Abstract
Background and Aim:
Induced hypotension limits intra-operative blood loss to provide better visibility of the surgical field and diminishes the incidence of major complications during functional endoscopic sinus surgery (FESS). We aimed at comparing nitroglycerine, esmolol and dexmedetomidine for inducing controlled hypotension in patients undergoing FESS.
Material and Methods:
One hundred and fifty American Society of Anesthesiologists physical status I or II adult patients undergoing FESS under general anesthesia were randomly allocated to three groups of 50 patients each. Group E received esmolol in a loading and maintenance dose of 1 mg/kg over 1 min and 0.5-1.0 mg/kg/h, respectively. Group D received a loading dose of dexmedetomidine 1 μg/kg over 10 min followed by an infusion 0.5-1.0 μg/kg/h, and group N received nitroglycerine infusion at a dose of 0.5-2 μg/kg/min so as to maintain mean arterial pressure (MAP) between 60 and 70 mmHg in all the groups. The visibility of the surgical field was assessed by surgeon using Fromme and Boezaart scoring system. Hemodynamic variables, total intra-operative fentanyl consumption, emergence time and time to first analgesic request were recorded. Any side-effects were noted. The postoperative sedation was assessed using Ramsay Sedation Score.
Result:
The desired MAP (60-70 mmHg) could be achieved in all the three study groups albeit with titration of study drugs during intra-operative period. No significant intergroup difference was observed in Fromme's score during the intra-operative period. The mean total dose of fentanyl (μg/kg) used was found to be significantly lower in group D compared to groups E and N (1.2 ± 0.75 vs. 3.6 ± 1.3 and 2.9 ± 1.1 respectively). The mean heart rate was significantly lower in group D compared to groups E and N at all times of measurement (P < 0.05). The MAP was found to be significantly lower in group D compared to groups E and N after infusion of study drugs, after induction, just after intubation and 5 min after intubation (P < 0.05). The Ramsay Sedation Scores were significantly higher in group D (score 3 in 46%) when compared to group E (score 2 in 50%) and group N (score 2 in 54%) (P < 0.001). The emergence time was significantly lower in group E and group N compared to group D. Time to first analgesic request was significantly longer in group D.
Conclusion:
Dexmedetomidine and esmolol provided better hemodynamic stability and operative field visibility compared to nitroglycerin during FESS. Dexmedetomidine provides an additional benefit of reducing the analgesic requirements and providing postoperative sedation.
Keywords: Controlled hypotension, dexmedetomidine, esmolol, functional endoscopic sinus surgery, nitroglycerine
Introduction
Rhino-sinusitis, an important cause of significant discomfort and morbidity is commonly treated with FESS nowadays.[1,2,3] However, there can be serious complications associated with this procedure during peri-operative period like orbital cellulitis, optic nerve injuries, meningitis, etc. whose incidence can increase with excessive bleeding during surgery.[4,5] Hence, it is mandatory to keep the surgical field as free of blood as possible to improve visibility of anatomical landmarks and structures. This can be achieved with the use of topical vasoconstrictors, with local anesthesia or use of controlled hypotension with general anesthesia.
Controlled hypotension involves reducing arterial blood pressure 30-40% below its normal range or reducing mean arterial pressure (MAP) to 65 mmHg reversibly and maintaining it at that level throughout the surgery.[6] A variety of medications can be used to induce intra-operative hypotension including vasodilators like sodium nitroprusside,[7] nitroglycerin[8] and hydralazine; inhaled anesthetics like isoflurane[9] and sevoflurane; intravenous anesthetics like propofol; beta adrenergic antagonists like esmolol;[10] trimethaphan, adenosine and α2 agonists. Some of the reported disadvantages with the use of these agents include resistance to vasodilators, tachyphylaxis with nitroglycerin, cyanide toxicity with the use of nitroprusside and delayed recovery from anesthesia with the use of high doses of inhaled anesthetics.[11]
Esmolol and nitroglycerine have been frequently compared for controlled hypotension during nasal surgery.[8,12] Dexmedetomidine has also gained wide acceptance for induced hypotension because of its sedation, analgesia and anxiolysis.[11,13,14] There are no studies comparing the efficacy of these three drugs in achieving controlled hypotension. Therefore, this randomized study was planned using these three drugs for inducing and maintaining controlled hypotension in patients undergoing functional endoscopic sinus surgery (FESS) under general anesthesia.
