Abstract
Background and Aims:
Patients with brainstem tumors have many associated systemic abnormalities and are prone to develop perioperative complications. We studied the problems associated with brainstem tumors and their influence on the postoperative neurological outcome.
Material and Methods:
Retrospective review of records of patients who underwent surgery for brainstem tumors over a period of 8 years was done. Preoperative variables, perioperative complications and neurological outcome as assessed by Glasgow Outcome Scale at the time of hospital discharge were noted. Association between perioperative factors and the unfavorable neurological outcome was evaluated.
Results:
Data of 70 patients were retrieved, 7 patients were excluded from the study because of incomplete data and data analysis was carried out for 63 patients. We found that lower cranial nerve palsies (32%) and hydrocephalus (43%) were common preoperatively. Various intraoperative problems encountered were hemodynamic instability (56%), major blood loss requiring blood transfusion (40%) and venous air embolism (11%), and postoperative problems were meningitis (51%), hypokalemia (38%), chest infection (21%), seizure (11%), deterioration of Glasgow Coma Scale (GCS, 11%), hyponatremia (8%), hydrocephalus (6%), respiratory distress (3%) and operatives site hematoma (3%). Fifty-six (89%) patients had favorable outcome at hospital discharge whereas, 7 (11%) had an unfavorable outcome. There was no association between pre- and intra-operative factors and the neurological outcome. Deterioration of GCS, chest infection, and the need for reintubation and tracheostomy were associated with unfavorable neurological outcome.
Conclusion:
Patients of brainstem tumors are at increased risk of perioperative complications. Some of the postoperative complications were associated with unfavorable neurological outcome.
Keywords: Brainstem tumor, intraoperative complications, neurological outcome, postoperative complications
Introduction
Brainstem tumor patients generally present with many associated systemic abnormalities and are prone to develop complications in the perioperative period. Various causes of these complications are contrast agents, electrolyte disturbances, loss of airway reflexes, pulmonary aspiration, hydrocephalus, chest infection, and the need for prolonged intravenous fluids, diuretics, and ventilator support.[1,2,3,4,5] Associated systemic abnormalities and perioperative complications can have significant adverse effects on patient outcome. The scarcity of data about the systemic abnormalities in these patients prompted us to conduct a retrospective review to enumerate the perioperative problems in these patients and the factors associated with unfavorable neurological outcome.
Material and Methods
This is a retrospective review of the patients who underwent surgery for brainstem tumors at our institute between January 2002 and December 2009. After approval of the ethics committee, data were collected by reviewing the patients' medical records including; anesthetic records and perioperative notes. Following preoperative data were collected: age, sex, weight, American Society of Anesthesiologists physical status, associated systemic illness, cough and gag reflexes, type of tumor, and neurological deficits. The intraoperative data of blood transfusion and complications such as hemodynamic instability (hypotension requiring vasopressor therapy), venous air embolism (VAE, sudden and sustained fall in EtCO2 by 5 mmHg or more), and hypothermia (core temperature <36°C) were recorded. Postoperative data regarding mechanical ventilation, lower cranial nerve (LCN) function deterioration, Glasgow Coma Scale (GCS) deterioration, occurrence of respiratory distress, residual tumor, reintubation, and tracheostomy, occurrence of various complications (meningitis, electrolyte disturbances, chest infection, postoperative hydrocephalus, seizure, postoperative hematoma), duration of Intensive Care Unit (ICU) and hospital stay and Glasgow Outcome Score (GOS) at the time of hospital discharge were recorded.[6]
On the basis of GOS at the time of hospital discharge, patients were divided into two groups as favorable GOS group (GOS score: 4 or 5) and unfavorable GOS group (GOS score: 1-3).[6]
Statistical analysis
Statistical analysis was performed using software Stata 9.0 (College Station, Texas, USA). Data are expressed as mean ± standard deviation or number (%). t-test and Fisher's exact test were carried out for continuous and categorical variables respectively, to compare different variables between the two groups. P <0.05 was considered as significant.
