Abstract
Background and Aims:
There is limited data on the impact of perioperative fluid therapy guided by dynamic preload variables like stroke volume variation (SVV) on outcomes after abdominal surgery. We studied the effect of SVV guided versus central venous pressure (CVP) guided perioperative fluid administration on outcomes after major abdominal surgery.
Material and Methods:
Sixty patients undergoing major abdominal surgeries were randomized into two equal groups in this prospective single blind randomized study. In the standard care group, the CVP was maintained at 10-12 mmHg while in the intervention group a SVV of 10% was achieved by the administration of fluids. The primary end-points were the length of Intensive Care Unit (ICU) and hospital stay. The secondary end points were intraoperative lactate, intravenous fluid use, requirement for inotropes, postoperative ventilation and return of bowel function.
Results:
The ICU stay was significantly shorter in the intervention group as compared to the control group (2.9 ± 1.15 vs. 5.4 ± 2.71 days). The length of hospital stay was also shorter in the intervention group, (9.9 ± 2.68 vs. 11.96 ± 5.15 days) though not statistically significant. The use of intraoperative fluids was significantly lower in the intervention group than the control group (7721.5 ± 4138.9 vs. 9216.33 ± 2821.38 ml). Other secondary outcomes were comparable between the two groups.
Conclusion:
Implementation of fluid replacement guided by a dynamic preload variable (SVV) versus conventional static variables (CVP) is associated with lesser postoperative ICU stay and reduced fluid requirements in major abdominal surgery.
Keywords: Abdominal surgery, central venous pressure, stroke volume variation
Introduction
The most ideal volume of fluid replacement during major abdominal surgery remains elusive. Restrictive fluid strategy contributes to better outcomes in major surgeries while goal-directed therapy targeting volume have shown improved results also. Excessive fluid restriction with hypovolemia may cause organ dysfunction, increased postoperative morbidity, and death.[1,2] Central venous pressure (CVP) as a static preload variable has been challenged in its utility to predict fluid responsiveness[3] and a systematic review of 24 studies showed that the CVP is a poor predictor of blood volume and that changes in the CVP do not predict response to fluid administration.[4] Goal-directed fluid management with hemodynamic targets and oxygenation indices have been associated with improved outcomes and reduction in hospital stay in patients undergoing high-risk surgeries.[5,6,7]
Variations in stroke volumes have been used as predictors of fluid responsiveness in patients who are mechanically ventilated. Mechanical ventilation induces cyclic variations in cardiac preload, which is reflected in the cyclic changes in systolic arterial pressure, arterial pulse pressure and left ventricular stroke volume.[8] While cyclical changes associated with ventilation does not produce changes in cardiac output in inadequately filled patients it can cause significant changes with hypovolemia.[8] Essentially, patients with wide variations in the stroke volume will be on the steep portion of the Frank Starling's curve that plots the effects of preload on the stroke volume.
The stroke volume variation (SVV) is the measure of the variations in stroke volume associated with ventilation, and the fluctuations are larger with hypovolemia. As an alternative to static variables like CVP, SVV can be used for guiding fluid therapy in patients receiving mechanical ventilation. This has been found to be a good predictor of fluid responsiveness.[9,10]
The FloTrac Vigileo™ is a minimally invasive cardiac output monitor that calculates the arterial pulsatility as the standard deviation of the arterial pressure wave. The device calculates the resistance and compliance of the vasculature by a proprietary algorithm from which values of stroke volume, cardiac output, systemic vascular resistance (SVR) and SVV are calculated.[11] In this study, we used the SVV measured from the FloTrac Vigileo™ as a guide for fluid replacement versus CVP targets during major abdominal surgery.
The primary outcomes were the length of Intensive Care Unit (ICU) and hospital stay postoperatively. The secondary outcomes were levels of intraoperative serum lactate, inotrope use during surgery, intraoperative fluids, postoperative ventilation and time to return of bowel function.