Material and Methods
This prospective randomized study was carried out after the approval of Institutional Ethics Committee. Hundred and fifty patients belonging to American Society of Anesthesiologists physical status class I or II, aged between 18 and 55 years and posted for elective FESS under general anesthesia were included in the study. Fifty patients were allocated to each of the three groups randomly, based on computer generated numbers. The operating surgeon and the anesthesiologist doing the peri-operative monitoring were blinded to the study drug by wrapping the syringes with number codes. Patients with uncontrolled hypertension, cardiovascular diseases including rhythm disturbances, renal or hepatic dysfunction, coagulation defects or patients on medications affecting coagulation system were excluded from the study.
A thorough preanesthetic evaluation was performed and an informed written consent was taken from all the patients by the investigator a day prior to the surgery. The patients received nil per oral instructions as per the standard protocol and were premedicated with alprazolam 0.25 mg and ranitidine 150 mg orally in the night and in the morning of day of surgery. After shifting the patients to the operating room, noninvasive blood pressure, five lead electrocardiography and pulse oximetry were started. Baseline vitals were recorded including heart rate (HR), MAP and oxygen saturation. After securing an intravenous line, preloading was carried out with lactated ringer's solution 5 ml/kg. The patients were randomly allocated by computer generated numbers in to three groups:
Group D: Received dexmedetomidine loading dose of 1 μg/kg given over 10 min, followed by a continuous infusion of 0.5-1.0 μg/kg/h.
Group N: Received an infusion of nitroglycerine 0.5-2 μg/kg/min.
Group E: Received a loading dose of esmolol 1 mg/kg infused over 1 min, followed by a continuous infusion of 0.5-1.0 mg/kg/h.
All the infusions were titrated to maintain a MAP between 60 and70 mmHg.
The loading doses of dexmedetomidine and esmolol were administered before the induction of anesthesia. Nitroglycerine was started as infusion without any bolus dose. The duration of infusion was constant in all the three groups. The induction of anesthesia was done with thiopentone sodium 5 mg/kg and fentanyl 2 μg/kg, followed by vecuronium 0.1 mg/kg intravenously. After tracheal intubation, anesthesia was maintained with one minimum alveolar concentration isoflurane in nitrous oxide and oxygen mixture (60:40) and top-up doses of vecuronium as and when required. An oropharyngeal pack was kept after the intubation.[15] An additional dose of fentanyl 1 μg/kg was given intra-operatively with an increase in HR and MAP of more than 20% from baseline values.
To further reduce the amount of surgical bleeding and for surgeon's convenience, all the patients were positioned in approx. 30° reverse trendelenburg position. Two ml of lignocaine-adrenaline (1:100,000) mixture was infiltrated at the surgical site by the surgeon in all the patients.
Heart rate, MAP, SpO2 and EtCO2 were monitored throughout the surgery and recorded at baseline, after loading dose of the study drug, after induction, after intubation, 5 min after intubation, at an interval of 5 min intra-operatively, after reversal, after extubation and 5 min after extubation. HR <45 beats/min was considered as bradycardia, and was managed with 0.5 mg atropine intravenously. MAP <60 mmHg was initially managed with a 50% reduction in the infusion dose of the study drug and further stoppage of the infusion if no response was obtained in 5 min. Mephentermine 6 mg intravenously was administered for the resistant hypotension.
The visibility of the operative field was assessed by the surgeon according to the scale proposed by Fromme and Boezaart.[16]
Five minutes before the end of surgery, all the study drugs were discontinued. At the end of the surgery, the nasal packing was done keeping a cut piece of small size endotracheal tube to allow the patient to breathe through the nose postoperatively.[17] The residual neuromuscular blockade was antagonized with neostigmine 0.05 mg/kg and glycopyrrolate 0.1 mg/kg intravenously and extubation was done when the patient was fully awake, breathing regularly with adequate tidal volume. Total intra-operative fentanyl consumption, duration of surgery and total anesthesia time were recorded. Emergence time, defined as the interval between discontinuation of the anesthetics to response of eye opening to the verbal command,[18] was also recorded. The postoperative sedation was assessed with Ramsay Sedation Score.[19] The postoperative side-effects such as nausea and vomiting, shivering and dry mouth were observed and recorded. The time for the first analgesic request after the surgery was also recorded.
