Abstract
Background and Aims:
We compared interscalene brachial plexus block (ISBPB) using peripheral nerve stimulation (PNS) and ultrasound (US) techniques. The primary outcomes were the incidence of hemidiaphragmatic paresis (HDP) and the duration of the block. Secondary outcomes were the block success rate, time to conduct the block, onset of sensory block, and dermatomal spread, postoperative pain by Numeric Rating Scale (NRS), duration of postoperative analgesia and incidence of complications.
Material and Methods:
We conducted a prospective, randomized, and observer-blinded study in 60 patients undergoing shoulder arthroscopy under block plus general anesthesia. ISBPB was performed with 10 ml of 0.5% bupivacaine using either PNS (Group PNS, n = 30) or US (Group US, n = 30). Hemidiaphragmatic function, the primary outcome, was assessed by ultrasonographic evaluation of diaphragmatic movement and pulmonary function tests using a bedside spirometer (forced vital capacity, forced expiratory volume in 1 s and peak expiratory flow rate). General anesthesia was administered to all the patients for surgery. P < 0.05 test was considered to be statistically significant.
Results:
Twelve patients in Group PNS had HDP and none in Group US (P < 0.0001). PFTs were also significantly reduced in Group PNS (P < 0.0001). The time to conduct the block and sensory onset time both were less in Group US (P < 0.05). The groups did not differ in block success rate, duration of analgesia, and NRS. Other complications like incidence of Horner's syndrome and vascular puncture were comparable in both the groups.
Conclusions:
PNS guided ISBPB with 10 ml of 0.5% bupivacaine is associated with a higher incidence of HDP as compared to US guided ISBPB. There is no significant difference in quality or duration of analgesia in the two groups.
Keywords: Hemidiaphragmatic paresis, local anesthetic, peripheral nerve stimulator, ultrasound
Introduction
Although shoulder surgeries can be done under interscalene brachial plexus block (ISBPB) alone,[1] for shoulder arthroscopy that involves a beach chair or lateral position and a lot of irrigation, block alone may be uncomfortable.[2,3] Hence, in certain institutes a combination of interscalene block with general anesthesia is utilized for shoulder arthroscopy.
Interscalene brachial plexus block affects mainly the C5, 6, 7 spinal roots at their trunks and thus provides complete anesthesia of shoulder.[4] The phrenic nerve is primarily composed of the anterior branch of spinal root C4 with variable contributions from spinal roots C3 and C5.[5] It courses caudally over the ventral surface of the anterior scalene muscle and prevertebral fascia that covers it.[5] It is either this close proximity of phrenic nerve to brachial plexus or a cephalad spread of the local anesthetic to the C3-C5 roots of the cervical plexus that appears to be responsible for its block and the resultant hemidiaphragmatic paresis (HDP) which is associated with changes in respiratory mechanics.[6,7]
Studies have compared the influence of various techniques such as peripheral nerve stimulator (PNS) versus ultrasound (US) guided block, and the volume of local anesthetics on the incidence of HDP.[8,9,10]
Previous studies with varied local anesthetic volumes at either cricoid level or at the level of C7 have documented a high incidence of HDP.[11,12,13,14,15] We decided to do the current study with 10 ml of local anesthetic (0.5% bupivacaine) for giving ISBPB at a cricoid level either by US or PNS technique. The primary outcome of the study was the incidence of HDP, which was done by assessment of hemidiaphragmatic function by US and spirometry tests. The secondary outcomes were to study block success rate, time taken to conduct the block, sensory dermatomal spread, incidence of complications (vascular puncture, Horner's syndrome), duration of hemidiaphragmatic dysfunction and postoperative pain scores.
Material and Methods
After approval from Institutional Ethical Committee, 60 patients scheduled for shoulder arthroscopy were recruited for the study. A written, informed consent was obtained from all the subjects. Inclusion criteria were patients with age between 18 and 50 years of either sex belonging to American Society of Anesthesiologists physical status I/II. Exclusion criteria were patients with local anesthetic allergy, preexisting lung diseases or hemidiaphragmatic dysfunction, neuropathy, coagulopathy, pregnancy. Patients were randomly allocated using a computer generated random number table into either Group US (n = 30, received US guided block) or Group PNS (n = 30, received PNS guided block).
