. 1995;62:259–74. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen KG, Lineberry TW, Galardy CW, Kung S, Lapid MI, Palmer BA, et al. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol. 2013;27:444–50. doi: 10.1177/0269881113478283. [DOI] [PubMed] [Google Scholar]
- 10.Sprenger T, Valet M, Woltmann R, Zimmer C, Freynhagen R, Kochs EF, et al. Imaging pain modulation by subanesthetic S-(+)−ketamine. Anesth Analg. 2006;103:729–37. doi: 10.1213/01.ane.0000231635.14872.40. [DOI] [PubMed] [Google Scholar]
- 11.Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth. 2011;58:911–23. doi: 10.1007/s12630-011-9560-0. [DOI] [PubMed] [Google Scholar]
- 12.Elia N, Tramèr MR. Ketamine and postoperative pain - A quantitative systematic review of randomised trials. Pain. 2005;113:61–70. doi: 10.1016/j.pain.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Stubhaug A, Breivik H, Eide PK, Kreunen M, Foss A. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthesiol Scand. 1997;41:1124–32. doi: 10.1111/j.1399-6576.1997.tb04854.x. [DOI] [PubMed] [Google Scholar]
- 14.Adriaenssens G, Vermeyen KM, Hoffmann VL, Mertens E, Adriaensen HF. Postoperative analgesia with i.v. patient-controlled morphine: Effect of adding ketamine. Br J Anaesth. 1999;83:393–6. doi: 10.1093/bja/83.3.393. [DOI] [PubMed] [Google Scholar]
- 15.Barreveld AM, Correll DJ, Liu X, Max B, McGowan JA, Shovel L, et al. Ketamine decreases postoperative pain scores in patients taking opioids for chronic pain: Results of a prospective, randomized, double-blind study. Pain Med. 2013;14:925–34. doi: 10.1111/pme.12086. [DOI] [PubMed] [Google Scholar]
- 16.Guillou N, Tanguy M, Seguin P, Branger B, Campion JP, Mallédant Y. The effects of small-dose ketamine on morphine consumption in surgical intensive care unit patients after major abdominal surgery. Anesth Analg. 2003;97:843–7. doi: 10.1213/01.ANE.0000075837.67275.36. [DOI] [PubMed] [Google Scholar]
- 17.Javery KB, Ussery TW, Steger HG, Colclough GW. Comparison of morphine and morphine with ketamine for postoperative analgesia. Can J Anaesth. 1996;43:212–5. doi: 10.1007/BF03011736. [DOI] [PubMed] [Google Scholar]
- 18.Murdoch CJ, Crooks BA, Miller CD. Effect of the addition of ketamine to morphine in patient-controlled analgesia. Anaesthesia. 2002;57:484–8. doi: 10.1046/j.0003-2409.2001.02409.x. [DOI] [PubMed] [Google Scholar]
- 19.Remérand F, Le Tendre C, Baud A, Couvret C, Pourrat X, Favard L, et al. The early and delayed analgesic effects of ketamine after total hip arthroplasty: A prospective, randomized, controlled, double-blind study. Anesth Analg. 2009;109:1963–71. doi: 10.1213/ANE.0b013e3181bdc8a0. [DOI] [PubMed] [Google Scholar]
- 20.Subramaniam K, Akhouri V, Glazer PA, Rachlin J, Kunze L, Cronin M, et al. Intra- and postoperative very low dose intravenous ketamine infusion does not increase pain relief after major spine surgery in patients with preoperative narcotic analgesic intake. Pain Med. 2011;12:1276–83. doi: 10.1111/j.1526-4637.2011.01144.x. [DOI] [PubMed] [Google Scholar]
- 21.Craven R. Ketamine. Anaesthesia. 2007;62(Suppl 1):48–53. doi: 10.1111/j.1365-2044.2007.05298.x. [DOI] [PubMed] [Google Scholar]
- 22.Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995;6:869–72. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- 23.Bell RF, Dahl JB, Moore RA, Kalso E. Perioperative ketamine for acute postoperative pain. Cochrane Database Syst Rev. 2006;1:CD004603. doi: 10.1002/14651858.CD004603.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Takeshita H, Okuda Y, Sari A. The effects of ketamine on cerebral circulation and metabolism in man. Anesthesiology. 1972;36:69–75. doi: 10.1097/00000542-197201000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Idvall J, Ahlgren I, Aronsen KR, Stenberg P. Ketamine infusions: Pharmacokinetics and clinical effects. Br J Anaesth. 1979;51:1167–73. doi: 10.1093/bja/51.12.1167. [DOI] [PubMed] [Google Scholar]
- 26.Morris C, Perris A, Klein J, Mahoney P. Anaesthesia in haemodynamically compromised emergency patients: Does ketamine represent the best choice of induction agent? Anaesthesia. 2009;64:532–9. doi: 10.1111/j.1365-2044.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- 27.Carstensen M, Møller AM. Adding ketamine to morphine for intravenous patient-controlled analgesia for acute postoperative pain: A qualitative review of randomized trials. Br J Anaesth. 2010;104:401–6. doi: 10.1093/bja/aeq041. [DOI] [PubMed] [Google Scholar]
- 28.Guignard B, Coste C, Costes H, Sessler DI, Lebrault C, Morris W, et al. Supplementing desflurane-remifentanil anesthesia with small-dose ketamine reduces perioperative opioid analgesic requirements. Anesth Analg. 2002;95:103–8. doi: 10.1097/00000539-200207000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Kwok RF, Lim J, Chan MT, Gin T, Chiu WK. Preoperative ketamine improves postoperative analgesia after gynecologic laparoscopic surgery. Anesth Analg. 2004;98:1044–9. doi: 10.1213/01.ANE.0000105911.66089.59. [DOI] [PubMed] [Google Scholar]