Results
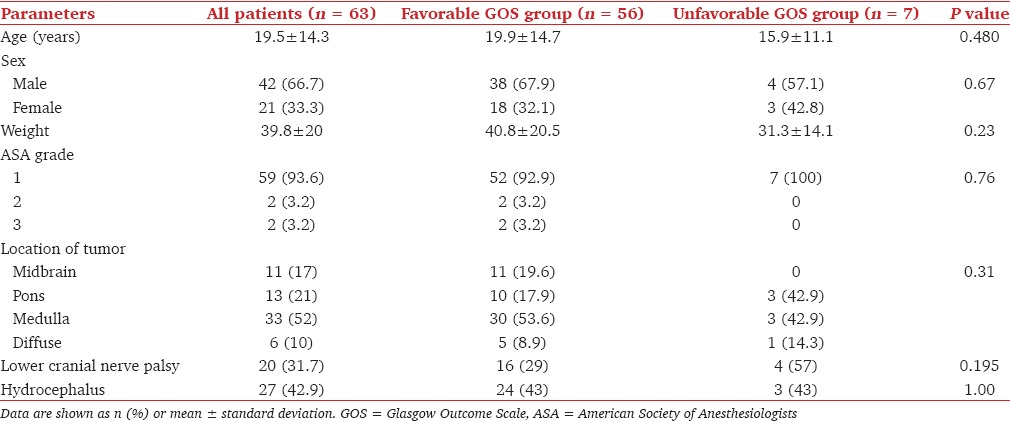
A total of 70 patients were operated during this period. Seven patients were excluded from the study because of incomplete data, and data analysis was done for 63 patients. The demographic and preoperative characteristics of the patients are given in Table 1. Twenty (31.7%) patients presented with the involvement of LCNs with impaired gag reflex. Twenty-seven (42.9%) patients had hydrocephalus.
Table 1.
Preoperative factors and outcome
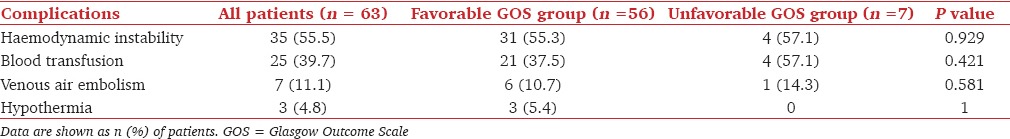
Majority of patients were operated in prone (36 patients, 57%) or sitting position (17 patients, 27%) but, some patients were operated in supine position (8 patients, 13%) and lateral position (2 patients, 3%) as well. Balanced anesthesia technique consisting of O2: N2O, isoflurane, fentanyl, and vecuronium/rocuronium, was used in all cases. Along with routine monitoring (heart rate, electrocardiography, pulse oximetry, temperature and end-tidal CO2), monitoring of invasive blood pressure and central venous pressure was also carried out in all patients. The intraoperative complications are given in Table 2. Seven (11.1%) patients had VAE during surgery and they were all operated in sitting position. VAE did not result in any clinically significant alteration in hemodynamic parameters. Hemodynamic instability requiring vasopressor therapy occurred in 35 (55.5%) patients.
Table 2.