HDP was evaluated by measuring the movement of diaphragm in supine position with a real time M mode US of hemidiaphragm using a 17 mm 1-5 MHz curved array US probe (Sonosite Nanomaxx). A caudad motion of the diaphragm during inspiration was considered normal. A 50% reduction in diaphragmatic movement or paradoxical movement (the cephalad motion during inspiration) was considered to be HDP. Pulmonary function tests (PFTs) viz. forced vital capacity, forced expiratory volume in 1 s, and peak expiratory flow rate were recorded with bedside spirometer. Values recorded were the average of three measurements. The assessments were done by an independent observer at preblock (baseline) and at 5, 10, 15, and 20 min after ISBPB. After preblock assessment patients received sedation with intravenous midazolam 0.03 mg/kg for the administration of block. Verbal communication was maintained throughout the block procedure. The time taken to conduct the block was recorded.
Group NS
Patients were put in supine position with the head turned to the contralateral side. Under all aseptic precautions, ISBPB was performed with PNS (B Braun, 22G insulated needle). The needle was introduced in the interscalene groove at the level of the cricoid and advanced until contractions of either biceps (C5, 6 roots) or triceps (C6, 7, 8 roots) were obtained. When these contractions persisted at the stimulation current 0.2-0.5 mA, and after negative aspiration, 10 ml of 0.5% bupivaciane was injected slowly.
Group US
Interscalene brachial plexus block was given with an in-plane approach using 22G short bevel needle at the level of the cricoid cartilage. A 13-6 MHz linear array probe (Sonosite Nanomaxx) was placed first to obtain an image of sternocleidomastoid and scalene muscle in short axis view. The needle was introduced in-plane from the lateral side of the probe to reach between the anterior and middle scalene muscle. The brachial plexus was identified as the most hypoechoic nerve structures lying in the groove. C5-C6 roots were confirmed by tracing and seeing them joining to form the superior trunk of the brachial plexus. The needle tip was adjusted caudally toward the C6 trunk, and 10 ml of 0.5% bupivacaine was injected slowly with negative aspiration. The drug was given with multi-injection technique in a controlled fashion to avoid medial and rostral spread. If such a spread was observed in real-time, the needle tip was directed more caudally. Any collection of local anesthetic anterior to scalenus anterior was observed at the end of injection.
The end of the local anesthetic injection was taken as time zero. The onset time of sensory anesthesia in dermatomes C4-C8 (C4 - top of the shoulder, C5 - skin over deltoid, C6 - tip of thumb, C7 - tip of middle finger, C8 - tip of little finger) were noted. The block was declared successful when complete sensory anesthesia was achieved in dermatomes C5 and C6. Other complications like vascular puncture and incidence of Horner's syndrome were also recorded.
All patients received general anesthesia 20 min after parameter monitoring was completed. Patients were induced with conventional doses of propofol and intubation was facilitated by vecuronium. Anesthesia was maintained with nitrous oxide, oxygen, and isoflurane mixture in a closed circuit with vecuronium top ups. Standard monitoring with an electrocardiogram, noninvasive blood pressure, capnogram, and pulse oximetry was carried out. In addition, depth of anesthesia was monitored with entropy and whenever required fentanyl (maximum 2 mcg/kg) was administered intravenously. At the end of the surgery, patients were reversed, extubated and shifted to recovery. In the immediate postoperative period, HDP and PFTs were evaluated and thereafter continued to monitor at hourly intervals till 6 h in the recovery. Evaluation of postoperative pain was done using a Numeric Rating Scale (NRS). Thereafter, NRS was recorded on an hourly basis and whenever pain score was >4, rescue analgesic in the form of tramadol 2 mg/kg was given intravenously. The time when rescue analgesia was demanded was recorded as the duration of postoperative analgesia. Patients were discharged to the ward with a prescription of paracetamol infusion to be started at 8 h after surgery. HDP was evaluated again on postoperative day 1.