Statistical analysis
Data were compiled analyzed using the Statistical Package for the Social Sciences 15.0 (SPSS Inc., Chicago IL, USA) statistical software package. Pearson Chi-square was used to examine the categorical data. MAP and HR within each group were analyzed using analysis of variance with Bonferroni's correction. Multivariate analysis was carried out to adjust surgeon's biased opinion and other confounding variables. To detect a significant difference of 10 mmHg in the MAP and also the associated simultaneous clarity of the surgical field between the groups, the overall sample size was estimated at 142 with a power of 80% and alpha error of 5%. However, 50 patients were selected for each group keeping in consideration the possible dropouts and for better validation of results. A P < 0.05 was considered significant.
Results
A total of 150 patients were included in the study and were divided randomly into three groups of 50 patients each (n = 50).
The demographic variables among the three groups are shown in Table 1 and there was no statistically significant difference among the three groups with regard to demographic variables, duration of surgery and total anesthesia time. The mean total dose of fentanyl in μg/kg used was significantly lower in group D compared to groups N and E (1.2 ± 0.75 vs. 3.6 ± 1.3 and 2.9 ± 1.1 respectively) [Table 1].
Table 1.
The demographic variables
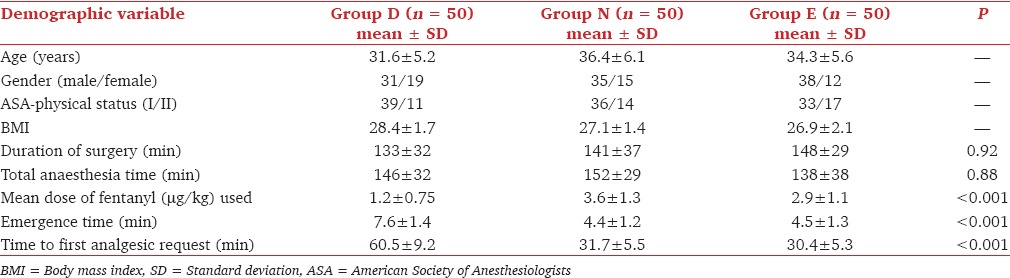
The mean HR was significantly lower in group D compared to groups N and E at all the times of measurements (P < 0.05) [Table 2].
Table 2.
Comparison of mean HR (per minute) in group D, N and E
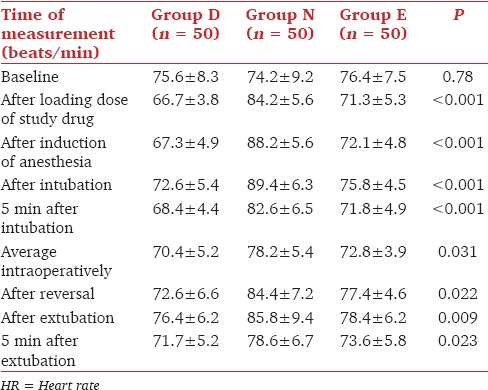
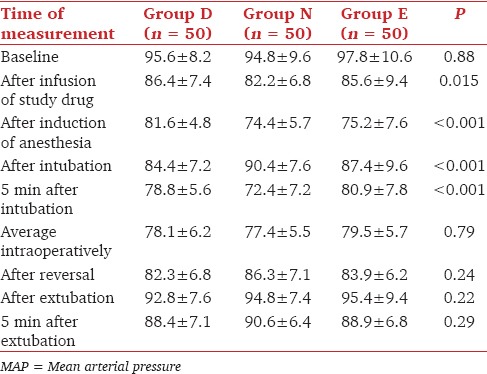
The MAP was significantly lower in group D compared to groups N and E after infusion of study drugs, after induction of anesthesia, after intubation and 5 min after intubation (P < 0.05) [Table 3]. However, the desired MAP for intra-operative induced hypotension could be achieved in all the three groups. There was no statistically significant difference in the Fromme's score in the three groups. None of the patients experienced bradycardia, resistant hypotension or hypertension during the study period. None of them required additional atropine or mephentermine.
Table 3.
Comparison of MAP (mmHg) in group D, N and E
The emergence time was significantly shorter in group E and group N compared to group D [Table 1].