Intraoperative complications and outcome
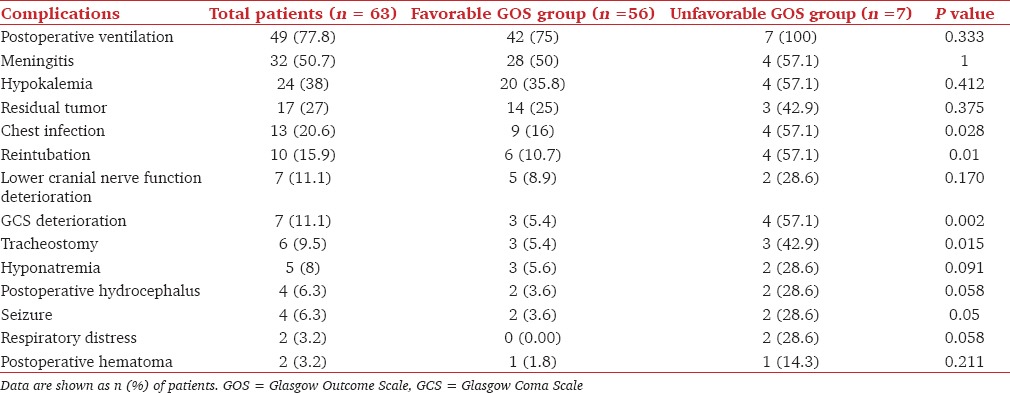
The postoperative complications are given in Table 3. Fourteen patients were extubated immediately after surgery, and 49 were electively ventilated. Of the 20 patients who presented with the involvement of LCNs, 7 (35%) patients had further deterioration in their LCNs in the postoperative period, as shown by impaired cough reflex. All of these 7 patients had brainstem handling manifesting with hemodynamic disturbances during the intraoperative period. All the 13 (20.6%) patients who developed chest infection in the postoperative period, had preexisting LCN palsies. Ten (15.8%) patients who were extubated after surgery required reintubation in the ICU, 2 due to acute onset respiratory distress, 7 due to deterioration in the sensorium and 1 due to generalized seizures. Three of them eventually needed tracheostomy due to the requirement of prolonged ventilation. In addition, another 3 patients required tracheostomy due to a superimposed chest infection, weaning failure, and prolonged ventilation. Two patients (3.2%) developed operative site hematoma in the postoperative period. One underwent surgical evacuation whereas, the other was managed conservatively. In our study, meningitis occurred in 32 (50.8%) patients and of them, 27 (84.4%) had associated cerebrospinal fluid (CSF) leak. Four (6.3%) patients had generalized seizure in the postoperative period, and in 3 of them hyponatremia (Na < 120) was the reason.
Table 3.
Postoperative complications and outcome
Mean duration of ICU stay was 4.3 days (range: 2-16 days) and mean duration of hospital stay was 14.1 days (range: 4-60 days). Of 63 patients, 56 (88.9%) patients had favorable outcome at hospital discharge whereas, 7 (11.1%) patients had an unfavorable outcome. Four (6.3%) patients died in the postoperative period, 2 patients (3.2%) remained severely disabled and 1 patient (1.5%) was discharged in a vegetative state.
Factors which were found to be associated with unfavorable GOS were deterioration of GCS, chest infection, need for reintubation and tracheostomy [Table 3].
Discussion
In this study of patients with brainstem tumors, we examined the incidence of various perioperative factors and their influence on the neurological outcome at the time of hospital discharge. We observed a high incidence of preoperative hydrocephalus (43%) and preexisting LCN palsies in (32%) our study population. Similar incidence of hydrocephalus and LCN palsies were also observed in earlier studies.[1,4,7,8,9,10,11,12]
Various intraoperative problems encountered in our patients were hemodynamic instability requiring vasopressor therapy, occurrence of VAE, major blood loss requiring blood transfusion. We did not find any significant association between preoperative and intraoperative factors on neurological outcome.
We observed a significant association between postoperative complications and unfavorable neurological outcome. Various postoperative factors found to be associated with unfavorable outcome were GCS deterioration, chest infection, and the need for reintubation and tracheostomy.