Statistical analysis
Al-Kaisy et al. showed that by reducing the dose of bupivacaine by 50% for ISBPB, the risk of having HDP decreased from 80 to 17%.[11] We conducted a preliminary study in twenty-five patients using US and found that none of the patients suffered from HDP. On the basis of above two findings, the 95% confidence interval was calculated using the modified Wald method to be 0-0.16. The incidence of HDP in ISBPB with PNS is 100%.[12] Using Fischer exact sample size calculation (power 90%), it was estimated to study 28 patients in each group. We included 30 patients in each group to compensate for the possibility of dropouts.
The data were analyzed by using SPSS (Statistical Package for Social Sciences) version 18.0 (SPSS Inc, Chicago, IL, USA). To compare the change over time in diaphragmatic movements and pulmonary functions within groups repeated measures analysis of variance was used. Nominal nonparametric data were analyzed using χ2 or Fischer exact test. Mann-Whitney U-test was utilized to compare the difference of nonparametric variables between groups. Student's t-test was used where applicable. P < 0.05 test was considered to be statistically significant.
Results
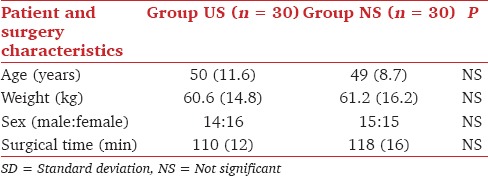
All 60 patients completed the study. Patients' demographic profile and surgery characteristics were comparable in both the groups [Table 1].
Table 1.
Demographic profile (mean with SD in parenthesis)
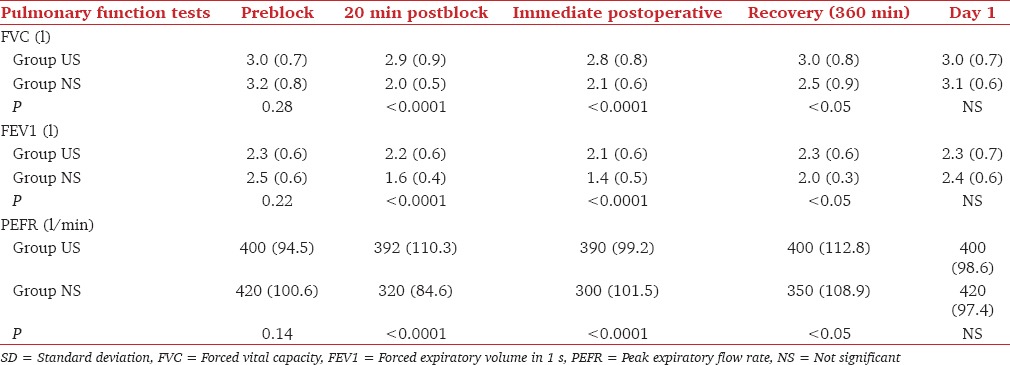
The incidence of HDP, Horner's syndrome and vascular puncture are given in Table 2. Ten of the 12 patients with HDP had a reduced and 2 paradoxical diaphragmatic motion. The maximum duration of hemidiaphragmatic dysfunction was 360 min. PFTs were significantly reduced in Group PNS at all times after baseline [Table 3].
Table 2.
Incidence of HDP and other complications
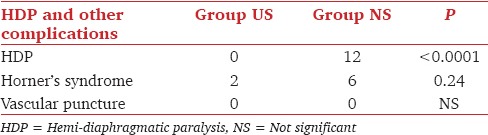
Table 3.
Changes in pulmonary function tests (mean with SD in parenthesis)
The C5 dermatome block was achieved in all patients of both groups. Group US had a higher incidence of C7 and C8 level block, and a lower incidence of C4 level block compared to Group PNS (P < 0.05). C3 block was also seen in 11 patients in Group NS as against none in Group US (P < 0.05) [Figure 1].
Figure 1.