Material and Methods
This study was a prospective, single-blinded, randomized study. Based on the key article by Donati et al.[7] with a mean difference of 2.1 days for the hospital stay (11.3 ± 3.8 vs. 13.4 ± 6.1) with 95% confidence limit the minimum sample size was calculated as 45 in each group. However due to constraints in time only 60 patients were included in the study. After approval from the hospital ethics committee and informed consent, 60 patients belonging to American Society of Anesthesiologists (ASA) physical status I and II, undergoing major abdominal surgery that included the Whipple's procedure, low anterior resection, retroperitoneal tumor and gastrectomy were recruited for the study. Those with ASA physical status III and above, age <18 years, patients with a history of cardiac arrhythmias, body mass index of >40 kg/m2 and those undergoing combined abdominal and open thoracic surgery were excluded. Patients were allotted to either control group or intervention group by a closed envelope method.
In the theatre, a large bore intravenous (IV) cannula (18G) was placed, and the pulse-oximeter and electrocardiogram were attached. The radial artery was cannulated (20G) under local anesthesia. A thoracic epidural catheter was placed between T8 and T12 levels and checked for intravascular or intrathecal placement by administering a test dose (3 ml of lidocaine 2% with 1 in 200,000 adrenaline). All patients were induced with a standard IV anesthesia protocol that included midazolam 0.02-0.04 mg/kg body weight, glycopyrrolate 0.2 mg, fentanyl 2 mcg/kg and propofol titrated to loss of verbal response. Intubation was accomplished 3 min after the administration of vecuronium 0.1 mg/kg. Tidal volumes of 6-8 ml/kg were used and the respiratory rate of the ventilator was adjusted to maintain EtCO2 between 30 and 40 mm Hg. A positive end-expiratory pressure of 4 cm H2 O was applied uniformly to all patients. Anesthesia was maintained with isoflurane 1-1.5%, air and oxygen (2:1) with intermittent muscle relaxants. Central venous cannulation was performed in the right internal jugular or right subclavian vein by the Seldinger technique. The depth of insertion of the catheter was 12-15 cm. Continuous epidural analgesia was administered by an infusion of 0.25% bupivacaine with 2 mcg/ml fentanyl as an additive at a rate between 4 and 8 ml/h.
The IV fluid therapy was guided by the CVP in the control group (Group C) to maintain it between 10 and 12 mm of Hg. In the intervention group (Group I), the guide for fluid replacement was the SVV which was maintained <10%. The SVV was measured using a Flotrac Vigileo™ third generation device and fluid replacements were administered until the SVV was corrected to <10%. The arterial blood gas (ABG) analysis was done every 2 h during the surgery.
The type of fluids replaced in the perioperative period was at the discretion of the anesthesiologist. The crystalloids used included Ringer's lactate and normal saline and all patients received 1.0 L of hydroxyethyl starch (130/0.4) prior to the administration of blood or products. Packed red blood cells were infused if blood loss exceeded allowable limits and ABG values showed a decrease in hematocrit to <27%. Vasopressor (noradrenaline) was added to maintain the mean arterial pressure (MAP) >65 mm Hg if the hypotension could not be corrected by volume replacement and the measured value of SVR was low. An inotrope (dopamine/dobutamine) was added if the blood pressure did not respond to fluid boluses and if the SVR was normal.
Intraoperative parameters recorded included the total volume of IV fluids (colloids/crystalloids/blood), 2 hourly ABG analysis from which serum lactate was obtained. We also recorded the MAP, CVP, SVV (intervention group), and the requirement of vasoactive agents and the total duration of surgery in both groups of patients.
At the end of surgery, patients were extubated after administration of neostigmine 0.05 mg/kg and glycopyrrolate 10 mcg/kg. If the patients were hemodynamically unstable or hypothermic, they were ventilated until reassessment the next morning. All patients were shifted to a surgical ICU managed by an intensivist who was blinded to the study allocation.
Postoperative parameters monitored were the need for postoperative ventilation, or the requirement of ventilatory assistance (BiPAP) after extubation. Time to return of bowel function was assessed by time to passage of flatus or functioning of stoma. The duration of ICU stay and time to discharge from the hospital were noted as primary end points.
The numerical data were analyzed using Mann-Whitney U nonparametric test. The categorical data were analyzed using Fisher's exact test. Corresponding “P” values were also calculated. P <0.05 was considered statistically significant.