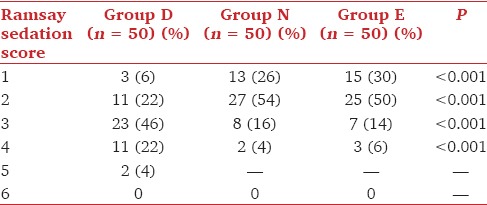
No serious side-effects were observed in any of the three groups. The incidence of nausea and vomiting was comparable in the three groups. The major side-effect observed in group D was dry mouth (26%) [Table 4]. The Ramsay Sedation Scores were significantly higher in group D compared with groups N and E with majority of patients having a sedation score of 3 (46%) in group D while most of the patients had a score of 2 in groups N and E (54% in group N and 50% in group E) [Table 5] two patients in group D had sedation score of 5 and were deeply sedated. The time to first analgesic request was significantly prolonged in group D when compared to other groups [Table 1].
Table 4.
Side-effect profile of patients in groups D, N and E

Table 5.
Degree of sedation (Ramsay Sedation Score) during postoperative period
Discussion
An important technique to reduce bleeding during the surgery is controlled reduction in blood pressure to such levels so that bleeding is minimal, but at the same time perfusion to the vital organs is well-maintained. This is the underlying concept for controlled hypotensive anesthesia.[20] Reduced bleeding in the operative site improves the quality of the surgical field, decreases the number of manipulations as well as the incidence of major complications and shortens the surgical time.[10,21]
Dexmedetomidine, a selective α2 adrenoceptor agonist, causes reduction in blood pressure, slowing of HR, sedation and analgesia. The fall in blood pressure is mainly due to inhibition of central sympathetic outflow and also due to stimulation of presynaptic α2 adrenoceptors decreasing norepinephrine release.[22] An important advantage is its minimal respiratory depressant effect with potent sedative and analgesic effects compared with opioids and other sedatives. A few studies have shown that dexmedetomidine decreases the bleeding in surgeries within the framework of hemodynamic stability.[11,13,14,23]
The heart rate was higher in group N due to reflex tachycardia associated with nitroglycerine infusion. Dexmedetomidine caused a lower heart rate due to its sympatholytic effect.[24]
The MAP also showed a significant reduction in group D compared to group N and E, but only at three observation times, that is, after induction of anesthesia, after intubation and 5 min after intubation. This observation suggested that dexmedetomidine is effective in blunting the hemodynamic response of stress during laryngoscopy as has been shown by other studies.[25,26] The MAP however, was equally lowered in all the three groups suggesting equal efficacy of all the three drugs in lowering the MAP, thereby providing comparable surgical field as suggested by the Fromme and Boezaart's score.
Cincikas and Ivaskevicius[27] used nitroglycerine infusion (0.79 ± 0.34 μg/kg/min) to maintain MAP of 50-60 mmHg during endoscopic nasal surgery and observed reduced surgical bleeding and improved surgical view quality. Guven et al.[28] used dexmedetomidine for conscious sedation for FESS and reported better hemodynamic stability and improved surgical field.
The analgesic efficacy of dexmedetomidine has been appreciated in diverse settings.[29,30,31,32,33] Similarly, we found that intra-operative fentanyl requirement was significantly reduced in the dexmedetomidine group as compared to the other two groups. The patients in group D had a longer emergence time, as reported in other studies as well.[11,34] We also observed a significant delay in the first postoperative analgesic request in group D as compared to the other two groups. It has been shown that perioperative analgesic requirements are significantly reduced with intra-operative use of dexmedetomidine infusion.[11,35] The patients in the dexmedetomidine group had significantly higher sedation scores compared to group N and E. Shams et al.[11] also reported higher postoperative sedation scores with the intra-operative use of dexmedetomidine. The sedative and analgesic sparing effects of dexmedetomidine are mediated through its action in the locus coeruleus and dorsal horn of spinal cord respectively.[36] The postoperative sedation is often desirable, but may sometimes prolong the emergence time.[34] The incidence of postoperative shivering was significantly lower in the dexmedetomidine group, as acknowledged earlier also.[37] The most frequent reported side-effect with dexmedetomidine is dry mouth, which is not bothersome and can be easily managed.
Conclusion
Dexmedetomidine and esmolol provided better hemodynamic stability and comparable operative field visibility to nitroglycerine during FESS. Dexmedetomidine provides an additional benefit of reducing the analgesic requirements and providing postoperative sedation.
Footnotes
Source of Support: Nil
Conflicts of Interest: None declared.