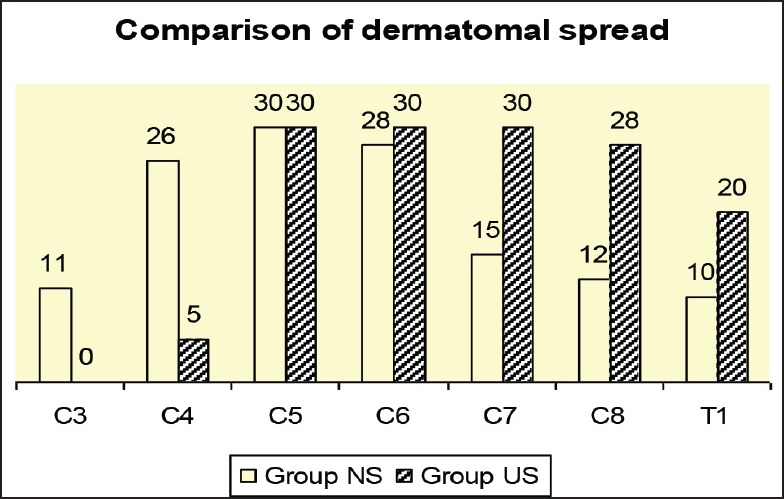
Dermatomal spread
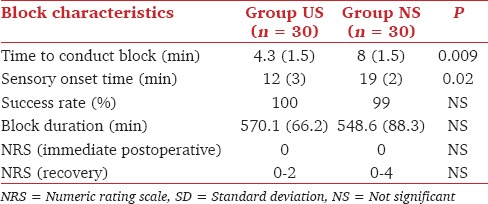
Block characteristics are given in Table 4.
Table 4.
Block characteristics (mean with SD in parenthesis)
Discussion
HDP associated with ISBPB can cause a compromise in ventilation in patients with limited pulmonary reserve like the morbidly obese, those with chronic obstructive lung diseases, and the elderly.[13]
Traditionally, ISBPB was given with landmark technique eliciting paresthesia with 40 ml of LA that resulted in 100% incidence of HDP.[12] Attempts were made to decrease the incidence of HDP by decreasing the LA volume from 40 to 20 ml and then to 10 ml, using landmark or PNS technique.[7,8,9,10] It was observed that with PNS technique and using 10 ml of LA, there was a 20% decrease in the incidence of HDP, but the block had inconsistent C3-C6 dermatomal spread.[9] Therefore it is still uncertain whether 10 ml is sufficient for complete anesthesia of the shoulder.[14]
With the advent of US, interventions were carried out by changing the site of drug injection or by further decreasing the LA volume.[10,15,16] Previous efforts to determine the minimum effective LA dose for ISBPB with least decrease in hemidiaphragmatic function demonstrated a dose-response relationship. Gautier P et al. found that, a minimum LA volume of 5 ml was effective for adequate anesthesia.[17] Renes et al. compared PNS and US guided interscalene block at the level of C7 and found a significant reduction in the incidence of HDP in the latter group.[15] They hypothesized that at C7 level the injection site is farther away from upper cervical roots and phrenic nerve compared to the cricoid level. However, the safety of administering ISBPB at C7 level near C7 tubercle, which has vertebral vessels in close vicinity is to be challenged. Sinha et al. compared US guided 20 versus 10 ml of LA injected at the cricoid level and found that the low volume did not decrease the incidence of HDP.[16] Our results are contradict these findings. The difference in the results can be ascribed to the fact that we injected the drug caudally in a controlled fashion, and observed the spread over anterior scalene muscle at the end of injection. This may indicate direct spread to be one of the causative factors for the HDP at the cricoid level. We postulate that with PNS, the needle is inserted more proximal to the scalenus anterior which could lead to spreading of LA toward scalenus anterior muscle and blocking of the phrenic nerve. We, however, did not ascertain this spread at the end of LA injection in PNS group.
Kessler et al.[18] found by US that the distance between the phrenic nerve and C5 root was 1.8 mm at the cricoid and 10.8 mm at a point 3 cm caudal. They suggested that phrenic nerve block was independent of LA volume at the cricoid level because of the proximity of the phrenic nerve to the C5 root.