- 30.Loftus RW, Yeager MP, Clark JA, Brown JR, Abdu WA, Sengupta DK, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113:639–46. doi: 10.1097/ALN.0b013e3181e90914. [DOI] [PubMed] [Google Scholar]
- 31.Mathisen LC, Aasbø V, Raeder J. Lack of pre-emptive analgesic effect of (R)-ketamine in laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 1999;43:220–4. doi: 10.1034/j.1399-6576.1999.430218.x. [DOI] [PubMed] [Google Scholar]
- 32.Persson J, Hasselstrom J, Maurset A, Oye I, Svensson JO, Almqvist O, et al. Pharmacokinetics and non-analgesic effects of S- and R-ketamines in healthy volunteers with normal and reduced metabolic capacity. Eur J Clin Pharmacol. 2002;57:869–75. doi: 10.1007/s002280100353. [DOI] [PubMed] [Google Scholar]
- 33.Roytblat L, Korotkoruchko A, Katz J, Glazer M, Greemberg L, Fisher A. Postoperative pain: The effect of low-dose ketamine in addition to general anesthesia. Anesth Analg. 1993;77:1161–5. doi: 10.1213/00000539-199312000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Weinbroum AA. A single small dose of postoperative ketamine provides rapid and sustained improvement in morphine analgesia in the presence of morphine-resistant pain. Anesth Analg. 2003;96:789–95. doi: 10.1213/01.ANE.0000048088.17761.B4. [DOI] [PubMed] [Google Scholar]
- 35.Persson J. Wherefore ketamine? Curr Opin Anaesthesiol. 2010;23:455–60. doi: 10.1097/ACO.0b013e32833b49b3. [DOI] [PubMed] [Google Scholar]
- 36.Clements JA, Nimmo WS. Pharmacokinetics and analgesic effect of ketamine in man. Br J Anaesth. 1981;53:27–30. doi: 10.1093/bja/53.1.27. [DOI] [PubMed] [Google Scholar]
- 37.Dallimore D, Anderson BJ, Short TG, Herd DW. Ketamine anesthesia in children - Exploring infusion regimens. Paediatr Anaesth. 2008;18:708–14. doi: 10.1111/j.1460-9592.2008.02665.x. [DOI] [PubMed] [Google Scholar]
- 38.Murphy EJ. Acute pain management pharmacology for the patient with concurrent renal or hepatic disease. Anaesth Intensive Care. 2005;33:311–22. doi: 10.1177/0310057X0503300306. [DOI] [PubMed] [Google Scholar]
- 39.Dundee JW, Fee JP, Moore J, McIlroy PD, Wilson DB. Changes in serum enzyme levels following ketamine infusions. Anaesthesia. 1980;35:12–6. doi: 10.1111/j.1365-2044.1980.tb03713.x. [DOI] [PubMed] [Google Scholar]
- 40.Noppers IM, Niesters M, Aarts LP, Bauer MC, Drewes AM, Dahan A, et al. Drug-induced liver injury following a repeated course of ketamine treatment for chronic pain in CRPS type 1 patients: A report of 3 cases. Pain. 2011;152:2173–8. doi: 10.1016/j.pain.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 41.Chiew YW, Yang CS. Disabling frequent urination in a young adult. Ketamine-associated ulcerative cystitis. Kidney Int. 2009;76:123–4. doi: 10.1038/ki.2009.139. [DOI] [PubMed] [Google Scholar]
- 42.Menigaux C, Guignard B, Fletcher D, Sessler DI, Dupont X, Chauvin M. Intraoperative small-dose ketamine enhances analgesia after outpatient knee arthroscopy. Anesth Analg. 2001;93:606–12. doi: 10.1097/00000539-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Dahmani S, Michelet D, Abback PS, Wood C, Brasher C, Nivoche Y, et al. Ketamine for perioperative pain management in children: A meta-analysis of published studies. Paediatr Anaesth. 2011;21:636–52. doi: 10.1111/j.1460-9592.2011.03566.x. [DOI] [PubMed] [Google Scholar]
- 44.Urban MK, Ya Deau JT, Wukovits B, Lipnitsky JY. Ketamine as an adjunct to postoperative pain management in opioid tolerant patients after spinal fusions: A prospective randomized trial. HSS J. 2008;4:62–5. doi: 10.1007/s11420-007-9069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNicol ED, Schumann R, Haroutounian S. A systematic review and meta-analysis of ketamine for the prevention of persistent post-surgical pain. Acta Anaesthesiol Scand. 2014;58:1199–213. doi: 10.1111/aas.12377. [DOI] [PubMed] [Google Scholar]
- 46.Dahl V, Ernoe PE, Steen T, Raeder JC, White PF. Does ketamine have preemptive effects in women undergoing abdominal hysterectomy procedures? Anesth Analg. 2000;90:1419–22. doi: 10.1097/00000539-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 47.Menigaux C, Fletcher D, Dupont X, Guignard B, Guirimand F, Chauvin M. The benefits of intraoperative small-dose ketamine on postoperative pain after anterior cruciate ligament repair. Anesth Analg. 2000;90:129–35. doi: 10.1097/00000539-200001000-00029. [DOI] [PubMed] [Google Scholar]
- 48.Wulfsohn NL. Ketamine dosage for induction based on lean body mass. Anesth Analg. 1972;51:299–305. [PubMed] [Google Scholar]