Infratentorial surgeries carries a higher rate of CSF leak, which may contribute to a higher rate of postoperative meningitis.[13,14,15] In our study, meningitis occurred in 32 (51%) patients and 27 (84%) of these patients had CSF leak. Several factors which can predispose to CSF leak include tumor size, a dural invasion of the tumor, hydrocephalus, blood in CSF, and brain edema.[13,14,15]
Brainstem tumors patients are prone to develop a number of complications in the postoperative period leading to prolonged mechanical ventilation. Factors responsible for increased risk of respiratory complications are damage to the respiratory center, LCN palsies, altered sensorium, pulmonary aspiration, need for reintubation and prolonged ventilation. Chevron et al., in a prospective case-control study, found that a low consciousness level correlated with the need for reintubation.[16] The incidence of reintubation in neurosurgical patients, in general has been described as 0.56-8.2% and has been attributed to both anesthetic and surgical factors.[17,18] However, none of these studies were conducted in patients of brainstem tumor. Brainstem tumor patients may be more prone to reintubation because of the damage to the respiratory center, LCN palsies, and altered sensorium. This was the reason of high incidence of reintubation in our study (16%), as compared with the low incidence reported in general neurosurgical population.
Thirteen (21%) patients developed chest infection in the postoperative period. All of these patients had impaired LCNs and gag reflex in the preoperative period. The impaired gag reflex is known to cause chest infection due to impaired swallowing, pooling of saliva in the oral cavity and silent aspiration. High incidence of postoperative elective ventilation, decreased sensorium, and reintubation are the other possible reasons for the postoperative chest infections.[19] Jallo et al. in a retrospective analysis of 41 children and adolescents with brainstem medullary tumors, reported loss of LCN in 46% of them, 11% of patients had pneumonia in the postoperative period and all of them required tracheostomy.[4] Sogame et al. in their study in elective intracranial surgery reported that the predicting factors for postoperative pulmonary complications are prolonged mechanical ventilation (≥48 h), time spent in ICU (>3 days), decreased in level of consciousness, duration of surgery (≥300 min), and preexisting chronic lung diseases.[19]
Generalized seizures following neurosurgery may lead to deterioration of patient's condition and the need for intubation and mechanical ventilation. Suri et al. in a retrospective study of posterior fossa surgeries (for diverse types of pathologies) in 511 patients reported 5.9% incidence of seizures.[20] Our incidence of 6% is in agreement with their observation. Whereas, in their series, most common cause of seizures was tension pneumocephalus, it was hyponatremia in our series. The main reason for different etiology of seizures in their series versus ours could be the more frequent employment of sitting position in their study (48.9% vs. 27%).
The retrospective nature of the study and the small sample size are the limitations of our study. A larger sample size with a multifactorial regression analysis would have shown independent predictors of the unfavorable neurological outcome.
Conclusion
The patients of brainstem tumors are at increased risks of perioperative complications. We found that the occurrence of GCS deterioration, chest infection, reintubation, and tracheostomy in the postoperative period is associated with unfavorable outcome. Further, larger prospective studies are required for determination of independent predictors of unfavorable outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wang G, Zhang J, Sun M, Wang C, Long DM. Surgical management of brainstem mass lesions: Respiratory insufficiency occurrence and recovery. Neurosurg Q. 2001;11:302–13. [Google Scholar]