With the use of US, the drug is precisely deposited around target structures, and hence a lesser volume of LA appears sufficient.[19] Riazi et al.[10] found a lower incidence of HDP with 5 ml of LA compared to 20 ml using US. US allows real-time visualization with scrupulous drug deposition thus hastening the onset and easing the administration of block.[20] Our finding of adequate sensory anesthesia in both groups is consistent with results from previously conducted studies. However, we observed more complete block of C5-C7 with US compared to PNS. C3 block was only seen and C4 was more commonly blocked with PNS. The association of HDP and reduced PFTs that we elucidated in our study are in concordance with others. LA volume as low as 3 ml for ISBPB has been implicated in causing respiratory distress from concurrent phrenic nerve block.[21] Although none of the patients in Group US suffered from HDP, the inability to predict which patients are prone to develop this complication prevents it from being used in patients with severe respiratory compromise.[22]
There are certain limitations to our study. We included only sensory blockade monitoring and instituted general anesthesia in all patients; hence our results cannot be extrapolated to surgeries that need to be done under ISBPB alone. We did not perform an ultrasonographic evaluation to determine LA spread over anterior scalene muscle in the PNS group, which would have helped in deducing the mechanism of HDP in PNS group. Another limitation was that there was no blinding of the patient.
Conclusion
The incidence of hemidiaphragmatic paralysis is high with PNS guided ISBPB compared to US guided even with 10 ml of local anesthetic volume. Nevertheless caution is warranted while using US ISBPB in patients with limited pulmonary reserve.
Footnotes
Source of Support: Nil
Conflicts of Interest: None declared.
References
- 1.Bishop JY, Sprague M, Gelber J, Krol M, Rosenblatt MA, Gladstone J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87:974–9. doi: 10.2106/JBJS.D.02003. [DOI] [PubMed] [Google Scholar]
- 2.Janssen H, Stosch Rv, Pöschl R, Büttner B, Bauer M, Hinz JM, et al. Blood pressure response to combined general anaesthesia/interscalene brachial plexus block for outpatient shoulder arthroscopy. BMC Anesthesiol. 2014;30(14):50. doi: 10.1186/1471-2253-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozzeybek D, Oztekin S, Mavioglu O, Karaege G, Ozkardesler S, Ozkan M, et al. Comparison of the haemodynamic effects of interscalene block combined with general anaesthesia and interscalene block alone for shoulder surgery. J Int Med Res. 2003;31:428–33. doi: 10.1177/147323000303100512. [DOI] [PubMed] [Google Scholar]
- 4.Salcedo E, Shay P, Berrigan M. The pre-emptive analgesic effect of interscalene blockprior to shoulder surgery (scientific poster) Reg Anesth. 1996;21:107. [Google Scholar]
- 5.Arthur F Dalley, Anne MR Agur. Grant's Atlas of Anatomy. 6th ed. Baltimore, MD: Williams and Wilkins; 1972. Plate 663. [Google Scholar]
- 6.Urmey WF, McDonald M. Hemidiaphragmatic paresis during interscalene brachial plexus block: Effects on pulmonary function and chest wall mechanics. Anesth Analg. 1992;74:352–7. doi: 10.1213/00000539-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Fujimura N, Namba H, Tsunoda K, Kawamata T, Taki K, Igarasi M, et al. Effect of hemidiaphragmatic paresis caused by interscalene brachial plexus block on breathing pattern, chest wall mechanics, and arterial blood gases. Anesth Analg. 1995;81:962–6. doi: 10.1097/00000539-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Sala-Blanch X, Lázaro JR, Correa J, Gómez-Fernandez M. Phrenic nerve block caused by interscalene brachial plexus block: Effects of digital pressure and a low volume of local anesthetic. Reg Anesth Pain Med. 1999;24:231–5. [PubMed] [Google Scholar]