Results
Demographic parameters and duration of anesthesia were comparable in both groups [Table 1]. The primary outcome measure, the postoperative ICU stay, was significantly shorter in the intervention group as compared to the control group [Table 2]. The length of hospital stay was also shorter in the intervention versus the control group however this value was not significant statistically [Table 2].
Table 1.
Comparison of demographic data
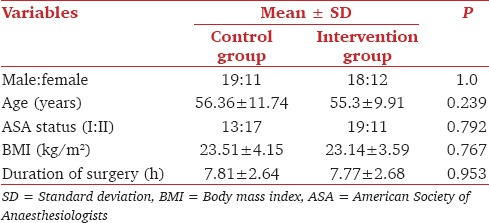
Table 2.
Comparison of ICU and hospital stay in days

The mean volume of IV fluids given in the intervention group to maintain the SVV <10% was significantly lesser than the volume of IV fluids administered in the control group [Table 3]. The mean intraoperative blood loss in the intervention and control groups were comparable [Table 4]. The lactate levels during surgery were similar between the two groups [Table 3].
Table 3.
Comparison of secondary outcome variables
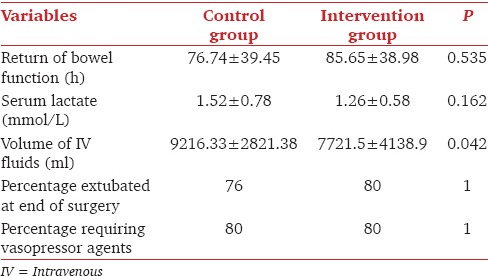
Table 4.
Comparison of intraoperative blood loss and postoperative renal function
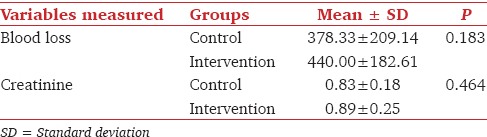
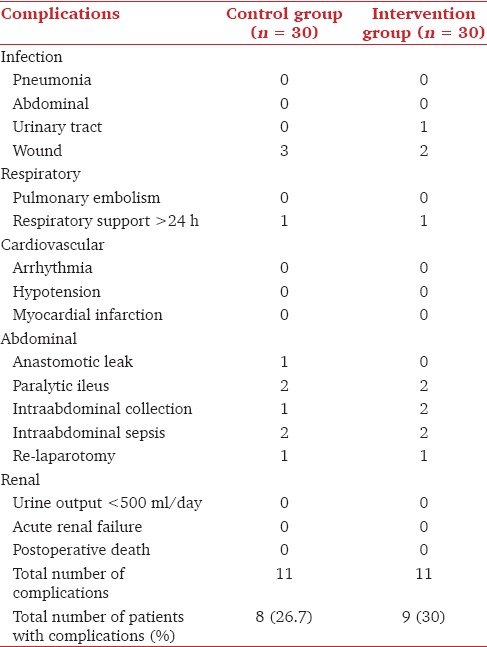
All patients had urine output >0.5 ml/kg/h during surgery and postoperative creatinine levels were comparable [Table 4]. The numbers of patients needing inotropes, postoperative ventilation and the time to return of bowel function were not different between the two groups (P > 0.05, Table 3). Nine patients in the intervention and eight patients in the control group developed postoperative complications [Table 5].
Table 5.
Distribution of postoperative complications among groups
Discussion
We found that the fluid replacement according to the SVV protocol resulted in a significant reduction in the postoperative ICU stay. We hypothesized that the fluid administration targeted to the SVV optimized the cardiac output and tissue perfusion in this group of patients similar to several studies.[12,13,14] In the study by Lopes et al.[15] fluid administration based upon pulse pressure variation (PPV) resulted in a significantly shorter postoperative stay in the intervention group.
Studies comparing volume replacements by specific strategies had aimed to keep the CVP between 8 and 15 mm Hg.[16,17] We aimed a CVP between 8 and 12 mmHg in our patients as epidural vasodilatation demanded adequate fluid replacement prior to the use of vasopressors. Inadequate fluid replacement may result in the use of inotropes that could result in tachycardia, increased myocardial oxygen demand and risk for arrhythmias.