- 2.Dubey A, Sung WS, Shaya M, Patwardhan R, Willis B, Smith D, et al. Complications of posterior cranial fossa surgery - An institutional experience of 500 patients. Surg Neurol. 2009;72:369–75. doi: 10.1016/j.surneu.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Abbott R, Shiminski-Maher T, Epstein FJ. Intrinsic tumors of the medulla: Predicting outcome after surgery. Pediatr Neurosurg. 1996;25:41–4. doi: 10.1159/000121095. [DOI] [PubMed] [Google Scholar]
- 4.Jallo GI, Shiminski-Maher T, Velazquez L, Abbott R, Wisoff J, Epstein F. Recovery of lower cranial nerve function after surgery for medullary brainstem tumors. Neurosurgery. 2005;56:74–7. doi: 10.1227/01.neu.0000144782.39430.12. [DOI] [PubMed] [Google Scholar]
- 5.Warnick RE. Surgical complications and their avoidance. In: Winn HR, editor. Youmans Neurological Surgery. Vol. 1. Philadelphia: Saunders; 2004. pp. 931–40. [Google Scholar]
- 6.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–4. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 7.Raimondi AJ, Tomita T. Hydrocephalus and infratentorial tumors. Incidence, clinical picture, and treatment. J Neurosurg. 1981;55:174–82. doi: 10.3171/jns.1981.55.2.0174. [DOI] [PubMed] [Google Scholar]
- 8.Vandertop WP, Hoffman HJ, Drake JM, Humphreys RP, Rutka JT, Amstrong DC, et al. Focal midbrain tumors in children. Neurosurgery. 1992;31:186–94. doi: 10.1227/00006123-199208000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Chitnavis B, Phipps K, Harkness W, Hayward R. Intrinsic brainstem tumours in childhood: A report of 35 children followed for a minimum of 5 years. Br J Neurosurg. 1997;11:206–9. doi: 10.1080/02688699746258. [DOI] [PubMed] [Google Scholar]
- 10.Guillamo JS, Monjour A, Taillandier L, Devaux B, Varlet P, Haie-Meder C, et al. Brainstem gliomas in adults: Prognostic factors and classification. Brain. 2001;124(Pt 12):2528–39. doi: 10.1093/brain/124.12.2528. [DOI] [PubMed] [Google Scholar]
- 11.Amano T, Inamura T, Nakamizo A, Inoha S, Wu CM, Ikezaki K. Case management of hydrocephalus associated with the progression of childhood brain stem gliomas. Childs Nerv Syst. 2002;18:599–604. doi: 10.1007/s00381-002-0637-5. [DOI] [PubMed] [Google Scholar]
- 12.Roujeau T, Di Rocco F, Dufour C, Bourdeaut F, Puget S, Rose CS, et al. Shall we treat hydrocephalus associated to brain stem glioma in children? Childs Nerv Syst. 2011;27:1735–9. doi: 10.1007/s00381-011-1538-2. [DOI] [PubMed] [Google Scholar]
- 13.Bryce GE, Nedzelski JM, Rowed DW, Rappaport JM. Cerebrospinal fluid leaks and meningitis in acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1991;104:81–7. doi: 10.1177/019459989110400115. [DOI] [PubMed] [Google Scholar]
- 14.Bennett M, Haynes DS. Surgical approaches and complications in the removal of vestibular schwannomas. Otolaryngol Clin North Am. 2007;40:589. doi: 10.1016/j.otc.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Friedman RA, Cullen RD, Ulis J, Brackmann DE. Management of cerebrospinal fluid leaks after acoustic tumor removal. Neurosurgery. 2007;61(3 Suppl):35–9. doi: 10.1227/01.neu.0000289709.87802.12. [DOI] [PubMed] [Google Scholar]
- 16.Chevron V, Ménard JF, Richard JC, Girault C, Leroy J, Bonmarchand G. Unplanned extubation: Risk factors of development and predictive criteria for reintubation. Crit Care Med. 1998;26:1049–53. doi: 10.1097/00003246-199806000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Meng L. Reintubation during postoperative care in patients undergoing neurosurgical procedures. J Neurosurg Anesthesiol. 2010;22:436. [Google Scholar]
- 18.Vidotto MC, Sogame LC, Gazzotti MR, Prandini M, Jardim JR. Implications of extubation failure and prolonged mechanical ventilation in the postoperative period following elective intracranial surgery. Braz J Med Biol Res. 2011;44:1291–8. doi: 10.1590/s0100-879x2011007500146. [DOI] [PubMed] [Google Scholar]
- 19.Sogame LC, Vidotto MC, Jardim JR, Faresin SM. Incidence and risk factors for postoperative pulmonary complications in elective intracranial surgery. J Neurosurg. 2008;109:222–7. doi: 10.3171/JNS/2008/109/8/0222. [DOI] [PubMed] [Google Scholar]
- 20.Suri A, Mahapatra AK, Bithal P. Seizures following posterior fossa surgery. Br J Neurosurg. 1998;12:41–4. doi: 10.1080/02688699845500. [DOI] [PubMed] [Google Scholar]