- 9.Urmey WF, Gloeggler PJ. Pulmonary function changes during interscalene brachial plexus block: Effects of decreasing local anesthetic injection volume. Reg Anesth. 1993;18:244–9. [PubMed] [Google Scholar]
- 10.Riazi S, Carmichael N, Awad I, Holtby RM, McCartney CJ. Effect of local anaesthetic volume (20 vs 5 ml) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth. 2008;101:549–56. doi: 10.1093/bja/aen229. [DOI] [PubMed] [Google Scholar]
- 11.al-Kaisy AA, Chan VW, Perlas A. Respiratory effects of low-dose bupivacaine interscalene block. Br J Anaesth. 1999;82:217–20. doi: 10.1093/bja/82.2.217. [DOI] [PubMed] [Google Scholar]
- 12.Urmey WF, Talts KH, Sharrock NE. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography. Anesth Analg. 1991;72:498–503. doi: 10.1213/00000539-199104000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Rau RH, Chan YL, Chuang HI, Cheng CR, Wong KL, Wu KH, et al. Dyspnea resulting from phrenic nerve paralysis after interscalene brachial plexus block in an obese male - A case report. Acta Anaesthesiol Sin. 1997;35:113–8. [PubMed] [Google Scholar]
- 14.Krone SC, Chan VW, Regan J, Peng P, Poate EM, McCartney C, et al. Analgesic effects of low-dose ropivacaine for interscalene brachial plexus block for outpatient shoulder surgery - A dose-finding study. Reg Anesth Pain Med. 2001;26:439–43. doi: 10.1053/rapm.2001.25914. [DOI] [PubMed] [Google Scholar]
- 15.Renes SH, Rettig HC, Gielen MJ, Wilder-Smith OH, van Geffen GJ. Ultrasound-guided low-dose interscalene brachial plexus block reduces the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med. 2009;34:498–502. doi: 10.1097/AAP.0b013e3181b49256. [DOI] [PubMed] [Google Scholar]
- 16.Sinha SK, Abrams JH, Barnett JT, Muller JG, Lahiri B, Bernstein BA, et al. Decreasing the local anesthetic volume from 20 to 10 mL for ultrasound-guided interscalene block at the cricoid level does not reduce the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med. 2011;36:17–20. doi: 10.1097/aap.0b013e3182030648. [DOI] [PubMed] [Google Scholar]
- 17.Gautier P, Vandepittec C, Ramquet C, De Coopman M, Xu D, Hadzic A. The minimum effective anesthetic volume of 0.75% ropivacaine in ultrasound guided interscalene brachial plexus block. Anesth Analg. 2011;113:951–5. doi: 10.1213/ANE.0b013e31822b876f. [DOI] [PubMed] [Google Scholar]
- 18.Kessler J, Schafhalter-Zoppoth I, Gray AT. An ultrasound study of the phrenic nerve in the posterior cervical triangle: Implications for the interscalene brachial plexus block. Reg Anesth Pain Med. 2008;33:545–50. [PubMed] [Google Scholar]
- 19.Kapral S, Greher M, Huber G, Willschke H, Kettner S, Kdolsky R, et al. Ultrasonographic guidance improves the success rate of interscalene brachial plexus blockade. Reg Anesth Pain Med. 2008;33:253–8. doi: 10.1016/j.rapm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Chan VW. Applying ultrasound imaging to interscalene brachial plexus block. Reg Anesth Pain Med. 2003;28:340–3. doi: 10.1016/s1098-7339(03)00189-5. [DOI] [PubMed] [Google Scholar]
- 21.Koscielniak-Nielsen ZJ. Hemidiaphragmatic paresis after interscalene supplementation of insufficient axillary block with 3 mL of 2% mepivacaine. Acta Anaesthesiol Scand. 2000;44:1160–2. doi: 10.1034/j.1399-6576.2000.440922.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith HM, Duncan CM, Hebl JR. Clinical utility of low-volume ultrasound-guided interscalene blockade: Contraindications reconsidered. J Ultrasound Med. 2009;28:1251–8. doi: 10.7863/jum.2009.28.9.1251. [DOI] [PubMed] [Google Scholar]