Although the length of hospital stay appeared to be lesser in the intervention group, this was not different from the control statistically. This is contrary to the study by Lopes et al.,[15] where a difference in both ICU stay and hospital stay were observed. In their study, the control group did not have targets for CVP while the intervention group targeted PPV unlike our study where even the control had a targeted fluid administration based on the CVP. We believe that this fluid optimization may have improved tissue perfusion in the control group also.
In our study, the intervention group (SVV) received significantly less fluid than CVP group. This finding although contradictory to other studies targeting the PPV[15] or the systolic pressure variation[18] could be explained by the fact that we allowed a liberal fluid administration. We also think that in all patients, the epidural analgesia at a thoracic level could have caused vasodilatation and hypotension that increased fluid resuscitation.
We used serum lactate levels as a surrogate for adequacy of tissue perfusion in the intraoperative period. Although the mean serum lactate values were lower in the intervention group (1.26 vs. 1.52) this was not significant. The levels of lactate in both groups were well within the normal range despite the long duration of surgery and large volume shifts suggesting the overall beneficial effects of adequate volume replacements during major abdominal surgeries.
The blood loss in both the groups was comparable in our study and the use of colloids and blood products were not different between the two groups. Only 4 patients in the control and 3 in the intervention group needed a transfusion (6 units vs. 5 units). There was no statistically significant difference between the two groups in the requirement of vasoactive agents.
Patients in both groups maintained urine output of >0.5 ml/kg/h and none developed postoperative acute kidney injury as reflected by a normal and comparable creatinine values in the postoperative period.
Brandstrup et al.[19] observed that patients managed with a restrictive intraoperative fluid regimen had fewer complications and a shorter postoperative stay in the ICU. This was also reported by Lobo et al.[20] who showed that a restrictive fluid strategy while targeting oxygenation indices in high-risk surgery reduced the complications after surgery. In our study, the patients in the SVV group received significantly less fluids and also had a significantly shorter postoperative ICU stay than the control, although the fluid replacements were above the volumes used in a restrictive strategy.
The difference between the two groups in the time to return of bowel function was not statistically significant in our study. Wakeling et al.[16] demonstrated that esophageal Doppler guided fluid management minimized the time to return of bowel function and postoperative hospital stay. In their study the intervention group received significantly more colloids than the control group.
The incidence of postoperative complications was not significantly different between the two groups in our study (26.7% vs. 30%, Table 5). One patient in the control group and 1 patient in the intervention needed re-laparotomy for sepsis and 1 patient in the control for postoperative anastomotic leak. There were no differences in the duration of postoperative ventilation or need for respiratory assist devices. In this study, we used the FloTrac Vigileo™, a third generation device for assessment of SVV. The SVV derived from this was found to have a good correlation with the SVV obtained from PiCCO plus[21] which uses a femoral arterial line and has shown to be a reliable predictor of SVV in several studies.[22] The threshold value for SVV using the FloTrac was 9.6% in this study[21] and hence we aimed to keep the values of SVV <10% in the intervention group.
Our study had limitations in the numbers of patients and in the fact that we had excluded higher risk patients undergoing major surgeries. A larger multicenter trial including higher ASA grades could be considered in the background of these results.