References
- 1.Slavin RG. Sinusitis in adults. J Allergy Clin Immunol. 1988;81:1028–32. doi: 10.1016/0091-6749(88)90174-1. [DOI] [PubMed] [Google Scholar]
- 2.Stammberger H. Endoscopic endonasal surgery—concepts in treatment of recurring rhinosinusitis. Part II. Surgical technique. Otolaryngol Head Neck Surg. 1986;94:147–56. doi: 10.1177/019459988609400203. [DOI] [PubMed] [Google Scholar]
- 3.Stammberger H, Posawetz W. Functional endoscopic sinus surgery. Concept, indications and results of the Messerklinger technique. Eur Arch Otorhinolaryngol. 1990;247:63–76. doi: 10.1007/BF00183169. [DOI] [PubMed] [Google Scholar]
- 4.Stankiewicz JA. Complications of endoscopic intranasal ethmoidectomy. Laryngoscope. 1987;97:1270–3. doi: 10.1288/00005537-198711000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Maniglia AJ. Fatal and other major complications of endoscopic sinus surgery. Laryngoscope. 1991;101:349–54. doi: 10.1002/lary.1991.101.4.349. [DOI] [PubMed] [Google Scholar]
- 6.Van Aken H, Miller D. Delibrate hypotension. In: Miller RD, editor. Anesthesia. New York, USA: Churchill Livingstone Inc; 2000. pp. 1470–90. [Google Scholar]
- 7.Degoute CS, Ray MJ, Manchon M, Dubreuil C, Banssillon V. Remifentanil and controlled hypotension; comparison with nitroprusside or esmolol during tympanoplasty. Can J Anaesth. 2001;48:20–7. doi: 10.1007/BF03019809. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava U, Dupargude AB, Kumar D, Joshi K, Gupta A. Controlled hypotension for functional endoscopic sinus surgery: Comparison of esmolol and nitroglycerine. Indian J Otolaryngol Head Neck Surg. 2013;65:440–4. doi: 10.1007/s12070-013-0655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandal P. Isoflurane anaesthesia for functional endoscopis sinus surgery. Indian J. Anaesth. 2003;47:37–40. [Google Scholar]
- 10.Degoute CS. Controlled hypotension: A guide to drug choice. Drugs. 2007;67:1053–76. doi: 10.2165/00003495-200767070-00007. [DOI] [PubMed] [Google Scholar]
- 11.Shams T, El Bahnasawe NS, Abu-Samra M, El-Masry R. Induced hypotension for functional endoscopic sinus surgery: A comparative study of dexmedetomidine versus esmolol. Saudi J Anaesth. 2013;7:175–80. doi: 10.4103/1658-354X.114073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guney A, Kaya FN, Yavascaoglu B, Gurbet A, Selmi NH, Kaya S, et al. Comparison of esmolol to nitroglycerine in controlling hypotension during nasal surgery. EAJM. 2012;44:99–105. doi: 10.5152/eajm.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turan G, Dincer E, Ozgultekin A, Uslu C, Akgun N. Comparison of dexmedetomidine, remifentanyl and esmolol in controlled hypotensive anaesthesia. Eur J Anaesthesiol. 2008;25:65–6. [Google Scholar]
- 14.Erbesler ZA, Bakan N, Karaoren GY, Erkmen MA. A comparison of the effects of esmolol and dexmedetomidine on the clinical course and cost for controlled hypotensive anaesthesia. Turk J Anaesth Reanim. 2013;41:156–61. doi: 10.5152/TJAR.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajwa SS. Prevention of aspiration of blood with a unique pharyngeal packing method. Anesth Essays Res. 2012;6:251–2. doi: 10.4103/0259-1162.108361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fromme GA, MacKenzie RA, Gould AB, Jr, Lund BA, Offord KP. Controlled hypotension for orthognathic surgery. Anesth Analg. 1986;65:683–6. [PubMed] [Google Scholar]
- 17.Bajwa SJ, Kaur J, Singh A, Parmar SS, Singh S. Postoperative airway management after nasal endoscopic sinus surgery: A comparison of traditional nasal packing with nasal airway. Anesth Essays Res. 2013;7:116–22. doi: 10.4103/0259-1162.114017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung F. Are discharge criteria changing? J Clin Anesth. 1993;5:64S–8. doi: 10.1016/0952-8180(93)90011-3. [DOI] [PubMed] [Google Scholar]
- 19.De Jonghe B, Cook D, Appere-De-Vecchi C, Guyatt G, Meade M, Outin H. Using and understanding sedation scoring systems: Asystematic review. Intensive Care Med. 2000;26:275–85. doi: 10.1007/s001340051150. [DOI] [PubMed] [Google Scholar]