Conclusion
Fluid replacements targeting to minimize SVV during major abdominal surgery resulted in a shorter postoperative ICU stay and lesser intraoperative fluid requirement as compared to CVP guided fluid replacement. Implementation of fluid replacement guided by a dynamic preload variable (SVV) appeared to have distinct advantages over conventional static variables (CVP) on postoperative ICU stay in abdominal surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Boyd O, Bennett ED. Achieving the goal. Crit Care Med. 1999;27:2298–9. doi: 10.1097/00003246-199910000-00045. [DOI] [PubMed] [Google Scholar]
- 2.Mythen MG, Webb AR. Intra-operative gut mucosal hypoperfusion is associated with increased post-operative complications and cost. Intensive Care Med. 1994;20:99–104. doi: 10.1007/BF01707662. [DOI] [PubMed] [Google Scholar]
- 3.Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64–8. doi: 10.1097/01.CCM.0000249851.94101.4F. [DOI] [PubMed] [Google Scholar]
- 4.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness. A systematic review of the literature and the tale of seven mares? Chest. 2008;134:172–8. doi: 10.1378/chest.07-2331. [DOI] [PubMed] [Google Scholar]
- 5.Mythen MG, Webb AR. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg. 1995;130:423–9. doi: 10.1001/archsurg.1995.01430040085019. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair S, James S, Singer M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: Randomised controlled trial. BMJ. 1997;315:909–12. doi: 10.1136/bmj.315.7113.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donati A, Loggi S, Preiser JC, Orsetti G, Münch C, Gabbanelli V, et al. Goal-directed intraoperative therapy reduces morbidity and length of hospital stay in high-risk surgical patients. Chest. 2007;132:1817–24. doi: 10.1378/chest.07-0621. [DOI] [PubMed] [Google Scholar]
- 8.Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005;103:419–28. doi: 10.1097/00000542-200508000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Marx G, Cope T, McCrossan L, Swaraj S, Cowan C, Mostafa SM, et al. Assessing fluid responsiveness by stroke volume variation in mechanically ventilated patients with severe sepsis. Eur J Anaesthesiol. 2004;21:132–8. doi: 10.1017/s0265021504002091. [DOI] [PubMed] [Google Scholar]
- 10.Hofer CK, Müller SM, Furrer L, Klaghofer R, Genoni M, Zollinger A. Stroke volume and pulse pressure variation for prediction of fluid responsiveness in patients undergoing off-pump coronary artery bypass grafting. Chest. 2005;128:848–54. doi: 10.1378/chest.128.2.848. [DOI] [PubMed] [Google Scholar]
- 11.Manecke GR. Edwards Flo Trac TM sensor and Vigileo TM monitor: Easy, accurate, reliable cardiac output assessment using the arterial pulse wave. Exp Rev Med Devices. 2005;2:523–7. doi: 10.1586/17434440.2.5.523. [DOI] [PubMed] [Google Scholar]
- 12.Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97:820–6. doi: 10.1097/00000542-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445] Crit Care. 2005;9:R687–93. doi: 10.1186/cc3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer J, Boldt J, Mengistu AM, Röhm KD, Suttner S. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: A randomized, controlled trial. Crit Care. 2010;14:R18. doi: 10.1186/cc8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopes MR, Oliveira MA, Pereira VO, Lemos IP, Auler JO, Jr, Michard F. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: A pilot randomized controlled trial. Crit Care. 2007;11:R100. doi: 10.1186/cc6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakeling HG, McFall MR, Jenkins CS, Woods WG, Miles WF, Barclay GR, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth. 2005;95:634–42. doi: 10.1093/bja/aei223. [DOI] [PubMed] [Google Scholar]
- 17.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 18.Buettner M, Schummer W, Huettemann E, Schenke S, van Hout N, Sakkal SG. Influence of systolic-pressure-variation-guided intraoperative fluid management on organ function and oxygen transport. Br J Anaesth. 2008;101:194–9. doi: 10.1093/bja/aen126. [DOI] [PubMed] [Google Scholar]
- 19.Brandstrup B, Tonnesen H, Beier-Holgersen R, Hjortso E, Ording H, Lindorff-Larsen K. Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens. A randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–8. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lobo MS, Ronchi SL, Oliviera NG, Brandao PG, Froes A, Cunrath GS, et al. Restrictive strategy of intraoperative fluid maintenance during optimization of oxygen delivery decreases major complications after high-risk surgery. Crit Care. 2011;15:R226. doi: 10.1186/cc10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofer CK, Senn A, Weibel L, Zollinger A. Assessment of stroke volume variation for prediction of fluid responsiveness using the modified FloTrac and PiCCOplus system. Crit Care. 2008;12:R82. doi: 10.1186/cc6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiesenack C, Fiegl C, Keyser A, Prasser C, Keyl C. Assessment of fluid responsiveness in mechanically ventilated cardiac surgical patients. Eur J Anaesthesiol. 2005;22:658–65. doi: 10.1017/s0265021505001092. [DOI] [PubMed] [Google Scholar]