- 20.Tobias JD. Controlled hypotension in children: A critical review of available agents. Paediatr Drugs. 2002;4:439–53. doi: 10.2165/00128072-200204070-00003. [DOI] [PubMed] [Google Scholar]
- 21.Baker AR, Baker AB. Anaesthesia for endoscopic sinus surgery. Acta Anaesthesiol Scand. 2010;54:795–803. doi: 10.1111/j.1399-6576.2010.02259.x. [DOI] [PubMed] [Google Scholar]
- 22.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 23.Kol IO, Kaygusuz K, Yildirim A, Dogan M, Gursoy S, Yucel E, et al. Controlled hypotension with desflurane combined with esmolol or dexmedetomidine during tympanoplasty in adults: A double-blind, randomized, controlled trial. Curr Ther Res Clin Exp. 2009;70:197–208. doi: 10.1016/j.curtheres.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basar H, Akpinar S, Doganci N, Buyukkocak U, Kaymak C, Sert O, et al. The effects of preanesthetic, single-dose dexmedetomidine on induction, hemodynamic, and cardiovascular parameters. J Clin Anesth. 2008;20:431–6. doi: 10.1016/j.jclinane.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H, Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Ann Card Anaesth. 2012;15:39–43. doi: 10.4103/0971-9784.91480. [DOI] [PubMed] [Google Scholar]
- 26.Bajwa SJ, Kaur J, Singh A, Parmar S, Singh G, Kulshrestha A, et al. Attenuation of pressor response and dose sparing of opioids and anaesthetics with pre-operative dexmedetomidine. Indian J Anaesth. 2012;56:123–8. doi: 10.4103/0019-5049.96303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cincikas D, Ivaskevicius J. Application of controlled arterial hypotension in endoscopic rhinosurgery. Medicina (Kaunas) 2003;39:852–9. [PubMed] [Google Scholar]
- 28.Guven DG, Demiraran Y, Sezen G, Kepek O, Iskender A. Evaluation of outcomes in patients given dexmedetomidine in functional endoscopic sinus surgery. Ann Otol Rhinol Laryngol. 2011;120:586–92. doi: 10.1177/000348941112000906. [DOI] [PubMed] [Google Scholar]
- 29.Huncke TK, Adelman M, Jacobowitz G, Maldonado T, Bekker A. A prospective, randomized, placebo-controlled study evaluating the efficacy of dexmedetomidine for sedation during vascular procedures. Vasc Endovascular Surg. 2010;44:257–61. doi: 10.1177/1538574410363621. [DOI] [PubMed] [Google Scholar]
- 30.Bajwa S, Kulshrestha A. Dexmedetomidine: An adjuvant making large inroads into clinical practice. Ann Med Health Sci Res. 2013;3:475–83. doi: 10.4103/2141-9248.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bajwa SJ, Arora V, Kaur J, Singh A, Parmar SS. Comparative evaluation of dexmedetomidine and fentanyl for epidural analgesia in lower limb orthopedic surgeries. Saudi J Anaesth. 2011;5:365–70. doi: 10.4103/1658-354X.87264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karaaslan K, Yilmaz F, Gulcu N, Colak C, Sereflican M, Kocoglu H. Comparison of dexmedetomidine and midazolam for monitored anesthesia care combined with tramadol via patient-controlled analgesia in endoscopic nasal surgery: A prospective, randomized, double-blind, clinical study. Curr Ther Res Clin Exp. 2007;68:69–81. doi: 10.1016/j.curtheres.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richa F, Yazigi A, Sleilaty G, Yazbeck P. Comparison between dexmedetomidine and remifentanil for controlled hypotension during tympanoplasty. Eur J Anaesthesiol. 2008;25:369–74. doi: 10.1017/S0265021508003761. [DOI] [PubMed] [Google Scholar]
- 35.Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. 2006;53:646–52. doi: 10.1007/BF03021622. [DOI] [PubMed] [Google Scholar]
- 36.Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84:873–81. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Bajwa SJ, Gupta S, Kaur J, Singh A, Parmar S. Reduction in the incidence of shivering with perioperative dexmedetomidine: A randomized prospective study. J Anaesthesiol Clin Pharmacol. 2012;28:86–91. doi: 10.4103/0970-9185.92452. [DOI] [PMC free article] [PubMed] [Google Scholar]


